Abstract
Vertebrate brains are organized in modules which process information from sensory inputs selectively. Therefore they are probably under different evolutionary pressures. We investigated the impact of environmental influences on specific brain centres in bats. We showed in a phylogenetically independent contrast analysis that the wing area of a species corrected for body size correlated with estimates of habitat complexity. We subsequently compared wing area, as an indirect measure of habitat complexity, with the size of regions associated with hearing, olfaction and spatial memory, while controlling for phylogeny and body mass. The inferior colliculi, the largest sub-cortical auditory centre, showed a strong positive correlation with wing area in echolocating bats. The size of the main olfactory bulb did not increase with wing area, suggesting that the need for olfaction may not increase during the localization of food and orientation in denser habitat. As expected, a larger wing area was linked to a larger hippocampus in all bats. Our results suggest that morphological adaptations related to flight and neuronal capabilities as reflected by the sizes of brain regions coevolved under similar ecological pressures. Thus, habitat complexity presumably influenced and shaped sensory abilities in this mammalian order independently of each other.
Keywords: hippocampus, inferior colliculi, main olfactory bulb, wing morphology, phylogenetic contrasts
1. Introduction
Animals are moulded by their environment. This is true within orders, families and even genera at large ecological scales (e.g. aquatic versus terrestrial organisms), to closely related species occupying narrow niches (e.g. Darwin’s finches (Lack 1969; Grant & Grant 1989)). Morphology reflects the environment an organism inhabits, and consequently much about ecological niches can be deduced from the way that animals are shaped. Parts of the mammalian brain are adapted to solving different tasks and respond to selective regimes, including environmental influences (Eisenberg & Wilson 1978; Barton et al. 1995; Hutcheon et al. 2002). One well-investigated example showing that brain centres do vary in size under selective pressure is the hippocampus, which becomes larger with increasing demands on spatial memory (e.g. birds (Krebs et al. 1989); rodents (Jacobs et al. 1990) and London taxi-drivers (Maguire et al. 2000)). The extent to which mammalian brain regions develop independently has been the subject of a controversial debate. While some authors argue that the set-up of a common ancestor’s brain constrains development (Finlay & Darlington 1995), others think that selection acts on brains and brain regions independently of phylogeny (‘mosaic theory’ (e.g. Harvey & Krebs 1990; Barton et al. 1995; Barton & Harvey 2000)). The following scenarios can be imagined. Either the whole brain of an organism changes in size or selection operates on individual neuro-cognitive systems. In the latter case, the ancestral blueprint may limit changes in brain size, according to the phylogenetic constraint hypothesis (Harvey & Krebs 1990). Or, according to the mosaic theory (Barton & Harvey 2000), selection should act on parts of the brain independently of the rest and of phylogenetic constraints (Harvey & Krebs 1990; Barton & Harvey 2000).
Bats (Chiroptera) are an exceptionally species-rich and widely distributed order and are particularly fascinating, as far as morphological adaptations are concerned (Swartz et al. 2003). The ability to fly in connection with the use of echolocation for orientation (in the suborder microchiroptera) is generally viewed as a prerequisite for the manifold niche differentiations (Neuweiler 1993; Altringham 1996). Wing measures and especially wing area reflect flight performance and the ecological niche of flying animals in general (e.g. Altshuler & Dudley 2002; Hoffmann et al. 2002; Tobalske et al. 2003) and of bats in particular (Norberg 1986, 1994; Norberg & Rayner 1987; Fenton & Bogdanowicz 2002). At one extreme of morphological adaptations, species hunt insects in open space relying on speed. Such fast-flying bats have small wing areas relative to body mass, resulting in low agility and manoeuvrability (Norberg & Rayner 1987; Norberg 1994). At another extreme, some bats typically forage in highly structured habitat while flying slowly or perching, detecting their food (animals or fruit) at short range through echolocation calls, olfaction or passive listening. Their wings are broad and large, rendering them highly manoeuvrable (Norberg & Rayner 1987; Neuweiler 1990). Although the study of wing morphology in bats and their adaptation to habitat complexity represents one of the best documented examples of functional ecology, a proper phylogenetic analysis of the correlation of wing morphology with habitat complexity was lacking. Thus, we validated the reliability of this measure in a comparative approach using appropriate statistical methods of phylogenetically independent contrasts.
Habitat should not only influence morphological adaptation to flight, but also sensory requirements. Previous comparative studies on the neurobiology of bats dealt with taxonomy, echolocation and dietary specialization (Eisenberg & Wilson 1978; Jolicoeur & Baron 1980; Pirlot & Jolicoeur 1982; Jolicoeur et al. 1984; Neuweiler 1989, 1993; Barton et al. 1995; Hutcheon et al. 2002). Preferences of the two main dietary subgroups, plant- and animal-eating bats, were found to correlate with sensory specialization, reflected by size changes of the corresponding brain centres (Hutcheon et al. 2002). However, this may be at least partly a consequence of the underlying effect of sensory adaptations to habitat, and only indirectly connected with diet (Harvey & Krebs 1990).
Here, we aimed to correlate the influence of ecological factors with the evolution of sensory adaptations to the environment. We associated wing morphology as an indirect measure of the complexity of a bat’s foraging habitat and the brain centres connected with three sensory channels (hearing: auditory nuclei and inferior colliculi; smell: main olfactory bulb; and spatial memory: hippocampus), while controlling for phylogeny and body mass.
We made the following predictions regarding the influence of wing area on the size of the investigated brain parts.
Bats foraging in complex habitats must distinguish prey from background clutter, while simultaneously recognizing and avoiding obstacles. This puts higher demands on hearing abilities than detection of prey and orientation in open space. Consequently, we expected an increase in the size of auditory nuclei and/or inferior colliculi along with increasing wing area (habitat complexity) in all echolocating bats (animal-eating species and phytophagous Phyllostomidae), but no association in the exclusively frugivorous suborder Megachiroptera with the single family Pteropodidae, whose members do not echolocate. Hearing ability influences the size of the inferior colliculi in bats (Baron et al. 1996). They are the main switchboard for all incoming auditory information, incorporating the acoustic fovea (Neuweiler 1993).
The main olfactory bulb is assumed to be of importance to phytophagous bats for the detection of food sources and the determination of the ripeness of fruit (Baron et al. 1996). However, whether olfaction alone is sufficient for the localization of food is controversial (Baron et al. 1996; Hutcheon et al. 2002), and we did not expect an influence of habitat complexity on the size of this brain part.
Finally, hippocampus size is directly related to spatial memory (Krebs et al. 1989). We expected a positive correlation of wing area with hippocampus size. Dense habitats contain many obstacles and changes occur relatively frequently, making spatial memory a valuable tool for orientation. While frugivorous bats have bigger hippocampi than animal-eating bats (Hutcheon et al. 2002), we expected an influence of habitat structure in all species.
2. Methods
Using wing morphology data from Norberg & Rayner (1987) and brain volumes from Baron et al. (1996) we analysed a total of 97 species from 12 families. Data for body mass (in grams), wing area (in centimetres squared), volume of hippocampus and main olfactory bulb were available for all 97 species. The volume of auditory nuclei was obtained in 75 species and the volume of inferior colliculi in 69 species. Brain components were in millimetres cubed. Data were log transformed to obtain a normal distribution. Dietary information was assigned according to Nowak (1994) and in case of the genus Tonatia, according to Reid (1997).
Bat species can be categorized into guilds according to the complexity of their foraging habitats (Patterson et al. 2003). We selected 30 species grouped into four categories (1, open aerial foragers; 2, edge and gap foragers; 3, background-cluttered and narrow space habitat; and 4, highly cluttered habitat (see Kalko et al. 1996); guilds according to Kalko et al. (1996) and J. Fahr, personal communication). We then compared these guilds according to their wing area to verify the reliability of this measure for habitat complexity. We treated the four categories as continuous characters. This assumes a continuous spectrum of habitat complexity, representing discrete approximations (for a similar approach see Purvis et al. 2000; Safi & Kerth 2004).
2.1 Statistical analyses
Statistical tests were based on phylogenetically independent contrasts (Felsenstein 1985; Pagel 1999), generated with the software CAIC (Purvis & Rambaut 1995; http://www.bio.ic.ac.uk/evolve/software/caic/). Using this approach we acknowledge the fact that species are not independent entities and have a common history represented by a hierarchical and branched phylogeny. We used the recent phylogeny provided by Jones et al. (2002) to infer relationships between the species used in this analysis. Because branch lengths were not known, we set them to equal length (Garland et al. 1992). The plots of the absolute values of the standardized independent contrasts versus the standard deviation showed no correlation for all variables analysed in this study. This suggested that the arbitrarily equalized branch lengths standardized the contrasts and were reasonable for use in our analyses (Diaz-Uriarte & Garland 1996, 1998). As all variables represented continuous data, the ‘crunch’ algorithm of the CAIC package was used (Purvis & Rambaut 1995). The results were tested in general linear models for type-III sums of squares using SAS v. 6.12 (SAS Institute Inc. 1993). Wing area was the main predictor, and body mass the covariate. Regressions were forced through the origin (Purvis & Rambaut 1995).
In the analysis with CAIC (Purvis & Rambaut 1995), we first tested the data for all species together. As recommended by Garland et al. (1992) we analysed the data on species level and using phylogenetically independent contrasts. Species level data were assumed to be independent or to stem from a star phylogeny, where all species have the same ancestor and equal branch lengths.
Then we separated the data for CAIC into subgroups according to our predictions. We investigated the effect of increasing wing area on the auditory nuclei and inferior colliculi of echolocators and pteropodids separately, because differences may exist between their hearing brains. We also individually analysed the size of the main olfactory bulb of phyllostomid bats, pteropodid bats and all other bats in association with wing area, as frugivorous bats are thought to rely on their sense of smell very much. Pteropodids and phyllostomids were separated owing to fundamental differences in orientation mode (echolocation versus vision), which might indirectly influence the role of olfaction for orientation. Finally, for the analysis of hippocampus size, phytophagous and non-phytophagous bats were separated. Although we expected an influence of habitat structure in all bats, the hippocampus is assumed to play a more important role in frugivores (Hutcheon et al. 2002). We only present the results on subsets of the entire data for the phylogenetically independent contrasts. The data and the tree used are available from the corresponding author upon request.
3. Results
3.1 Verification of wing area as a measure for habitat complexity
At species level, wing area corrected for body mass showed a significant positive correlation with guild (n=30, F3,0.1=5.29, p=0.006). A significant positive correlation was maintained after correcting for phylogenetic dependence and controlling for body mass (ncontrasts=9, F1,0.12=18.35, p=0.004). These analyses justify the use of wing area as a reliable correlate of habitat complexity.
3.2 Auditory nuclei and inferior colliculi
At species level, wing area showed a significant positive correlation with the size of the auditory nuclei, both corrected for body mass (table 1). However, after controlling for phylogeny, the effect of wing area on auditory nuclei was no longer significant (table 1).
Table 1.
The effect of log wing area on four different brain regions corrected for body weight (covariate) for all species. Estimate denotes the slope of a given source on the independent variable in the model.
| species independent |
contrast level |
|||||||||
| estimate | d.f. | SS3 | F | p | estimate | d.f. | SS3 | F | p | |
| log auditory nucleia | ||||||||||
| log wing area | 0.19 | 1 | 0.10 | 4.32 | 0.04 | 0.03 | 1 | <0.001 | 0.26 | 0.61 |
| log body weight | 0.37 | 1 | 0.07 | 2.81 | 0.10 | 0.50 | 1 | 0.10 | 46.5 | <0.01 |
| error | 71 | 30 | ||||||||
| log inferior colliculib | ||||||||||
| log wing area | 0.25 | 1 | 0.06 | 2.28 | 0.14 | 0.25 | 1 | 0.04 | 24.6 | <0.01 |
| log body weight | 0.28 | 1 | 0.11 | 4.40 | 0.04 | 0.37 | 1 | 0.05 | 28.8 | <0.01 |
| error | 68 | 30 | ||||||||
| log main olfactory bulbc | ||||||||||
| log wing area | −0.29 | 1 | 0.08 | 1.07 | 0.30 | 0.04 | 1 | 0.002 | 0.34 | 0.56 |
| log body weight | 1.20 | 1 | 3.50 | 47.50 | <0.01 | 0.81 | 1 | 0.35 | 72.03 | <0.01 |
| error | 94 | 41 | ||||||||
| log hippocampusc | ||||||||||
| log wing area | 0.40 | 1 | 0.14 | 4.29 | 0.04 | 0.43 | 1 | 0.18 | 108.0 | <0.01 |
| log body weight | 0.53 | 1 | 0.68 | 20.70 | <0.01 | 0.40 | 1 | 0.08 | 50.7 | <0.01 |
| error | 94 | 41 | ||||||||
a nspecies=75; ncontrasts=32.
b nspecies=69; ncontrasts=32.
c nspecies=97; ncontrasts=43.
When we separated the pteropodids from all other bats in a phylogenetically corrected analysis, size of auditory nuclei still did not correlate with wing area in echolocating species, while it increased significantly with wing area in the Pteropodidae (table 2).
Table 2.
The effect of log wing area corrected for body size on (a) log auditory nuclei and (b) log inferior colliculi for echolocating bats and pteropodid bats using phylogenetically independent contrasts.
| echolocating bats |
pteropodid bats |
|||||||||
| estimate | d.f. | SS3 | F | p | estimate | d.f. | SS3 | F | p | |
| (a) log auditory nuclei | ||||||||||
| log wing area | 0.03 | 1 | <0.01 | 0.48 | 0.50 | 0.68 | 1 | 0.01 | 7.86 | 0.01 |
| log body weight | 0.64 | 1 | 0.08 | 91 | <0.01 | 0.09 | 1 | <0.01 | 0.25 | 0.63 |
| error | 16 | 12 | ||||||||
| (b) log colliculi inferiores | ||||||||||
| log wing area | 0.21 | 1 | 0.02 | 20.4 | <0.01 | 0.47 | 1 | <0.01 | 7.09 | 0.03 |
| log body weight | 0.52 | 1 | 0.05 | 51.9 | <0.01 | 0.20 | 1 | <0.01 | 2.47 | 0.15 |
| error | 20 | 10 | ||||||||
There was no correlation between wing area and the size of inferior colliculi at species level (table 1). When using independent contrasts, a strong positive correlation between wing area and the size of the inferior colliculi was found (table 1).
In a separate analysis, both echolocating and pteropodid bats showed a significant increase in the size of the inferior colliculi with increasing wing area; however, the effect was much stronger for echolocating bats (table 2).
3.3 Main olfactory bulb
The volume of the main olfactory bulb was not affected by wing area either at species level or using independent contrasts (table 1).
We analysed three subgroups: pteropodids, phytophagous phyllostomids and non-phytophagous bats. Only the pteropodids showed a significant relationship between wing area and mass of olfactory bulb (table 3). In addition, the main olfactory bulb shows a tendency to be reduced in non-phytophagous bats in relation to wing area.
Table 3.
Effect of wing area on log main olfactory bulb using phylogenetically independent contrasts.
| phytophagous bats |
non-phytophagous bats |
||||||||||||||
| phyllostomids |
pteropodids |
all remaining species |
|||||||||||||
| estimate | d.f. | SS3 | F | p | estimate | d.f. | SS3 | F | p | estimate | d.f. | SS3 | F | p | |
| log main olfactory bulb | |||||||||||||||
| log wing area | −0.02 | 1 | <0.01 | 0.00 | 0.96 | 0.53 | 1 | <0.01 | 5.36 | 0.04 | −0.16 | 1 | 0.02 | 4.17 | 0.05 |
| log body weight | 0.58 | 1 | <0.01 | 4.54 | 0.06 | 0.22 | 1 | <0.01 | 1.76 | 0.21 | 1.02 | 1 | 0.32 | 68.0 | <0.01 |
| error | 9 | 14 | 20 | ||||||||||||
3.4 Hippocampus
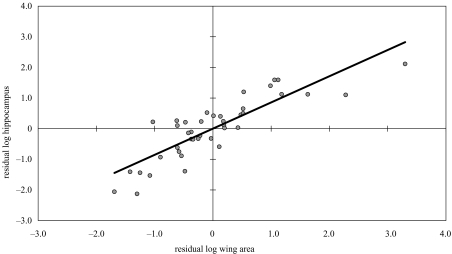
There was a correlation between size of hippocampus and wing area at species level (table 1). The independent contrasts also showed a significant increase in size of the hippocampus with increasing wing area (table 1; figure 1).
Figure 1.
Plot of residual contrasts in log wing area (residuals generated from a least-squares regression of contrasts in log wing area and log hippocampus against log body mass (Garland et al. 1992)) against contrasts in log hippocampus. Contrasts were generated using CAIC.
When using independent contrasts, wing area was positively correlated to an increase in size of hippocampus in phytophagous and all other bats (table 4).
Table 4.
Effect of log wing area on log hippocampus using phylogenetically independent contrasts.
| phytophagous bats |
non-phytophagous bats |
|||||||||
| estimate | d.f. | SS3 | F | p | estimate | d.f. | SS3 | F | p | |
| log hippocampus | ||||||||||
| log wing area | 0.30 | 1 | <0.01 | 5.80 | 0.01 | 0.47 | 1 | 0.07 | 82.1 | <0.01 |
| log body weight | 0.38 | 1 | <0.01 | 6.88 | 0.02 | 0.35 | 1 | 0.1 | 54.9 | <0.01 |
| error | 26 | 20 | ||||||||
3.5 Phylogenetic inertia
The independent contrast analyses and the species level analyses substantially deviated in the analyses concerning brain regions associated with hearing. This suggests that phylogenetic inertia is present and that corresponding correction required. A species level ANOVA, with volume of brain regions nested in family, revealed significant differences for all four brain regions, confirming the strong phylogenetic influence (auditory nuclei: nspecies=74, F21,5.02=43.2, p<0.001; inferior colliculi: nspecies=70, F21,4.62=36.0, p<0.001; main olfactory bulb: nspecies=102, F22,1.47=159.5, p<0.001; hippocampus: nspecies=102, F22,0.74=78.6, p<0.001).
4. Discussion
We were able to confirm most predictions concerning the influence of morphological adaptations to structure of foraging habitats measured by wing area on various brain regions. We also showed that phylogenetic constraints may act on the morphology of closely related species to a certain extent, making the use of independent contrasts a useful tool to reveal such effects.
Previous studies on encephalization and brain regions in bats (and other mammals) found an influence of diet on brain size (Eisenberg & Wilson 1978; Pirlot & Jolicoeur 1982; Jolicoeur et al. 1984; Harvey & Krebs 1990; Barton et al. 1995; Hutcheon et al. 2002). It has been speculated that it is not the nature of the food of animal taxa which directly influences brain size, but rather the variation in information storage and retrieval systems associated with diet (Eisenberg & Wilson 1978; Harvey & Krebs 1990). Our findings support these hypotheses and suggest that the surplus of information processing required in complex habitats may have influenced brain evolution (Harvey & Krebs 1990).
The size of the inferior colliculi reflects the hearing capacity of species better than any other brain structure (Baron et al. 1996). The fact that the inferior colliculi of echolocating bats were correlated with wing area may reflect their improved ability to deal with increasingly difficult acoustic environments. However, there may be additional influences on the size of the inferior colliculi: passive gleaners, which use prey-generated sounds for the detection of food in addition to echolocation, are closely associated with dense and complex habitats. Such species have two sensitive frequency ranges possibly resulting in larger inferior colliculi (Baron et al. 1996).
Echolocating bats have larger auditory nuclei than the non-echolocating pteropodids (Hutcheon et al. 2002). However, auditory nuclei were not influenced by habitat complexity. Environmental influences may act differently on each of the several centres summarized under ‘auditory nuclei’, and their functions may be only partly or not at all related to changes in the environment.
The increase of both auditory nuclei and inferior colliculi with wing area in the non-echolocating pteropodids is noticeable. It remains unclear whether selective pressure on hearing ability is responsible for this effect. The benefits of improved hearing in increasingly complex habitats despite a lack of echolocation have not, to our knowledge, been investigated to date.
Brains of pteropodid bats have been characterized as ‘olfactory’ and ‘visual’, whereas those of microchiropteran bats have been described as ‘auditory’ (Eisenberg & Wilson 1978; Barton et al. 1995; Hutcheon et al. 2002). Our results show an increasing main olfactory bulb with wing area only in the Pteropodidae. This suggests that, while olfaction may play a role in the localization of food (Möhres & Kulzer 1956; Luft et al. 2003), the importance of it is likely to increase in denser habitat in this suborder. The reason for the tendency towards reduction in the main olfactory bulb size of non-phytophagous bats is unclear. While it may indicate a phylogenetic constraint (Finlay & Darlington 1995), echolocation is unlikely to be the reason for this, because we would then expect the same pattern in the phytophagous phyllostomid bats who also use echolocation. But the phyllostomids seem to use olfaction only for the detection but not for the localization of food sources in denser habitat.
The hippocampus is responsible for spatial memory in various animal taxa (Krebs et al. 1989; Jacobs et al. 1990; Maguire et al. 2000). The ability of bats to memorize structures within their foraging area has long been recognized, and anecdotal evidence suggests that spatial memory is crucial for orientation in bats (Neuweiler 1993). Dense habitat is not only difficult to move in but changes in vegetation, which require improved spatial learning, occur more often here. Among the Chiroptera, phytophagous bats have the largest hippocampus (Hutcheon et al. 2002). They evidently benefit from enhanced spatial memory by remembering the location of unpredictable but stationary food resources such as flowering trees (Barton et al. 1995; Baron et al. 1996). The correlation with wing area in this group of bats suggests that at least two mechanisms (location and orientation) act together on hippocampus size. Thus the alleged effect of habitat complexity reflected by wing morphology presumably influenced the evolution of spatial memory.
The strong influence of the phylogenetic corrections on practically all of our results indicates that common ancestry influences external flight, as well as internal brain morphology. The phylogenetic independent analyses of the four brain regions in bats presented here show that the involved size changes in brain regions differed between specific structures and functional systems. Similarly as shown by Hutcheon et al. (2002) in the context of diet, the diverging influence of habitat structure on the two suborders and even within the suborder Microchiroptera, e.g. concerning the size of the olfactory bulb, indicate that specific brain regions can develop in a mosaic pattern (Barton et al. 1995; Barton & Harvey 2000) at least to a large extent when selective pressure necessitates it.
5. Conclusions
Our study shows that neuronal capacities in bats presumably coevolved with flight morphology, under selection imposed by habitat complexity. This was true for brain parts processing sensory input connected to hearing and spatial memory, but not for olfaction. Our study on the selective evolution of brain regions reveals a differentiated pattern of size increase in brain regions in relation to habitat complexity. These findings suggest that neuro-cognitive centres are under specific selection pressure according to the ‘mosaic theory’ (Barton & Harvey 2000). The fingerprints of the adaptive radiation in the order of Chiroptera thus cannot only be found on external morphology but also on neuronal units. Both morphology and brain regions, together with sensory capabilities, seem to be shaped to match the demands of a species’ ecology.
Acknowledgments
We are grateful to David Hosken for his invaluable support in statistical analyses and discussions that helped to improve this study. Rachel Page helped to initiate this work and contributed with useful comments. G. Kerth, S. Krackow, A. McElligott, M. Seid, S. Spehn and two anonymous referees provided helpful comments on the manuscript. K.S. was supported by the Graduiertenkollegium Zürich: Wissensgesellschaft und Geschlechterbeziehungen.
References
- Altringham J.D. Bats: biology and behaviour. Oxford University Press; 1996. [Google Scholar]
- Altshuler D.L., Dudley R. The ecological and evolutionary interface of hummingbird flight physiology. J. Exp. Biol. 2002;205:2325–2336. doi: 10.1242/jeb.205.16.2325. [DOI] [PubMed] [Google Scholar]
- Baron G., Stephan H., Frahm H.D. Comparative neurobiology in Chiroptera. Birkhäuser; Basel, Switzerland: 1996. [Google Scholar]
- Barton R.A., Harvey P.H. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- Barton R.A., Purvis A., Harvey P.H. Evolutionary radiation of visual and olfactory brain systems in primates, bats and insectivores. Phil. Trans. R. Soc. B. 1995;348:381–392. doi: 10.1098/rstb.1995.0076. [DOI] [PubMed] [Google Scholar]
- Diaz-Uriarte R., Garland T. Testing hypotheses of correlated evolution using phylogenetically independent contrasts: sensitivity to deviations from Brownian motion. Syst. Biol. 1996;45:27–47. [Google Scholar]
- Diaz-Uriarte R., Garland T. Effects of branch length errors on the performance of phylogenetically independent contrasts. Syst. Biol. 1998;47:654–672. doi: 10.1080/106351598260653. [DOI] [PubMed] [Google Scholar]
- Eisenberg J.F., Wilson D.E. Relative brain size and feeding strategies in the Chiroptera. Evolution. 1978;32:740–751. doi: 10.1111/j.1558-5646.1978.tb04627.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Fenton M.B., Bogdanowicz W. Relationships between external morphology and foraging behaviour: bats in the genus. Myotis. Can. J. Zool.-Revue Canadienne De Zoologie. 2002;80:1004–1013. [Google Scholar]
- Finlay B.L., Darlington R.B. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Garland T., Harvey P.H., Ives A.R. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst. Biol. 1992;41:18–32. [Google Scholar]
- Grant B.R., Grant P.R. Natural selection in a population of Darwin’s finches. Am. Nat. 1989;133:377–393. [Google Scholar]
- Harvey M.J., Krebs J.R. Comparing brains. Science. 1990;249:140–146. doi: 10.1126/science.2196673. [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A., Collins E., Woods R. Wing shape and wing size changes as indicators of environmental stress in Helicoverpa punctigera (Lepidoptera: Noctuidae) moths: comparing shifts in means, variances, and asymmetries. Environ. Entomol. 2002;31:965–971. [Google Scholar]
- Hutcheon J.M., Kirsch J.W., Garland T. A comparative analysis of brain size in relation to foraging ecology and phylogeny in the Chiroptera. Brain Behav. Evol. 2002;60:165–180. doi: 10.1159/000065938. [DOI] [PubMed] [Google Scholar]
- Jacobs L.F., Gaulin S.J.C., Sherry D.F., Hoffman G.E. Evolution of spatial cognition: sex-specific patterns of spatial-behavior predict hippocampal size. Proc. Natl Acad. Sci. USA. 1990;87:6349–6352. doi: 10.1073/pnas.87.16.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Baron G. Brain center correlations among Chiroptera. Brain Behav. Evol. 1980;17:419–431. doi: 10.1159/000121812. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Pirlot P., Baron G., Stephan H. Brain structure and correlation patterns in Insectivora, Chiroptera, and primates. System. Zool. 1984;33:14–29. [Google Scholar]
- Jones K.E., Purvis A., MacLarnon A., Bininda-Emonds O.R.P., Simmons N.B. A phylogenetic supertree of the bats (Mammalia: Chiroptera) Biol. Rev. 2002;77:223–259. doi: 10.1017/s1464793101005899. [DOI] [PubMed] [Google Scholar]
- Kalko E.K.V., Handley C.O., Handley D. Organization, diversity, and long-term dynamics of a neotropical bat community. In: Cody M., Smallwood J., editors. Long-term studies in vertebrate communities. Academic; Los Angeles, CA: 1996. pp. 503–553. [Google Scholar]
- Krebs J.R., Sherry D.F., Healy S.D., Perry V.H., Vaccarino A.L. Hippocampal specialization of food-storing birds. Proc. Natl Acad. Sci. USA. 1989;86:1388–1392. doi: 10.1073/pnas.86.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack D. Subspecies and sympatry in Darwin’s finches. Evolution. 1969;23:252–263. doi: 10.1111/j.1558-5646.1969.tb03509.x. [DOI] [PubMed] [Google Scholar]
- Luft S., Curio E., Tacud B. The use of olfaction in the foraging behaviour of the golden-mantled flying fox, Pteropus pumilus, and the greater musky fruit bat, Ptenochirus jagori (Megachiroptera: Pteropodidae) Naturwissenschaften. 2003;90:84–87. doi: 10.1007/s00114-002-0393-0. [DOI] [PubMed] [Google Scholar]
- Maguire E.A., Gadian D.G., Johnsrude I.S., Good C.D., Ashburner J., Frackowiak R.S.J., Frith C.D. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl Acad. Sci. USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhres F.P., Kulzer E. Über die Orientierung der Flughunde (Chiroptera-Pteropodidae) Zeitschrift für vergleichende Physiologie. 1956;38:1–29. [Google Scholar]
- Neuweiler G. Foraging ecology and audition in echolocating bats. Trends Ecol. Evol. 1989;4:160–166. doi: 10.1016/0169-5347(89)90120-1. [DOI] [PubMed] [Google Scholar]
- Neuweiler G. Auditory adaptations for prey capture in echolocating bats. Physiol. Rev. 1990;70:615–641. doi: 10.1152/physrev.1990.70.3.615. [DOI] [PubMed] [Google Scholar]
- Neuweiler G. Biologie der Fledermäuse. Thieme Verlag; Stuttgart, Germany: 1993. [Google Scholar]
- Norberg U.M. Evolutionary convergence in foraging niche and flight morphology in insectivorous aerial-hawking birds and bats. Ornis Scandinavica. 1986;17:253–260. [Google Scholar]
- Norberg U.M. Wing design, flight performance, and habitat use in bats. In: Wainwright P.C., Reilly S.M., editors. Ecological morphology. The University of Chicago Press; 1994. pp. 205–239. [Google Scholar]
- Norberg U.M., Rayner J.M.V. Ecological morphology and flight in bats (Mammalia, Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Phil. Trans. R. Soc. B. 1987;316:337–419. [Google Scholar]
- Nowak M.R. Walker’s bats of the world. The Johns Hopkins University Press; London: 1994. [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- Patterson B.D., Willig M.R., Stevens R.D. Trophic strategies, niche partitioning, and patterns of ecological organization. In: Kunz T.H., Fenton M.B., editors. Bat ecology. Chicago, IL: The University of Chicago Press; 2003. pp. 536–579. [Google Scholar]
- Pirlot P., Jolicoeur P. Correlations between major brain regions in Chiroptera. Brain Behav. Evol. 1982;20:172–181. doi: 10.1159/000121589. [DOI] [PubMed] [Google Scholar]
- Purvis A., Rambaut A. Comparative analysis by independent contrasts (CAIC): an Apple-Macintosh application for analyzing comparative data. Comput. Applic. Biosci. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Purvis A., Gittleman J.L., Cowlishaw G., Mace G.M. Predicting extinction risk in declining species. Proc. R. Soc. B. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. doi:10.1098/rspb.2000.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid F.A. A field guide to the mammals of Central America and Southeast Mexico. Oxford University Press; Oxford: 1997. [Google Scholar]
- Safi K., Kerth G. A comparative analysis of specialization and extinction risk in temperate-zone bats. Conserv. Biol. 2004;18:1293–1303. [Google Scholar]
- SAS Institute Inc. 1993 SAS technical report P-243. Cary, NC: SAS Institute Inc.
- Swartz S.M., Freeman P.W., Stockwell E.F. Ecomorphology of bats: comparative and experimental approaches relating structural design to ecology. In: Kunz T.H., Fenton M.B., editors. Bat ecology. Chicago: The University of Chicago Press; 2003. pp. 257–300. [Google Scholar]
- Tobalske B.W., Hedrick T.L., Biewener A.A. Wing kinematics of avian flight across speeds. J. Avian Biol. 2003;34:177–184. [Google Scholar]