Abstract
In functional neuroimaging, a local decrease in blood flow during an active task, relative to a “resting” baseline, is referred to as task-induced deactivation (TID). TID may occur when resources shift from ongoing, internally generated processing typical of “resting” states to processing required by an exogenous task. We previously found specific brain regions in which TID increased as task processing demands increased. When engaged in an exogenous cognitive task, reallocation of resources from areas involved in internal processing should result in suspension of that processing. Self-reported thought content has been used as an indicator of the extent of internal processing activity. We investigated the relationship between TID and task-unrelated thought (TUT) frequency using an auditory target detection task with seven levels of task difficulty. At varied intervals during task performance, subjects indicated whether they were experiencing a TUT. We expected TUT frequency to decrease as task demands increased and for this pattern to correlate with TID magnitude across conditions. Generally, fewer TUTs were reported during difficult task conditions than during easier conditions. As TID magnitude increased across task conditions, the frequency of TUTs declined (r = 0.90, P = 0.005). Four left hemisphere regions (posterior parieto-occipital cortex, anterior cingulate gyrus, fusiform gyrus, and middle frontal gyrus) showed strong relationships between TUTs and TID (r > 0.79, P < 0.05 corrected). As these regions have been implicated in semantic processing and self-referential thought, the findings support the suspension of internal cognitive processing as one mechanism for TID.
Keywords: fMRI, Task-induced deactivation, Resting state processing, Task-unrelated thoughts, Task difficulty
Introduction
Researchers studying brain function generally assume that presentation of a stimulus or task will induce increased neural activity in the brain relative to a resting state. Investigators using functional neuroimaging techniques to study human cognition have been surprised to find that many tasks also produce relative decreases in blood flow or blood-oxygenation indexes of neural activity, a phenomenon referred to as “task-induced deactivation” (TID). Several studies (Binder et al., 1999; Mazoyer et al., 2001; McKiernan et al., 2003; Shulman et al., 1997) demonstrated that TID occurs consistently in specific brain regions, including posterior cingulate cortex, dorsomedial prefrontal cortex, rostral anterior cingulate gyrus, orbitofrontal cortex, and angular gyrus. The mechanisms underlying these deactivations, however, are not fully understood.
Some researchers (Binder et al., 1999; Gusnard and Raichle, 2001; Mazoyer et al., 2001; McKiernan et al., 2003; Raichle et al., 2001; Shulman et al., 1997) have speculated that the “resting” condition is actually a state of organized cognitive activity involving many processes, including monitoring of the external environment, monitoring of body image and state, and processing emotions. Also included in the list of possible resting activities is the ongoing internal “thought” processing that humans experience during resting consciousness, sometimes referred to as “stream of consciousness” (James, 1890). Since these “thought” processes are generally self-initiated and self-referential and not related to specific exogenous task demands, we use the term “task-unrelated thoughts” (TUTs) to describe them (Giambra, 1989; Shaw and Giambra, 1993; Singer, 1988). This term is comparable to others such as “stimulus independent thoughts” (SITs) (Antrobus, 1968; Teasdale et al., 1993, 1995) and “free association” (Andreasen and O'Leary, 1995).
Based on our previous findings and the work of others (Binder et al., 1999; Mazoyer et al., 2001; McKiernan et al., 2003; Shulman et al., 1997), we suggested a mechanism of reallocation of brain processing resources to account for some instances of TID (McKiernan et al., 2003). This model proposes that internally generated cognitive activities (such as TUTs) are suspended due to reduced availability of resources when attention shifts from ongoing, internal processes to performance of an exogenous task. In support of this hypothesis, we observed a correlation between task difficulty and the magnitude of TID in many brain regions (McKiernan et al., 2003). TID in these areas may thus stem from the attenuation of internally generated processes when attentional resources are required for performance of an effortful exogenous task. From this perspective, processing demands of the exogenous task should also influence the propensity for TUTs, in that more demanding exogenous tasks leave fewer resources available for “stream of consciousness” processing.
TUTs may be experienced as self-generated thoughts or daydreams, often with verbal or visual content (McGuire et al., 1996). The content of TUTs can include all of the “monitoring” activities listed above as well as problem solving, planning, and retrieval of memories. TUTs have biological significance in that these activities all serve to enhance the potential for survival and can make the individual more efficient and effective in managing their environment and their actions. Research indicates that thought processes of this nature are salient features of the resting state (Antrobus, 1968; Antrobus et al., 1966; Giambra, 1989, 1995; Gusnard et al., 2001; James, 1890; McGuire et al., 1996; Pope and Singer, 1976; Posner and Rothbart, 1998; Singer, 1988; Teasdale et al., 1993, 1995). Self-reported thought content has been used as an indicator of the extent of internal processing activity (Antrobus, 1968; Antrobus et al., 1966; Giambra, 1989; McGuire et al., 1996; Pope and Singer, 1976; Shaw and Giambra, 1993; Singer, 1988; Teasdale et al., 1995). Early studies (Antrobus, 1968; Antrobus et al., 1966) described the effects of external task load on TUT frequency, noting that as more external information is presented, TUT frequency declines. Another study linked TUT frequency with processing resource availability by manipulating practice effects. Significantly more TUTs were reported when subjects had previously practiced motor or memory tasks than when practice was not completed (Teasdale et al., 1995). The theory of TID proposed above, as well as the results from these studies, suggests that TUT processing is interrupted by the presence of new information that requires priority processing. Thus, TUT processing is attention dependent and interruptible. It is also moderated by task difficulty, which determines the amount of processing resources required to be reallocated to the external task.
In a simulated scanning environment, we used a standardized query procedure to elicit thought content (TUT frequency) during the same auditory target detection task used in our previous experiment (McKiernan et al., 2003). As before, task difficulty was parametrically manipulated within each of three factors. These factors – stimulus presentation rate, target discriminability, and short-term memory load – were selected based on evidence that their manipulation influences task processing demands (Antrobus, 1968; Antrobus et al., 1966; Teasdale et al., 1993, 1995). We predicted a higher frequency of TUTs during rest than during any task condition. As task difficulty increased within each factor, we expected a decreased BOLD signal in TID regions and fewer TUTs. Additionally, TUT frequency was expected to correlate with the degree of TID, with the strongest correlations in brain regions associated with problem solving, planning, knowledge retrieval, and self-referential thought processing. Our goal is to link our previously established changes in TID in response to task difficulty manipulation with self-reported reductions in “stream of consciousness” processing.
Materials and methods
Subjects
Thirty neurologically healthy subjects (19 women and 11 men), ranging in age from 18 to 49 years (M = 27.7, SD = 7.2), participated in the study. All were right-handed as defined by a laterality quotient >50 on the Edinburgh Handedness Inventory (Oldfield, 1971). fMRI data from 50% of the subjects in this study were included in our first study describing TID magnitude as a function of task difficulty (McKiernan et al., 2003); the fMRI data from the other 15 subjects are reported here for the first time, as are the TUT data from all 30 subjects. Thus, the data presented here are substantially different than the data from our first study. Subjects received an hourly stipend for participating. The Medical College of Wisconsin Human Research Review Committee approved this study.
Cognitive task
Subjects performed an auditory target detection task, in which they pressed a button with their right index finger to identify target sounds (or target sound trains in short-term memory (STM) conditions). Stimuli were presented and data recorded using EPRIME software (EPRIME).
Each subject performed the auditory detection task on two different occasions: first during fMRI scanning (as previously described in McKiernan et al., 2003) for collection of TID data and then in a mock scanning environment for collection of TUT data. Subjects returned for the second session (mock scanning) 11–353 days (median = 78 days) after completing the first session. A complete description of task parameters and response requirements was reported previously (McKiernan et al., 2003). See Table 1 for a summary of the experimental parameters.
Table 1.
Parametric manipulation of task parameters
| Target difference from non-target | ITI (ms) | # Stims/24 s | |
|---|---|---|---|
| Stimulus presentation rate | |||
| Slower | 40% of octave | 2000 | 12 |
| Moderatea | 40% of octave | 1000 | 24 |
| Fast | 40% of octave | 600 | 40 |
| Target discriminability | |||
| Easya | 40% of octave | 1000 | 24 |
| Moderate | 28% of octave | 1000 | 24 |
| Difficult | 16% of octave | 1000 | 24 |
| Short-term memory load | |||
| Easya | 40% of octave | 1000 | 24 |
| Moderate | 40% of octave | 4000 | 24 |
| Difficult | 40% of octave | 4000 | 24 |
Common difficulty level across factors.
In the fMRI session, blocks of the task alternated with rest blocks every 24 s. Subjects completed two scanning runs of each of the seven task conditions. At the mock scanner for the TUT session, the target detection task was modified to include semi-randomly placed “TUT probes” (average interval between probes = 20 s, range = 16–24 s) to elicit current thought content. For each probe, the “scanner” noise (recorded from the 1.5T scanner) and the task (if during a task block) stopped to cue the subject to use their left middle or index finger to respond YES or NO to the question, “Was I having a task-unrelated thought?” Previous studies showed that the simple occurrence of a TUT is defined more reliably than its duration or intensity (Antrobus, 1968; Antrobus et al., 1966); thus, we employed a previously validated, simple decision procedure to probe thought content at a specific moment in time (Teasdale et al., 1993, 1995). We adapted this procedure to require only a binary decision (Yes or No) rather than a full verbal report of the thought content. A response from the subject prompted the recorded scanner noise and task to resume. Subjects completed two runs of each of the seven task conditions. In total, subjects responded to 28 probes for each task condition. They also responded to a total of 56 probes during rest blocks (8 during each of the 7 conditions).
Prior to collecting data, subjects were briefed on what constituted task-unrelated and task-related thoughts. A task-related thought was defined as any thought that was related to task completion; a TUT was defined as any other (non-task related) thought (e.g., what the subject did earlier that day, things they needed to do on the way home, were they feeling hungry, etc.). Each subject completed a short practice session before the testing began. Appendix A contains specific information regarding the instructions given to subjects.
Stimuli
As previously described (McKiernan et al., 2003), the non-target stimulus was a 200 ms complex auditory tone. The target stimulus was constructed by modulating, over time, all frequencies in the non-target stimulus using a linear ramp function with an excursion equal to 40% of an octave. For the experimental conditions in which target discriminability was manipulated (moderate discriminability and difficult discriminability), two additional target stimuli were constructed with frequency excursions equal to 28% and 16% of an octave, respectively.
fMRI parameters
fMRI data for this study were collected at the Medical College of Wisconsin on a 1.5-T GE Signa scanner using a 3-axis local gradient coil. Data were collected via a multislice gradient echo EPI protocol with TR = 3 s and TE = 40 ms. Voxel size was 3.75 × 3.75 × 6 mm. Twenty-one or twenty-three contiguous sagittal slices covering the whole brain were acquired. Ninety-six volumes were acquired during each task condition and each related rest condition.
Mock scanner
TUT data were collected at the Medical College of Wisconsin in a mock scanner environment. The mock scanner is a plywood replica of the actual scanner, including “head coil”, with an audio system to allow playback of scanner-produced sounds. Subjects were positioned in the mock scanner such that their subjective experience of the environment and the task was similar to the 1.5-T scanner.
Data analysis
Behavioral data
Accuracy and time-on-task during fMRI, accuracy and time-on-task during the TUT study, and TUT responses were recorded. Accuracy data are presented as a percentage of the number of correct responses minus the number of false alarms, divided by the number of targets. Reaction time data were used to calculate the percentage of the trial duration used by the subject (time-on-task). This value was computed by adding stimulus duration (200 ms) to mean reaction time (for correct responses only) and dividing by the length (ms) of the trial. This ratio allows us to assess the average percentage of total trial time spent on-task across a wide range of presentation and response rates. Since only correct responses are included in the analysis, we assume that the subject was on-task for the 200 ms stimulus presentation; thus, the ratio described above is valid for any condition of single stimulus presentation. Unlike the other conditions, however, the two more difficult levels of the short-term memory factor employed trains of stimuli. During the presentation of a sound train, there were two to four inter-stimulus intervals of 400 ms, and there is no specific measure of whether or not the subject was on-task during these periods. Thus, the time-on-task ratio cannot be calculated for these conditions and will therefore not be presented.
TUT frequency represents the number of positive probe responses divided by the number of valid probes per condition. Due to technical difficulties, there were a few occasions in which the probe response was not properly recorded, these trials were considered invalid.
fMRI data
All image analyses were accomplished using AFNI software (Cox, 1996a). Subject data were volumetrically registered using an iterative procedure that minimizes variance in voxel intensity differences between images (Cox, 1996b). The image analyses at both the subject and the group levels follow the same procedures outlined in our previous study (McKiernan et al., 2003), including resampling the anatomical and functional data into stereotaxic space (Talairach and Tournoux, 1988). The same anatomical regions of interest (ROIs) derived from the original group of 30 subjects in the previous study were used in the present study (note: 50% of the subjects overlap between studies). Briefly, the ROIs were identified by averaging individual correlation maps (representing correlation of activity with a predicted response vector with correlations then converted to z scores) from all subjects for all task conditions and thresholding the averaged map at an arbitrary value of z = − 1 (corresponding to regions that had greater activation during rest than during the task). This procedure produced eleven individual ROIs (in order of decreasing volume): left posterior parieto-occipital (PPO) cortex, which includes parts of the angular gyrus, dorsolateral occipital lobe and cuneus; right PPO cortex; right precuneus/superior parietal lobule; left precuneus/superior parietal lobule; left anterior cingulate/superior frontal gyrus; left posterior cingulate gyrus; left middle frontal gyrus; left middle occipital gyrus; right posterior cingulate gyrus; right anterior cingulate gyrus; and left fusiform gyrus.
The relative magnitude of deactivation within each ROI was measured using fit coefficients, which are computed by a least squares fit of the ideal response vector to the observed response in each voxel for each condition in each subject. For each task condition, average deactivation values for each ROI were measured in each subject by averaging the fit coefficients for voxels within the ROI (see McKiernan et al., 2003 for a detailed explanation).
Results
Four subjects were removed from the analyses based on inspection of TUT session data: three as outliers because they did not indicate any TUTs during rest and one due to poor performance on the auditory target detection task. Behavioral data from both fMRI and TUT sessions (N = 26) are presented in Table 2. Paired t tests comparing task performance across sessions revealed no differences in performance except on accuracy in the most difficult discriminability condition, which was higher during the fMRI session. This indicates that the task conditions in the fMRI session were satisfactorily replicated during the TUT session. It also suggests that the addition of the probes to the protocol did not significantly influence cognitive processing during the task conditions (i.e., subjects were not distracted by trying to “monitor” what they were thinking about). The rest conditions do not provide behavioral data for comparison; however, we assume that the probes did not significantly disrupt processing in this condition either. In accordance with the previous session, all analyses reported here were evaluated at P < 0.05 with a modified Bonferroni correction (Keppel, 1991) to accommodate multiple comparisons.
Table 2.
Summary data for the TID and TUT sessions
| TID session (N = 26) |
TUT session (N = 26) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time-on-task |
Percent accuracy |
TID magnitude |
Time-on-task |
Percent accuracy |
TUT frequency |
|||||||
| % | SD | M | SD | M | SD | % | SD | M | SD | % | SD | |
| Presentation rate | ||||||||||||
| Slower | 27.20 | 4.13 | 96.58 | 6.64 | −1.16 | 1.00 | 29.64 | 6.95 | 92.53 | 11.33 | 58.66 | 26.74 |
| Moderatea | 48.04 | 5.34 | 95.76 | 5.13 | −1.21 | 1.00 | 47.70 | 9.58 | 93.65 | 6.70 | 47.93 | 22.66 |
| Fast | 67.74 | 5.27 | 80.19 | 19.61 | −1.57 | .99 | 64.48 | 9.46 | 83.14 | 11.40 | 33.68 | 20.61 |
| Target discriminability | ||||||||||||
| Easya | 48.04 | 5.34 | 95.76 | 5.13 | −1.21 | 1.00 | 47.70 | 9.58 | 93.65 | 6.70 | 47.93 | 22.66 |
| Moderate | 49.20 | 4.91 | 91.52 | 20.51 | −1.40 | 1.04 | 47.60 | 5.09 | 89.15 | 10.69 | 43.45 | 23.16 |
| Difficult | 51.59 | 3.66 | 89.10 | 11.10 | −1.62 | 1.20 | 50.94 | 4.69 | 77.38 | 19.82 | 38.33 | 21.38 |
| Short-term memory load | ||||||||||||
| Easya | – | – | 95.76 | 5.13 | −1.21 | 1.00 | – | – | 93.65 | 6.70 | 47.93 | 22.66 |
| Moderate | – | – | 81.87 | 27.53 | −1.68 | 1.00 | – | – | 69.01 | 11.66 | 37.25 | 21.72 |
| Difficult | – | – | 84.62 | 26.50 | −1.54 | .97 | – | – | 88.76 | 12.07 | 41.12 | 22.51 |
Values significantly different across sessions at P < 0.05 corrected.
Common difficulty level across factors.
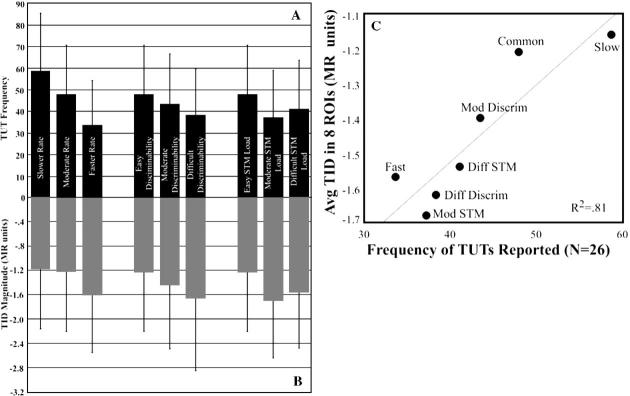
There were more TUTs reported during rest (90%) than during task conditions (43%) (t(25) = − 13.99, P < 0.001). For two of the three factors, the frequency of TUTs during the task decreased as task difficulty increased (rate: F(2,50) = 14.96, P < 0.001; STM: F(2,50) = 6.41, P = 0.003). There were no differences in TUT frequency across the three levels of task difficulty for the discriminability factor (F(2,50) = 2.38, P > 0.05). The average frequency of TUTs for each task condition is reported in Table 2 and Fig. 1A. The results of follow-up paired t tests to evaluate the frequency of TUTs between difficulty levels within each factor are presented in Table 3. On average, across factors and task/rest blocks, subjects took 1555 ms (SD = ±346 ms) to respond to a TUT probe.
Fig. 1.
(A) Average TUT frequency across task conditions. (B) TID values averaged across 8 ROIs for each task condition. (C) Correlation of TID and TUT values. Regression line represents best fit (R2 = 0.812). As TID magnitude increased (became more negative) across task conditions, the frequency of TUTs declined (r = 0.90, P = 0.005). Error bars in panels A and B represent standard deviation.
Table 3.
t values and significance levels for paired t tests to assess change in TID magnitude and TUT frequency as task difficulty varies within each factor
| TID (average of 8 ROIs) |
TUT |
|||
|---|---|---|---|---|
| Moderate | Difficult | Moderate | Difficult | |
| Presentation rate | ||||
| Slower | 0.32 | 3.64** | 3.02** | 4.44*** |
| Moderate | – | 2.22* | – | 3.29** |
| Target discriminability | ||||
| Easy | 1.57 | 2.79** | 1.44 | 1.82 |
| Moderate | – | 1.54 | – | 1.12 |
| STM Load | ||||
| Easy | 3.42** | 2.26* | 3.43** | 2.06 |
| Moderate | – | −1.38 | – | −1.50 |
P < 0.05
P < 0.01
P < 0.001 (corrected).
The effectiveness of manipulating task difficulty within each factor was evaluated using repeated measures ANOVAs (one for each ROI) to identify ROIs in which there were differences in the TID magnitude across the three difficulty levels of the task. The results of these analyses replicated the pattern previously reported (McKiernan, et al., 2003), with three exceptions (note: data from 15 of the subjects overlap between this study and the previous report). First, the previous study reported a total of seven ROIs in which TID varied as a function of task difficulty for at least one of the three factors. This study adds one more region, the left fusiform gyrus (F(2,58) = 4.08, P = 0.022) in which stimulus presentation rate affected TID. This brought the total number of ROIs to eight: left posterior parieto-occipital (PPO) cortex, which includes parts of the angular gyrus, dorsolateral occipital lobe and cuneus; right precuneus/superior parietal lobule; left anterior cingulate/superior frontal gyrus; left posterior cingulate gyrus; left middle frontal gyrus; right posterior cingulate gyrus; right anterior cingulate gyrus; and left fusiform gyrus. Second, in the left PPO cortex, the task difficulty manipulation no longer produced significant differences in TID across levels of the discriminability factor, but did do so for presentation rate (F(2,58) = 4.39, P = 0.017). Lastly, in the left posterior cingulate gyrus, manipulating target discriminability produced only a trend toward significance (F(2,58) = 3.20, P = 0.048) versus a previously significant result. These differences are likely due to the great inter-subject variability in TID magnitude values (see SD values in Table 2).
TID values measured in the fMRI study in each of the eight ROIs were averaged over all 26 subjects for each of the seven task conditions. Repeated measures ANOVAs for each of the three factors indicated that the magnitude of deactivation increased (became more negative) as task difficulty increased (rate: F(2,58) = 4.93, P = 0.011; discriminability: F(2,58) = 4.46, P = 0.016; STM: F(2,58) = 6.97, P = 0.002). Mean TID values for each task condition, averaged over all subjects and all ROIs, are reported in Table 2 and Fig. 1B. The results of follow-up paired t tests to evaluate the magnitude of TID changes between difficulty levels within each factor are presented in Table 3.
To assess the relationship between the frequency of TUTs and magnitude of TID, the average TUT frequency for each task condition was correlated with the average TID value. This correlation was significant (r = 0.90, P = 0.005) (see Fig. 1). To determine the specific ROIs in which TID magnitude was correlated with TUT frequency as task demands changed, TUT frequency for each task condition was correlated with the average TID for each condition on an ROI by ROI basis. TUT frequency and TID were correlated in four of the ROIs: left posterior parieto-occipital cortex (r = 0.93, P = 0.002), left fusiform gyrus (r = 0.90, P = 0.006), left anterior cingulate gyrus (r = 0.82, P = 0.023), and left middle frontal gyrus (r = 0.79, P = 0.037) (Fig. 2). Correlation between TUTs and TID approached significance in the right posterior cingulate gyrus (r = 0.76, P = 0.049) and right posterior parieto-occipital cortex (r = 0.75, P = 0.051).
Fig. 2.
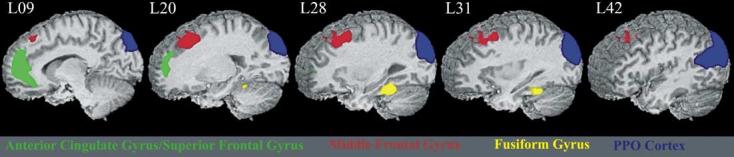
Left hemisphere regions of interest in which TID magnitude and TUT frequency are correlated across task conditions.
Discussion
As the auditory target detection task increased in difficulty (i.e., increased in processing demands), eight brain regions showed a correlated decline in fMRI BOLD signal (i.e., more negative TID values) relative to the resting state. Performance on the target detection task was equivalent during the fMRI and subsequent TUT session. TUT frequency also declined as the processing demands of the auditory task increased. In four of the eight brain regions of interest, decreases in fMRI BOLD signal correlated with decreases in the number of TUTs reported during task blocks. These findings support the hypothesis that reallocation of task processing resources may be a mechanism by which TID occurs in these brain regions. Withdrawal of processing resources from areas engaged by “stream of consciousness” processing should result in attenuation of both the brain activity in those areas as well as the cognitive processes they subserve. In accordance with our earlier work, we interpret these results to suggest that these four brain regions are actively engaged in organized cognitive processing during resting conditions, and this processing is interrupted (as measured by increased TID and reduced frequency of TUTs) in the presence of an exogenous task.
The manipulation of task difficulty within the discriminability factor did not significantly affect TUT frequency, although it did impact TID. The variability in subjects' TUT responses was fairly large. Given the highly subjective nature of the TUT decision, this variability is not surprising but may have influenced the potential for detecting differences across task conditions.
The correlation analyses were conducted at the group level because the measures contain inherently high levels of variability at the subject level. As seen in Table 2, the variability in each TID and TUT measure is fairly large, thus reducing the sensitivity of the analysis at the individual level. A follow-up study used a less subjective method of measuring resources available for ongoing thought processing, and these data will be assessed at the individual level.
These findings fit well with a previous study (Binder et al., 1999), in which many of the brain areas showing TID (left posterior parieto-occipital cortex, left dorsal prefrontal cortex, left fusiform gyrus) were also shown to be activated during a semantic task relative to a phonological control task, supporting claims that TID in these regions may be due to suspension of spontaneous semantic processes that occur during “rest”. Semantic processes rely on storage and retrieval of knowledge about concepts. Defined in this way, semantic processes are integral to problem solving, planning, thoughts, and visual imagery—all of which are salient examples of phenomena experienced in the “stream of consciousness”.
Although it is possible that task difficulty affects TID and TUTs via separate mechanisms thus allowing these phenomena to co-occur in some regions, but not in others, the connection between TID and TUTs occurring in the same brain regions and possibly via a single mechanism has precedents. The prefrontal/frontal regions that were found to deactivate in a pattern correlated with TUT suppression are similar to those found in a PET study investigating the relationship of regional cerebral blood flow and TUTs during articulation and reading tasks (McGuire et al., 1996). TUT frequency was positively correlated with rCBF in left dorsal prefrontal and rostral anterior cingulate cortex. These areas (peak activations reported in anterior cingulate cortex (BA 32) and middle and superior frontal gyri (BA8)) showed the same positive relationship as in the current study. The authors hypothesized that these areas are involved in self-initiated thoughts. Another study (Gusnard et al., 2001) reported increases in the dorsal medial prefrontal cortex to visual stimuli when making a self-referential judgment. The authors suggested that increased activity from baseline in the dorsal medial prefrontal cortex is associated with self-referential mental processes. Some of the region they define as dorsal medial prefrontal cortex overlaps with the anterior cingulate ROI in this study.
These findings also complement recent default mode network findings by Greicius and Menon (2004). The default mode network in the brain encompasses the brain regions that show TID; this network is thought to represent on-going, organized cognitive processing that occurs when the brain is not actively involved in another task. In fact, TID magnitude can be thought of as the manifestation of the activity level of the resting state or default mode network: when the network turns off, we see TID, and when it is active, we do not observe TID. Previous work by Greicius and colleagues has reported on default mode activity in this network (Greicius et al., 2003, 2004). From this default mode perspective, Greicius has shown that the default mode is still active at some level during easy or passive viewing tasks (Greicius and Menon, 2004). Our results support this finding in several ways. First, we do not see maximal deactivation (TID) in the easy task conditions, which supports the idea that there is some default mode processing still active. Second, our TUT results show that when the task is easy (i.e., low cognitive load), there are many TUTs reported. This also supports their hypothesis that the brain is able to engage in alternative processing even while performing an easy task. Our findings begin to connect the changing levels of activity in the brain's ongoing cognitive processing (i.e., stream of consciousness processing) with the goal of that processing (task related or not).
Conclusion
These results add to a growing understanding of the nature of task-induced deactivations commonly observed in functional neuroimaging studies. They suggest a link between the suspension of activity in specific brain regions and the attenuation of subjectively experienced thoughts, further supporting the suggestion that TID in these regions represents interruption of ongoing, cognitive processes present during resting states.
Acknowledgments
This research was supported by grants to JRB from the National Institute of Mental Health (PO MH51358) and National Institute of Neurological Disorders and Stroke (RO1 NS33576) and by the National Institutes of Health General Clinical Research Center (M01 RR00058) at MCW. We thank T. Prieto, T. Thelaner, B.D. Ward, and A. Moths for their technical assistance.
These data were presented in part at the 2001 Cognitive Neuroscience Society annual meeting.
Abbreviations
- TID
task-induced deactivation
- TUT
task-unrelated thought
- fMRI
functional magnetic resonance imaging
- ROI
region of interest
- rCBF
regional cerebral blood flow
Appendix A. Instructions for the TUT phase of the TID study at the mock scanner
The task you will be performing is the same target detection task that you completed at the last session at the 1.5-T scanner. This time, though, the task and scanner noise will stop at random intervals and you will be asked to decide what you were thinking about at the moment the task stopped. If you were thinking about the sounds or about performing the task, then we'll say you were having a task-related thought. If you were thinking about anything else, then you were having a task-unrelated thought. The question you need to answer whenever the scanner noise stops is, “Was I having a Task-Unrelated thought?”
Let's define what I mean by task-related and task-unrelated thoughts.
A task-related thought is any thought that is focused solely on the performance of the task. For example, at the time that the scanner noise stops, you may be thinking, “that was a target” or even just “yes”. You may have been thinking, “not a target” or “that cluster had 3 targets”, and these are both examples of task-related thoughts.
During this session, it is very possible that thoughts or images which are not related to the target detection task that you are performing may enter your mind. A task-unrelated thought is any thought that isn't specifically about your immediate task performance. For example, you may think of something you did last night, a friend or family member, or some event that has happened or will be happening in the future. You may think of things you need to do on your way home or something you need to make plans for. This also includes thoughts about when the scanner noise will stop, how much longer the task will last, and even thinking about whether you are thinking about a task-related or task-unrelated thought. Other examples are thoughts and images about things that have nothing to do with the task, such as whether you are hungry or tired, etc.
Please respond quickly and be as accurate as possible. We expect task-unrelated thoughts to occur, so it is perfectly acceptable to give that response.
We'll run through a practice session to be sure you feel comfortable with making this decision.
Here are the instructions for today's session:
Today you will be performing the same target detection task that you performed during the last session. This time, the task and scanner noise will stop at random intervals and you will be asked to decide immediately what you were thinking about at the moment the scanner noise stopped. If you answer the question “Was I having a task-unrelated thought?” with YES, then you will press the YES button. If the answer is NO, then you will press the NO button. You will use your right index finger to respond to targets. After you indicate the type of thought you were having, the task will start up again. Sometimes there will be a rest period in which you will hear the scanner noise, but there will be no task. During the rest period the scanner noise will stop after a random interval and you will make the same decision about whether your thought at that moment was task-related or task-unrelated. Like the last time, we will do 2 runs for each of the conditions.
References
- Andreasen NC, O'Leary DS. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am. J. Psychiatry. 1995;1576 doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Antrobus JS. Information theory and stimulus-independent thought. Br. J. Psychol. 1968;59:423–430. [Google Scholar]
- Antrobus JS, Singer JL, Greenber S. Studies in stream of consciousness—Experimental enhancement and suppression of spontaneous cognitive processes. Percept. Mot. Skills. 1966;23(2):399–417. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J. Cogn. Neurosci. 1999;11(1):80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996a;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW. Savoy. R.MGH-NMR Center; Boston, MA: 1996b. Algorithms for image registration, motion detection, and motion correction. [Google Scholar]
- EPRIME . EPRIME Beta 5.2. Psychology Software Tools; Pittsburgh: [Google Scholar]
- Giambra LM. Task-unrelated-thought frequency as a function of age: a laboratory study. Psychol. Aging. 1989;4(2):136–143. doi: 10.1037/0882-7974.4.2.136. [DOI] [PubMed] [Google Scholar]
- Giambra LM. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought. Conscious. Cogn. 1995;4(1):1–21. doi: 10.1006/ccog.1995.1001. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation [see comment] J. Cogn. Neurosci. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Neuroscience. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev., Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. Principles of Psychology. Dover; New York: 1890. [Google Scholar]
- Keppel J. Design and Analysis: A Researcher's Handbook. Prentice Hall; New Jersey: 1991. [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res. Bull. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. NeuroReport. 1996;7(13):2095–2099. [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation: an fMRI study. J. Cogn. Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pope KS, Singer JL. Regulation of the stream of consciousness: toward a theory of ongoing thought. In: Schwartz GE, Shapiro D, editors. Consciousness and Self-regulation. Plenum; New York: 1976. pp. 101–135. [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos. Trans. R. Soc. London, Ser. B Biol. Sci. 1998;353(1377):1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GA, Giambra L. Task-unrelated thoughts of college-students diagnosed as hyperactive in childhood. Dev. Neuropsychol. 1993;9(1):17–30. [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9(5):648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Singer JL. Sampling on-going consciousness and emotional experiences: implications for health. In: Horowitz MJ, editor. Psycho-dynamics and Cognition. University of Chicago Press; Chicago, IL: 1988. [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Teasdale JD, Proctor L, Lloyd CA, Baddley AD. Working memory and stimulus independent thought: effects of memory load and presentation rate. Eur. J. Cogn. Psychol. 1993;5:417–433. [Google Scholar]
- Teasdale JD, Dritschel BH, Taylor MJ, Proctor L, Lloyd CA, Nimmo-Smith I, Baddeley AD. Stimulus-independent thought depends on central executive resources. Mem. Cogn. 1995;23(5):551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]