Abstract
The evolution of communication is a fundamental biological problem. The genetic control of the signal and its reception must be tightly coadapted, especially in inter-individual sexual communication. However, there is very little experimental evidence for tight genetic linkage connecting the emission of a signal and its reception. We found that a single genomic transposon inserted in the desat1 gene of Drosophila melanogaster simultaneously affected the emission and the perception of sex-specific signals. This mutation greatly decreased the production of unsaturated hydrocarbons on the cuticle of mature flies of both sexes. These substances represent the sex pheromones necessary for mate discrimination: control males could not discriminate the sex of mutant desat1 flies. Moreover, mutant males were unable to discriminate the sex pheromones of control flies. Expression of desat1 was found in the peripheral tissues that produce and detect sex pheromones. Excision of the transposon rescued both the production and discrimination phenotypes, but the two effects did not always coincide. This indicates that the emission and perception of pheromones are coded by different products of the same gene, reflecting the pleiotropic activity of desat1.
Keywords: pheromonal communication, mate discrimination, desaturase, Drosophila, PGal4
1. Introduction
Communication is an omnipresent and often flamboyant feature of life, yet the genetic bases of the evolution of communication systems remain a mystery (Hauser 1996). The genetic control underlying the emission of the signal and its reception must be tightly coadapted or the system would collapse during evolution if either component were to diverge too greatly. This problem is particularly acute in the case of inter-individual sexual communication, which may be at the heart of many instances of speciation (Darwin 1883; Panhuis et al. 2001). There has been very little experimental investigation of the genetic architecture of such systems (Boake 1991; Bradbury & Vehrencamp 1998): those studies that do exist are based on statistical associations and suggest that the emission of the signal and its reception are coded by different genes, for example in frogs (Ryan & Wilczyynski 1988), crickets (Hoy et al. 1977; Ritchie 2000), moths (Löfstedt et al. 1989) and flies (Sureau & Ferveur 1999). Assortative mating can generate strong genetic covariance between the male and female components of mate recognition system, and the hypothesis of a tight ‘genetic coupling’ is considered unlikely under a model of signal-preference coevolution involving unlinked genes (Lande 1981). However, the rapid evolution of the correlation between sex pheromones and mating success in hybrid flies between the two sibling species Drosophila serrata and D. birchii suggests that the coevolution of sexual communication could also be the effect of a tight genetic linkage, or of pleiotropy (Blows 1999).
In D.melanogaster, an important part of sexual behaviour is also based on chemical communication, in particular the role of sex-specific cuticular unsaturated hydrocarbons (CHs) which act as short-range or contact sex pheromones (Antony & Jallon 1982). Predominant CHs are sexually dimorphic in both their occurrence and their effects. Mature female flies produce large amounts of 7,11-heptacosadiene (7,11-HD) and 7,11-nonacosadiene (7,11-ND; respectively, with 27 and 29 carbons and two double bonds) and much less 7-tricosene (7-T) and 7-pentacosene (7-P; respectively, with 23 and 25 carbons and a single double bond). By contrast, mature male flies predominantly produce 7-T and 7-P, but no dienes. Female pheromones tend to encourage conspecific male courtship, whereas male pheromones inhibit intraspecific male–male courtship (Ferveur & Sureau 1996). The simultaneous effect of these substances could enable males to distinguish one sex from the other, but this has not yet been shown. Female predominant CHs of the Canton-S strain (Cs) strongly enhance the copulatory activity of Cs males, but without a dose–response effect (Marcillac & Ferveur 2004). Small amounts of 7,11-dienes are sufficient to prevent interspecific courtship and mating with males of the two closely related species D.simulans and D.mauritiana (Savarit et al. 1999).
In D. melanogaster, the proportions of the principal CHs depend upon several genes, some of which have been mapped (Coyne et al. 1994; Ferveur & Jallon 1996). In particular, the proportions of unsaturated hydrocarbons in females are controlled by desat1 and desat2 (two genes closely located on chromosome 3; Coyne et al. 1999), which code for two related desaturase enzymes (Wicker-Thomas et al. 1997; Dallerac et al. 2000).
We describe the effects of a single transposable element in desat1 that simultaneously affects the production and perception of the pheromones necessary for mate discrimination in Drosophila. Removal of the transposon rescued both the production and discrimination phenotypes, but these effects did not always coincide, indicating that both aspects depend upon the pleiotropic activity of desat1. Furthermore, desat1 expression was found in the peripheral tissues that produce and detect sex pheromones.
2. Material and methods
2.1 Drosophila melanogaster stocks and crosses
All D.melanogaster strains were raised on yeast/cornmeal/agar medium and kept at 24±0.5°C with 65±5% humidity on a 12L:12D cycle. Canton-S (Cs) is a wild-type strain widely used as control. The w1118 strain carries the Cs genetic background with a mutation in the white gene that produces visual defects (Cook 1980). Crosses were performed using standard techniques and genetic tools (Lindsley & Zimm 1992). The desat11573-1 strain contains a PGal4 transposable element inserted in desat1 and causing the mutation (figure 1; Marcillac et al. 2005). To generate derivative lines of desat11573-1, we remobilized the PGal4 transposon (Marcillac et al. 2005). Within each line, white-eyed (w-; desat11573-exc/TM3, Sb Ser) males and females carrying the same excision allele (from a common ancestor) were mated together to produce a stable line. When these lines were stabilized, all desat11573 alleles were outcrossed with the Cs strain for five generations to homogenize the genetic background and yield red-eyed males.
Figure 1.
The transposable element is inserted in the desat1 gene. The triangle indicates the position of the PGal4 transposable element, at chromosomal site 87B10-11. We used Southern blotting to check that only one transposon was inserted in the genome of the desat11573-1 strain. The gene altered by the PGal4 (PGawB) transposon was mapped after cloning and sequencing of the two DNA fragments flanking the insertion point (…CGGCT GTTTT and GTTGA CATGC…). Comparison with the BDGP database <www.fruitfly.org> revealed that they share a complete identity with two contiguous sequences of the desat1 gene. The 3′ extremity of the transposon was mapped at −1691 bp of the transcription start (ATG). The black boxes represent the coding region (with the translation STOP) and the grey boxes alternative 5′UTR for the desat1 gene. The desat2 gene is located at less than 4 kb from the 5′UTR of desat1.
2.2 Behavioural tests
The simultaneous discrimination of single subject males toward two decapitated object flies was tested during a 5 min period. This allowed us to measure the role of wild-type and mutant sex pheromones on male discrimination, and to detect variations in male discrimination toward control sex pheromones. All flies were isolated 0–4 h after eclosion under CO2 anaesthesia. Subject male flies (i.e. those whose sexual responses to object flies were measured) were held individually in fresh glass food vials for 5 days before testing. Object flies from the control and mutant strains were similarly isolated and held in groups of five for the same period. All tests were performed in a room at 24±0.5°C with 65±5% humidity. Tests were performed simultaneously over several days for subject flies of each strain with male and female objects and always took place 1–4 h after lights on. Subject males were individually aspirated (without anaesthesia) under a watch glass used as an observation chamber (1.6 cm3). After 5 min, two headless object flies were simultaneously introduced.
Object flies were decapitated a few minutes prior to the test. This procedure allows courtship duration to be standardized by preventing copulation, and removes most behavioural and acoustic variables associated with the object fly, leaving only chemical, tactile, postural and morphological cues (Ferveur & Sureau 1996). Also, decapitated object flies rarely moved during the test, although they could sometimes jump. Briefly, anaesthetized 5 day old object flies were decapitated with a razor blade cleaned with ethanol between each treated genotype. They were kept by groups of 10 in food vials and allowed at least 15 min to recover. Only standing headless flies were used for the test. After simultaneous introduction of the two decapitated object flies, the courtship index (CI) toward each object fly was measured. CI is the proportion of time that the male spends in active courtship (wing vibration, licking and attempting copulation; the very brief episodes of tapping behaviour were not taken into account given that they are not easy to see under red light condition). No qualitative difference was noted between the courtship sequences of the different subject males. We designed the Simultaneous Discrimination Index to measure the difference of the courtship indices (SDI=CI1−CI2) that a single subject male directed towards two different simultaneously presented headless flies during a 5 min observation period. Some observations were carried out under a red light (25W with a Kodak safe-light filter no. 1) to remove all visual stimuli (Boll & Noll 2002). For each test, n=70–86 except for subject w1118 paired with Cs (n=42), and for subject 1573-1 males in white light (n=50).
2.3 Extraction and analyses of CHs
Male and female flies were prepared as for object flies in behavioural tests. CHs were extracted from 5 day old intact individual flies by gas chromatography (GC) following hexane extraction according to standard procedures (Antony & Jallon 1982; Ferveur 1991). Analyses were performed with a Varian CP3380 chromatograph, equipped with a Cp-sil 25 m capillary column with hydrogen as the carrier gas. All the D. melanogaster predominant CHs have already been identified and characterized (Pechiné et al. 1985). Twenty-four CHs were systematically detected in female flies, and 14 in male flies, both with a chain length ranging from 23 to 29 carbons. In addition, a hexacosane standard (not shown) was used to calculate the absolute amount (in nanograms) of CHs and to align and calibrate chromatograms. Each CH (or group of CHs) was characterized by its percentage relative to the total amount of CHs (ΣCHs). For the sake of clarity the only percentages shown here are for 7,11-dienes (7,11-HD;7,11-ND), for 7-T, for the sum of all unsaturated CHs (ΣDESAT) and for the sum of the three principal saturated CHs (ΣLIN=n-tricosane [23LIN];n-pentacosane [25LIN];n-heptacosane [27LIN]).
2.4 Molecular biology
We used Southern blotting—with a probe made with a fragment of the pBSK sequence belonging to the transposon—to check that only one transposon was inserted in the genome of the desat11573-1 strain (datum confirmed with the results obtained with inverse PCR). The gene altered by the PGal4 (PGawB) transposon was mapped after cloning and sequencing of the two DNA fragments flanking the insertion point. It revealed that they share a complete identity with two contiguous sequences of the desat1 gene (Marcillac et al. 2005).
Southern blotting allowed us to determine the size of the remaining part of the transposon in 1573-exc alleles: E1 and J2 alleles respectively retained a 6.2 kb and a 8.7 kb fragment, D′1 retained a 48 bp fragment, and the transposon was completely excised from the N2 allele. The DNA region that surrounds the insertion point was sequenced in these four desat11573-exc alleles and showed no alteration (Marcillac et al. 2005). Therefore, N2, which was precisely excised, can be considered as a control allele.
2.5 Histochemistry
The reporter UAS-CD8GFP transgene was used to visualize the activity of the desat11573-1 enhancer-trap strain either by direct detection of GFP or by immunohistochemistry carried out with the primary anti-CD8 antibody (1/50, RM2200, Caltag Laboratories, USA) coupled with a secondary anti-rat-Alexa488 (1/200, A-11006, Molecular Probes). For confocal microscopy, preparations were mounted in Vectashield medium (Vector Laboratories) and viewed with a Kr/Ar laser light source set for excitation at 488 nm (BioRad MRC1024), coupled with BioRad Laser Sharpe 2000 for imaging.
3. Results
3.1 The mutation substantially decreases sex pheromone production in flies of both sexes
We induced a mutation in desat1 by inserting a PGal4 transposable element in the complex DNA region of this gene that is transcribed but not translated (5′UTR; figure 1). The resulting strain desat11573-1 produced homozygous viable male and female flies with no obvious morphological anomaly or behavioural defect.
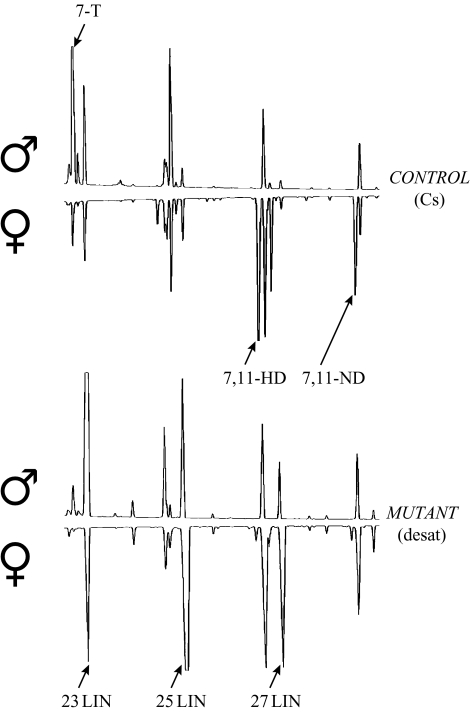
Female and male desat11573-1 mutant flies had radically decreased proportions of unsaturated hydrocarbons on their cuticle (table 1) including the main female and male pheromones (7,11-dienes and 7-tricosene, respectively). Conversely, mutants showed higher proportions of saturated hydrocarbons (n-alkanes), particularly n-pentacosane (25LIN; 32%) in females, and n-tricosane (23LIN; 48%) in males. The wild-type hydrocarbon phenotype could be simultaneously rescued in homozygous flies of both sexes by jumping out the PGal4 element. For example, desat11573-N2 and desat11573-D’1 alleles showed high levels of sex pheromones whereas desat11573-E1 and desat11573-J2 female and male flies retained a mutant phenotype (table 1). Out of a total of 132 excision strains, the CH profile was completely rescued for both sexes in ca. 75% cases, whereas the remainder showed a mutant phenotype similar to that of desat11573-1 (Marcillac et al. 2005). Mutant CH phenotype was generally shown by the alleles that retained a large fragment of the transposon (such as for E1 and J2), whereas rescue of CH profile occurred in alleles with a smaller fragment inserted (like D′1) or with a precisely excised transposon (like N2; see §2). Overall, sex differences in the CH profile of mutant flies were much smaller than in control flies (figure 2).
Table 1.
Principal male and female CHs in 5 day old control and mutant flies. (Values shown are the means (±s.e.m) for the percentage of 7-T (see figure 2), and for the sum of 11-dienes (7,11-HD;7,11-ND), for the sum of all unsaturated CHs (ΣDESAT), and for the sum of the three principal n-alkanes (23LIN;25LIN;27LIN=ΣLIN). All percentages are relative to the sum of all detected CHs (ΣCH; expressed in nanograms). Cs is a commonly used wild-type strain; the four excision strains were derived from the mutant 1573-1 strain after remobilization of the transposon (see § 2). n=10–12.)
| female |
male |
|||||||
| Σ CH (ng) | 7,11-dienes (%) | Σ DESAT (%) | Σ LIN (%) | Σ CH (ng) | 7-T (%) | Σ DESAT (%) | Σ LIN (%) | |
| control: Canton-S | 1583 (143) | 34%.5 (0.9) | 60.1 (2.5) | 10.6 (1) | 885 (80) | 33.5 (2.3) | 61.8 (3) | 18 (1.7) |
| mutant: desat11573-1 | 2400 (96) | 2 (0.1) | 6.1 (0.4) | 61.6 (0.6) | 2443 (119) | 3.4 (0.2) | 6.4 (0.3) | 67.5 (1.1) |
| excision: desat11573-N2 | 1642 (84) | 29.3 (1.7) | 48.5 (1.6) | 19.7 (1.8) | 1336 (66.3) | 33.5 (0.7) | 62.4 (1.1) | 16.9 (1.2) |
| excision: desat11573-D′1 | 1621 (59) | 32.7 (0.7) | 56.6 (1.3) | 17.6 (1.3) | 1549 (65.4) | 32.8 (1.2) | 59.1 (2) | 19.3 (1.7) |
| excision: desat11573-E1 | 2029 (99) | 1.8 (0.4) | 5.4 (0.9) | 54.5 (1.3) | 3218 (210) | 2.3 (0.4) | 3.8 (0.5) | 70.1 (0.8) |
| excision: desat11573-J2 | 2318 (97) | 4.2 (0.5) | 7.9 (0.6) | 56 (0.5) | 2481 (188) | 0.3 (0) | 3.1 (0.2) | 69.4 (0.5) |
Figure 2.
Gas chromatograms of individual male and female flies of control Canton-S and mutant desat11573-1 strains. Each peak corresponds to a single CH and sexually dimorphic CHs are cis 7-tricosene (7-T) in control males; cis, cis 7,11-heptacosadiene (7,11-HD) and cis, cis 7,11-nonacosadiene (7,11-ND) in control females. In mutant flies, n-tricosane (23LIN), n-pentacosane (25LIN) and n-heptacosane (27LIN) are the principal CHs.
3.2 The sex pheromones missing in mutant flies are necessary for male discrimination
A new behavioural paradigm allowed us to measure to what extent a subject male could simultaneously discriminate two decapitated object flies (of different sex or genotype; see §2). First, single Cs control males were simultaneously presented with two headless object flies and their behaviour recorded (figure 3a). The difference in courtship intensity directed towards the two objects was compared (with a t-test) either in white light or in red light. In white light, control males could discriminate between male and female flies, irrespective of whether these flies were mutant or wild-type (figure 3a(B,D); p < 0.0001). However, under red light conditions, in which flies are effectively blind (Boll & Noll 2002), cs males indiscriminately courted male and female desat1 mutants (figure 3a(C); p=n.s.), whereas they clearly preferred courting female flies when presented with male and female control flies (figure 3a(A); p < 0.0001). The inability of flies to discriminate desat1 flies on the basis of their chemical signal alone was confirmed by the behaviour of the visually impaired mutant w1118 (Cook 1980). When placed in white light, w1118 males were able to recognize the sex of wild-type flies (figure 3a(G); p=0.0003) but failed to discriminate between desat1 males and females (figure 3a(H); p=n.s.). As shown in figure 2, both stimulatory female sex pheromones and inhibitory male sex pheromones are disrupted in desat1 flies. The reciprocal role of normal female and male pheromones was confirmed in our simultaneous discrimination paradigm. In white light, Cs males were significantly less attracted to mutant desat1 females which lack stimulatory pheromones (figure 3a(E); p=0.027) than to control Cs females, whereas when they were presented with control and mutant males, they showed a significant preference for desat1 males which lack the inhibitory male pheromones (figure 3a(F); p=0.0098).
Figure 3.
Simultaneous discrimination of two object flies by subject control males. Tests were either carried out in red light (shaded), or in white light (plain). Each pair of mirrored bars represents the mean (±s.e.m.) courtship index that a 5-day-old subject male directed toward the two headless objects (CI1, CI2) simultaneously presented, during a 5 min observation period. The genotypes of subject males are shown above the histograms, the genotypes of object flies beneath each bar. Subjects are (a) Canton-S (Cs), and Cs with the white1118 mutation (w1118), (b) various desat11573-1 alleles (1573-1, -D′1, -N2, -E1, and-J2,; see table 1). Objects are desat11573-1 mutant (desat) and control (Cs) females and males. The t-value and the probability (with the Student’s t-test) for discrimination between the two objects is shown below each bar; d.f.=68–84 for each test except for subject w1118 paired with Cs (d.f.=40), and for subject 1573-1 males in white light (d.f.=48). The values for CI1/CI2 are as follows: for aA (43.3/9.4), aB (46/7.3), aC (25.2/17.9), aD (33.6/20.6), aE (33.3/21.3), aF (12/6.5), aG (35.2/11.8), aH (22.8/22), bA (21.9/17.5), bB (41.4/6.2), bC (41.8/17.6), bD (41/20.9), bE (29.8/16.1), bF (34/22.2).
3.3 The mutation also affects male discrimination of sex pheromones
Mutant desat11573-1 males were tested for their ability to discriminate males and females through the simultaneous detection of male inhibitory and female stimulatory pheromones (figure 3b). In red light, mutant males could not discriminate the sex of control flies (figure 3b(A); p=n.s.). However, in white light their ability to discriminate the sex of Cs control flies (figure 3b(B); p < 0.0001) was similar to that of control males, indicating that both their visual discrimination and their ability to court were unaffected. The wild-type discrimination phenotype could be rescued by jumping out the PGal4 element. For example, normal or quasi-normal sex discrimination in red light was shown by desat11573-N2 and desat11573-D′1 males (figure 3b(C,D); two excision alleles with a rescued hydrocarbon profile; table 1), whereas this performance was weaker in desat11573-E1 and desat11573-J2 males (figure 3b(E,F); two alleles with mutant hydrocarbon profiles). An ANOVA (F=2.518; d.f.=5, 234; p=0.0304) showed that the SDI (see §2) of wild-type male and female object flies was significantly lower (i) in males of the three mutant alleles (desat11573-1, -E1 and −J2) as compared to control Cs males and (ii) in mutant desat11573-1 flies as compared to the desat11573-N2 rescued allele, but not with the desat11573-D′1 rescued allele.
3.4 Adult expression of PGal4-desat11573-1
The adult expression pattern of desat11573-1 is shown in figure 4. The highest level of Gal4 expression was found in the oenocytes and throughout the fat body, two structures implicated in sex pheromone production in D.melanogaster (Ferveur et al. 1997; Savarit & Ferveur 2002). Gal4 expression was also detected in the legs (not shown; Robertson 1983), third antennal segments (Yamamoto et al. 1997) and proboscis (Boll & Noll 2002), which detect sex pheromones during courtship. Therefore, the tissue-specific expression of desat11573-1 enhancer-trap strain at the adult stage closely matches the altered phenotypes of mutant flies.
Figure 4.
Gal4 expression driven by the desat11573-1 strain. (a) Ventral view of a 7-day-old female abdomen. Expression was found in the ventral and lateral oenocytes (oe). The fat body showed a diffuse signal that declined during adulthood (not shown). (b) Right lateral view of a proboscis of a 1-day-old male. Gal4 expression was strong in the labial palp (lp; arrowhead), but weak in the maxillary palp (mp). (c) Posterior view of an antenna in a 7-day-old female. Expression was mostly found in the third segment (AIII; arrowhead), but not or weakly in the second segment (AII) or in the arista (ar). The reporter UAS-CD8GFP transgene was used to visualize the activity of desat11573-1 either by direct detection of the GFP (a,c) or by immunohistochemistry (b). Scale bar, 100μm.
4. Discussion
desat1 simultaneously affects the coding and decoding of a signal required for sexual communication. This does not mean that pheromone production and discrimination are based upon a single gene, but it does show that desat1 is not only involved in the pathways leading to the normal emission of sex pheromones, but also in their perception.
desat1 yields at least five transcripts, some of which, but not all, were drastically altered in the 1573-1 allele (Marcillac et al. 2005). Slight molecular variations between excision alleles may differentially affect the amount of one or several of these product(s) in different target tissues. The most obvious of these tissues are those associated with pheromone production, and our data show that the principal CHs missing in desat1 mutant females (probably 7,11-dienes) and males (7-tricosene) are required for mate discrimination by wild-type males in the absence of visual and other non-chemical cues. The site of the perception effect is less clear. Genetic feminization of male flies has implicated discrete brain regions in male discrimination of sex pheromones (Ferveur et al. 1995), but preliminary observations of the male’s peripheral sensory organs suggest that the desat1 signal is often associated with a specific accessory structure located at the base of a chemosensory hair. One possibility is that the mutation in desat1 might alter the activity of other gene(s) involved in sex pheromone reception, such as a pheromone-degrading enzyme, leading to a specific anosmia (Maïbèche-Coisne et al. 2004).
The fact that complete rescue of the discrimination and hydrocarbon mutant phenotypes did not necessarily coincide shows that the less effective discrimination of mutant flies was not directly caused by their own mutant hydrocarbon profile. This allows us to rule out the possibility that the mutant pheromones either cause an abnormal self-perception or mask the object fly’s pheromones. This interpretation is supported by a previous experiment where normal sex pheromone discrimination was shown by D.melanogaster males with genetically feminized pheromones (Ferveur et al. 1997).
We suggest that desat1 has pleiotropic functions and that its multiple products interact with genes involved in metabolic functions (i) in the oenocytes and (ii) in the accessory cells of some chemosensory organs. Pleiotropy has been considered as an unlikely cause of genetic correlation because of the phenotypic difference between the signal (produced by secretory cells) and its preference (processed by the nervous system; Boake (1991)). Drosophila CHs acting as sex pheromones can rapidly coevolve with mate recognition supporting the idea that the relationship between a trait and its preference could arise from the physical linkage between genes or be caused by pleiotropy (Blows 1999).
The fact that desat1 is directly or indirectly involved both in the emission of signals (female and male sex pheromones) and in the male structures necessary for their detection and discrimination casts a new light on previous studies of apparent incipient speciation in Drosophila. A mutation in the promoter of a neighbouring gene, desat2, which codes for another desaturase (Dallerac et al. 2000; Takahashi et al. 2001), has been correlated with a variable mating pattern shown by strains from Zimbabwe with unusual CHs (Ting et al. 2001), which has been interpreted as a case of incipient speciation (Fang et al. 2002). As well as changes in signal production, our data suggest that this phenomenon may be caused by simultaneous changes in signal detection. Speciation effects owing to variation in a desaturase gene and its effect on signal production have been postulated in moths, where the reactivation of a silent desaturase gene in ancestral Ostrinia nubilalis moths is thought to have allowed variant females to produce a new pheromone, leading to the appearance of a new species, O.furnicalis (Roelofs et al. 2002). The study of chemical communication in moths has revealed that in some species the broad tuning of male receptor sensitivity was correlated with changes in female pheromone composition (Baker 2002). It is not clear from our study whether desat1 has a direct or indirect effect on Drosophila male sensitivity to sex pheromones. Further tests with mutant genes that normally interact with desat1 will be required.
If other examples of sexual communication turn out to also involve the effect of a single gene on the emission and the perception of a signal, this would remove one level of difficulty in understanding the evolution of communication. However, our data show that even if the two aspects are affected by the same gene, coevolution of the sender and the receiver remains a necessity: the fact that rescue of the two mutant phenotypes did not necessarily coincide indicates that, in this system, different products of the same gene can act on the emission of the signal and its reception. Coevolution of the two characters, even if determined by slightly varying products from the same gene, will still be necessary, under the strong pressure exerted by both sexual and natural selection.
Acknowledgments
The authors thank F. Cézilly, R. J. Greenspan, A. J. Moore, and especially M. Cobb for their comments on the manuscript, R. Stocker and N. Gendre, and members of the UMR-5548 for technical help. This work was supported by the Centre National de la Recherche Scientifique, the French Ministry of Research and Education (for F.M.), the ARC (for Y.G) and the Burgundy Region.
References
- Antony C., Jallon J.M. The chemical basis for sex recognition in Drosophila melanogaster. J. Insect. Physiol. 1982;28:873–880. [Google Scholar]
- Baker T.C. Mechanism for saltational shifts in pheromone communication systems. Proc. Natl. Acad. Sci. USA. 2002;99:13368–13370. doi: 10.1073/pnas.222539799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blows M.W. Evolution of the genetic covariance between male and female components of mate recognition: an experimental test. Proc. R. Soc. B. 1999;266:2169–2174. doi: 10.1098/rspb.1999.0904. doi:10.1098/rspb.1999.0904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boake C. Coevolution of senders and receivers of sexual signals: genetic coupling and genetic correlations. Trends Ecol. Evol. 1991;6:225–227. doi: 10.1016/0169-5347(91)90027-U. [DOI] [PubMed] [Google Scholar]
- Boll W., Noll M. The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development. 2002;129:5667–5681. doi: 10.1242/dev.00157. [DOI] [PubMed] [Google Scholar]
- Bradbury J.W., Vehrencamp S.L. Principles of animal communication. Sinauer; Sunderland, MA: 1998. [Google Scholar]
- Cook R. The extent of visual control in the courtship tracking of Drosophila melanogaster. Biol. Cybernet. 1980;37:41–51. [Google Scholar]
- Coyne J.A., Mah K., Crittenden A.P. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science. 1994;265:1461–1464. doi: 10.1126/science.8073292. [DOI] [PubMed] [Google Scholar]
- Coyne J.A., Wicker-Thomas C., Jallon J.M. A gene responsible for a cuticular hydrocarbon polymorphism in Drosophila melanogaster. Genet. Res. 1999;73:189–203. doi: 10.1017/s0016672398003723. [DOI] [PubMed] [Google Scholar]
- Dallerac R., Labeur C., Jallon J.M., Knipple D.C., Roelofs W.L., Wicker-Thomas C. A delta 9 desaturase gene with a different substrate specificity is responsible for the cuticular diene hydrocarbon polymorphism in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2000;97:9449–9454. doi: 10.1073/pnas.150243997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. The descent of man and selection relation to sex. 3rd edn. Murray; London: 1883. [Google Scholar]
- Fang S., Takahashi A., Wu C.I. A mutation in the promoter of desaturase 2 is correlated with sexual isolation between Drosophila behavioral races. Genetics. 2002;162:781–784. doi: 10.1093/genetics/162.2.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J.-F. Genetic control of pheromones in Drosophila simulans. I. Ngbo, a locus on the second chromosome. Genetics. 1991;128:293–301. doi: 10.1093/genetics/128.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur J.-F., Jallon J.M. Genetic control of male cuticular hydrocarbons in Drosophila melanogaster. Genet. Res. 1996;67:211–218. doi: 10.1017/s0016672300033693. [DOI] [PubMed] [Google Scholar]
- Ferveur J.-F., Sureau G. Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc. R. Soc. B. 1996;263:967–973. doi: 10.1098/rspb.1996.0143. [DOI] [PubMed] [Google Scholar]
- Ferveur J.-F., Stortkühl K.F., Stocker R.F., Greenspan R.J. Genetic feminization of brain structures and changed sexual orientation in male Drosophila melanogaster. Science. 1995;267:902–905. doi: 10.1126/science.7846534. [DOI] [PubMed] [Google Scholar]
- Ferveur J.-F., Savarit F., O’Kane C.J., Sureau G., Greenspan R.J., Jallon J.M. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science. 1997;276:1555–1558. doi: 10.1126/science.276.5318.1555. [DOI] [PubMed] [Google Scholar]
- Hauser M.D. The evolution of communication. MIT Press; London: 1996. [Google Scholar]
- Hoy R.R., Hahn J., Paul R.C. Hybrid cricket auditory behavior: evidence for genetic coupling in animal communication. Science. 1977;195:82–84. doi: 10.1126/science.831260. [DOI] [PubMed] [Google Scholar]
- Lande R. Models of speciation by sexual selection on polygenic traits. Proc. Natl. Acad. Sci. USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D.L., Zimm G.G. The genome of Drosophila melanogaster. Academic Press; London: 1992. [Google Scholar]
- Löfstedt C., Hansson B.S., Roelofs W., Bengtsson B.O. No linkage between genes controlling female pheromone production and male pheromone response in the European corn borer, Ostrinia nubilalis Hubner (Lepidoptera; Pyralidae) Genetics. 1989;123:553–556. doi: 10.1093/genetics/123.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maïbèche-Coisne M., Nikonov A.A., Ishida Y., Jacquin-Joly E., Leal W.S. Pheromone anosmia in a scarab beetle induced by in vivo inhibition of a pheromone-degrading enzyme. Proc Natl. Acad. Sci. USA. 2004;101:11459–11564. doi: 10.1073/pnas.0403537101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcillac F., Ferveur J.-F. A set of female pheromones affects reproduction before, during and after mating in Drosophila. J. Exp. Biol. 2004;207:3927–3933. doi: 10.1242/jeb.01236. [DOI] [PubMed] [Google Scholar]
- Marcillac, F., Bousquet, F., Alabouvette, J., Savarit, F. & Ferveur, J.-F. 2005 A mutation with major effects on Drosophila melanogaster sex pheromones. Genetics (In the press.) (doi: 10.1534/genetics) [DOI] [PMC free article] [PubMed]
- Panhuis T.M., Butlin R., Zuk M., Tregenza T. Sexual selection and speciation. Trends Ecol. Evol. 2001;16:364–371. doi: 10.1016/s0169-5347(01)02160-7. [DOI] [PubMed] [Google Scholar]
- Pechiné J.M., Perez F., Antony C., Jallon J.M. A further characterization of Drosophila cuticular monoenes using a mass spectrometry method to localize double bonds in complex mixtures. Anal. Biochem. 1985;145:177–182. doi: 10.1016/0003-2697(85)90344-6. [DOI] [PubMed] [Google Scholar]
- Ritchie M.G. The inheritance of female preference functions in a mate recognition system. Proc. R. Soc. B. 2000;267:327–332. doi: 10.1098/rspb.2000.1004. doi:10.1098/rspb.2000.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H.M. Chemical stimuli eliciting courtship by males in Drosophila melanogaster. Experientia. 1983;39:333–335. [Google Scholar]
- Roelofs W.L., Liu W., Hao G., Jiao H., Rooney A.P., Linn C.E., Jr Evolution of moth sex pheromones via ancestral genes. Proc. Natl. Acad. Sci. USA. 2002;99:13621–13626. doi: 10.1073/pnas.152445399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.J., Wilczyynski W. Coevolution of sender and receiver: effects on local mate preference in cricket frogs. Science. 1988;240:1786–1788. doi: 10.1126/science.240.4860.1786. [DOI] [PubMed] [Google Scholar]
- Savarit F., Ferveur J.-F. Genetic study of the production of sexually dimorphic cuticular hydrocarbons in relation with the sex-determination gene transformer in Drosophila melanogaster. Genet. Res. 2002;79:23–40. doi: 10.1017/s0016672301005481. [DOI] [PubMed] [Google Scholar]
- Savarit F., Sureau G., Cobb M., Ferveur J.-F. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc. Natl. Acad. Sci. USA. 1999;96:9015–9020. doi: 10.1073/pnas.96.16.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau G., Ferveur J.-F. Coadaptation of pheromone production and behavioural responses in Drosophila melanogaster males. Genet. Res. 1999;74:129–137. doi: 10.1017/s0016672399003936. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Tsaur S.C., Coyne J.A., Wu C.I. The nucleotide changes governing cuticular hydrocarbon variation and their evolution in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 2001;98:3920–3925. doi: 10.1073/pnas.061465098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting C.T., Takahashi A., Wu C.I. Incipient speciation by sexual isolation in Drosophila concurrent evolution at multiple loci. Proc. Natl. Acad. Sci. USA. 2001;98:6709–6713. doi: 10.1073/pnas.121418898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker-Thomas C., Henriet C., Dallerac R. Partial characterization of a fatty acid desaturase gene in Drosophila melanogaster. Insect Biochem. Mol. Biol. 1997;27:963–972. doi: 10.1016/s0965-1748(97)00077-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto D., Jallon J.M., Komatsu A. Genetic dissection of sexual behavior in Drosophila melanogaster. A. Rev. Entomol. 1997;42:551–585. doi: 10.1146/annurev.ento.42.1.551. [DOI] [PubMed] [Google Scholar]