Abstract
Tyrosine phosphatases (PTPs) ε and α are closely related and share several molecular functions, such as regulation of Src family kinases and voltage-gated potassium (Kv) channels. Functional interrelationships between PTPε and PTPα and the mechanisms by which they regulate K+ channels and Src were analyzed in vivo in mice lacking either or both PTPs. Lack of either PTP increases Kv channel activity and phosphorylation in Schwann cells, indicating these PTPs inhibit Kv current amplitude in vivo. Open probability and unitary conductance of Kv channels are unchanged, suggesting an effect on channel number or organization. PTPα inhibits Kv channels more strongly than PTPε; this correlates with constitutive association of PTPα with Kv2.1, driven by membranal localization of PTPα. PTPα, but not PTPε, activates Src in sciatic nerve extracts, suggesting Src deregulation is not responsible exclusively for the observed phenotypes and highlighting an unexpected difference between both PTPs. Developmentally, sciatic nerve myelination is reduced transiently in mice lacking either PTP and more so in mice lacking both PTPs, suggesting both PTPs support myelination but are not fully redundant. We conclude that PTPε and PTPα differ significantly in their regulation of Kv channels and Src in the system examined and that similarity between PTPs does not necessarily result in full functional redundancy in vivo.
INTRODUCTION
Reversible phosphorylation of tyrosine residues in proteins is a major mechanism for regulation of protein structure and function. Phosphorylation is regulated by the opposing activities of two superfamilies of enzymes—the protein tyrosine kinases (PTKs) and the protein tyrosine phosphatases (PTPs). More than 100 PTP genes are known in higher organisms, of which 38 belong to the classical, tyrosine-specific PTP family (Alonso et al., 2004; Andersen et al., 2004). The number of tyrosine phosphorylation sites in target proteins is significantly larger than the numbers of known PTPs or PTKs (Manning et al., 2002; Alonso et al., 2004). Each enzyme therefore interacts with several substrates, and a given substrate may be targeted by more than one kinase or phosphatase. Consequently, it is not always clear whether these enzymes fulfill molecular and physiological roles that are entirely independent or that overlap at least in part.
Molecular and physiological studies of specific PTPs in recent years indicate that the issue of functional independence versus redundancy is complex. In many cases, specific substrates are currently known to be targeted by an individual PTP; in other cases, however, substrate sharing clearly occurs. A prime example of this is Src, which can be activated by dephosphorylation of its C-terminal inhibitory tyrosine by several PTPs, including PTP1B, SHP1, PTPα, and PTPε (Somani et al., 1997; Ponniah et al., 1999; Su et al., 1999; Bjorge et al., 2000; Gil-Henn and Elson, 2003; Pallen, 2003). Yet, targeting of a specific substrate by several PTPs may not necessarily imply that these PTPs are fully redundant in vivo, because their expression patterns, their physical access to the substrate, and regulation of their catalytic activities may vary.
Similar complexity is found at the physiological level when phenotypes of mice genetically lacking PTPs are analyzed. Several possible modes of functional interaction between pairs of PTPs may exist. Both PTPs may have physiological functions that are either partially or entirely overlapping, in which case single mutants may have relatively weak phenotypes due to functional compensation by the other PTP. Deletion of both PTPs might then exacerbate existing phenotypes or uncover novel phenotypes. We note that the developmental phenotypes of several single-gene PTP mutants, including some of the most informative ones, are in fact rather weak and do not lead to major histopathological abnormalities in mice (e.g., Schaapveld et al., 1997; Elchebly et al., 1999; Ponniah et al., 1999; Su et al., 1999; Klaman et al., 2000; Peretz et al., 2000). In other cases, both PTPs may antagonize each other's function, evident as phenotypes of reduced intensity in mice lacking both PTPs compared with mice lacking either PTP. Along these lines, simultaneous absence of CD45 and SHP-1 corrects several B-cell defects present in mice lacking either of these PTPs (Pani et al., 1997). Antagonistic interactions have been demonstrated also in axon guidance between several Drosophila PTPs (Desai et al., 1997; Schindelholz et al., 2001; Sun et al., 2001) and between PTPRO versus RPTPδ and RPTPγ in regulating nerve structure in chick lumbar spinal cord (Stepanek et al., 2005). Last, PTPs may have separate and nonoverlapping physiological functions, in which case the phenotype of mice lacking both PTPs would seem to be a simple combination of the two single-knockout phenotypes. We note that functional redundancy or antagonism between PTPs does not necessarily imply similar interrelationships at the molecular level.
We have chosen to examine this issue by comparing the abilities of two closely related PTPs, PTPε and PTPα, to regulate delayed rectifier voltage-gated potassium (Kv) channels in Schwann cells. The two major forms of PTPε protein are the receptor-type form (RPTPε) and the nonreceptor form (cyt-PTPε), which are produced by distinct promoters of the Ptpre gene (Elson and Leder, 1995a, b; Nakamura et al., 1996; Tanuma et al., 1999). Other protein forms of PTPε are p67 PTPε and p65 PTPε, whose production is regulated at the levels of transcription and posttranslational processing, respectively (Gil-Henn et al., 2000, 2001). RPTPε and cyt-PTPε differ only at their amino termini, resulting in each having a distinct pattern of subcellular localization and rendering them physiologically nonequivalent. Demonstrated roles of RPTPε include assisting Neu-induced mouse mammary tumor cells maintain their transformed phenotype by dephosphorylating and activating Src in vivo (Gil-Henn and Elson, 2003), and possibly down-regulating insulin receptor signaling (Moller et al., 1995; Andersen et al., 2001; Lacasa et al., 2005; Nakagawa et al., 2005). The cyt-PTPε form is important for proper adhesion of osteoclasts to bone and for subsequent bone resorption (Chiusaroli et al., 2004). cyt-PTPε can also down-regulate signaling mediated by the mitogen-activated protein kinase (Wabakken et al., 2002; Toledano-Katchalski et al., 2003) and by the Janus tyrosine kinase-signal transducer and activator of transcription (Tanuma et al., 2000, 2001, 2003) pathways and is required for proper functioning of mouse macrophages (Sully et al., 2001).
cyt-PTPε can also down-regulate the activity of Kv channels in Schwann cells (Peretz et al., 2000). Kv channels comprise a large and ubiquitous family and are key regulators of cellular functions such as action potential waveforms, neuronal firing patterns, synaptic integration, neurotransmitter release, volume regulation, and cell proliferation. Kv channels are comprised of tetramers of membrane-spanning α subunits, which may associate with up to four cytosolic regulatory β subunits (Pongs, 1995; Martens et al., 1999; Yi et al., 2001). Kv channels are activated by membrane depolarization, but their activity can be modulated after tyrosine phosphorylation of their α subunits by Src family PTKs (Fadool et al., 1997; Sobko et al., 1998a; Levitan, 1999; Peretz et al., 1999; MacFarlane and Sontheimer, 2000). We have shown that the α subunit Kv2.1 is a physiological substrate of cyt-PTPε. Kv2.1 is hyperphosphorylated in Schwann cells and sciatic nerve tissue of PTPε-deficient mice; phosphorylation of Kv2.1 by Src or Fyn up-regulates channel activity, whereas channel dephosphorylation by cyt-PTPε counters this effect (Peretz et al., 2000). The major site within Kv2.1 at which Src and cyt-PTPε exert their opposing effects is Y124 in the cytoplasmic N terminus of the channel (Tiran et al., 2003). A stable complex between Kv2.1 and the substrate-trapping mutant D245A cyt-PTPε can be isolated readily; a significant part of the stability of this complex is linked to the presence of the Y124 phosphorylation site in Kv2.1 (Peretz et al., 2000; Tiran et al., 2003). Increased Kv channel activity in Schwann cells of mice lacking PTPε is correlated with transiently reduced myelination of sciatic nerve axons of these mice (Peretz et al., 2000).
The PTP most closely related to PTPε is RPTPα, whose major protein form is a receptor-type molecule (Krueger et al., 1990). RPTPα activates Src by dephosphorylating its C-terminal inhibitory tyrosine residue in a manner similar to PTPε (for review, see Pallen, 2003). Activation of Src and related kinases by RPTPα mediates many of its physiological functions, such as regulation of integrin signaling and cell migration (Ponniah et al., 1999; Su et al., 1999; von Wichert et al., 2003; Zeng et al., 2003) and neurite elongation (Bodrikov et al., 2005). RPTPα has been suggested to down-regulate insulin receptor signaling (Moller et al., 1995; Lammers et al., 1997; Andersen et al., 2001; Lacasa et al., 2005), although lack of RPTPα does not seem to affect glucose homeostasis in vivo (Le et al., 2004). At the whole-animal level, gene-targeting studies have established that RPTPα functions in developmental positioning of hippocampal pyramidal neurons, learning, and neuroplasticity (Petrone et al., 2003; Skelton et al., 2003). Molecular targets of RPTPα responsible for these phenotypes remain to be identified. Interestingly, ectopically expressed RPTPα is capable of activating the Kv1.2 and Kv1.1 potassium channels upon signaling by the m1 muscarinic and serotonin receptors (Tsai et al., 1999; Imbrici et al., 2000). The difference between this effect and inhibition of Kv2.1 by cyt-PTPε may stem from differences in the biophysical attributes of the two channel subtypes, the biochemical properties of either PTP, or from differences in the cellular context or specific signaling pathways involved in either system.
A significant part of the above-mentioned information has been obtained by use of mice genetically lacking either PTPα or PTPε (Ponniah et al., 1999; Su et al., 1999; Peretz et al., 2000; Sully et al., 2001; Petrone et al., 2003; Bodrikov et al., 2005). Yet, lack of either PTP is tolerated well by mice and is not associated with major morbidity or mortality. Although PTPs α and ε are products of separate genes and have distinct expression patterns in vivo, these PTPs are highly related. PTPs α and ε are the only two members of the type IV family of receptor PTPs (Alonso et al., 2004; Andersen et al., 2004), and 83% of the amino acid residues in their cytosolic domains are either identical or similar, suggesting that functional redundancies may exist between them. To examine this issue in vivo, we derived and examined mice lacking both PTPε and PTPα. In this study, we examine regulation of Kv channel activity and Src in Schwann cells in the absence of either or both PTPs and conclude that some of the functions of these closely related PTPs are clearly divergent within a single cell type.
MATERIALS AND METHODS
Plasmids
Full-length mouse cDNAs for cyt-PTPε (Elson and Leder, 1995a), RPTPε (Elson and Leder, 1995b), and RPTPα (kind gift of the late Dr. M. Thomas) were used in this study. Lck-cyt-PTPε was prepared by polymerase chain reaction (PCR)-mediated addition of the N-terminal Lck myristoylation domain to the N terminus of cyt-PTPε; cyt-PTPα was prepared similarly by replacing the extracellular and transmembranal domains of RPTPα (amino acids 1–166) with the 12 N-terminal residues of cyt-PTPε. The following mutants were prepared by site-directed mutagenesis: R340M RPTPε, R283M cyt-PTPε, D245A cyt-PTPε, D437A RPTPα, D245A Lck-cyt-PTPε, and D437A cyt-PTPα. Numbering of the mutated residue in the latter two cDNAs is unchanged from cyt-PTPε and RPTPα, respectively, for clarity. All products of PCR and of site-directed mutagenesis were sequenced before use, and all contained a C-terminal FLAG tag. Also used were the cDNAs for rat Kv2.1 (gift of Drs. J. Barhanin and M. Lazdunski), Y124F Kv2.1 (Tiran et al., 2003), and chicken Y527F Src (gift of Dr. S. Courtneidge, Van Andel Research Institute, Grand Rapids, MI). All cDNAs were cloned into the pcDNA3 expression vector (Invitrogen, Carlsbad, CA).
Generation and Analysis of Mice
Mice were housed (up to 5 per cage) in the Weizmann Institute barrier mouse facility. Animals were handled in accordance with National Research Council regulations, Israeli Law, and Weizmann Institute regulations; all studies were approved by the Institutional Animal Care and Use Committee. Mice lacking PTPε (Peretz et al., 2000) or PTPα (Su et al., 1999) were crossed; progeny were interbred to generate the four genotypes used in this study—wild type (WT), PTPε-deficient (EKO or Ptpre−/−), PTPα-deficient (AKO or Ptpra−/−), and deficient in both PTPs (DKO or Ptpre−/−/Ptpra−/−)—in the same genetic background (C57Bl/6 × 129Sv/Ev).
Genotyping of mice by PCR for the PTPε-targeted allele was done using primers WT5′ (5′-ACTCCCAGACAGCTGCAAAGC-3′) and WT3′ (5′-CGCTACAGTGAACCACAATGG-3′), which amplify a 250-base pair fragment from the WT allele of PTPε, and primers SL3015′ (5′-GGATCCAATTGCAATGATCA-3′) and NEOKO3′ (5′-ACTGAAGGCTCTTTACTATTGC-3′), which amplify a 450-base pair fragment from the targeted PTPε allele. Reactions included 1-μl tail biopsy DNA, ∼30 pmol of each oligonucleotide, 0.25 mM of each dNTP (Sigma-Aldrich, St. Louis, MO), 1× polymerase reaction buffer [1.5 mM MgCl2, 20 mM (NH4)SO4, 75 mM Tris-HCl, pH 9.0, and 0.01% Tween 20], and 1.25 U of Taq polymerase (JMR Holdings, London, United Kingdom) in a final volume of 25 μl. Samples were denatured at 93°C for 2 min followed by 30 cycles of 93°C for 30 s, 52°C for 1 min, and 72°C for 1 min. Genotyping by PCR for the RPTPα-targeted allele was performed as described previously (Su et al., 1999). Mice were weaned at ∼3 wk of age and were weighed once a week starting 40 d after birth through adulthood (5–7 mo). The following tissues were stained with hematoxylin and eosin and examined by light microscopy: liver, stomach, small and large intestines, kidney, adipose connective tissue, uterus, skeletal muscle (diaphragm), various peripheral nerves, pancreas, adrenal, brown fat, various blood vessels, heart, thymus, spleen, lung, lymph nodes (mesenteric, peripheral, and inguinal), skull (coronal sections), skin (head), marrow (sternum), thyroid, salivary glands, tongue, pancreas, oviducts, urinary bladder, and brain. No gross abnormalities beyond those described for AKO mice (Su et al., 1999) were detected.
Electron Microscopy
Sciatic nerves from 5-d-old mice (6–11 mice from each genotype from at least 2 different litters born to different parents) were analyzed as described previously (Peretz et al., 2000). Samples from different mice were always taken from the same location, 5 mm from the proximal end of the nerve. Briefly, samples were fixed with 3% paraformaldehyde and 2% glutaraldehyde in 0.1 M cacodylate buffer, postfixed in 1% OsO4 and 2% uranyl acetate, dehydrated in a graded series of ethanols, and embedded in Epon-812. Transverse cross-sections of 70–90 nm thickness were cut with a Leica Ultracut UCT ultramicrotome (Leica, Wetzlar, Germany), stained with uranyl acetate and lead citrate, and examined on a Tecnai 12 transmission electron microscope at 120Kv. Cross-sections were examined from each mouse at 4000× magnification. Pictures were digitized with a MegaView III charge-coupled device camera and analyzed with AnalySIS software (SPSS, Chicago, IL). The thickness of the myelin sheath of all axon profiles in a given field was measured, and data for mice of the same genotype was pooled.
Cell Culture
Primary Schwann cells were prepared from sciatic nerves of 3- to 5-d-old pups as described previously (Sobko et al., 1998a). Age-matched WT, EKO, AKO, or DKO pups were obtained from separate matings of homozygous mice of the same genotypes. Schwann cells were grown in DMEM/F-12 (Invitrogen) supplemented with 10% fetal calf serum (FCS) (Invitrogen), 2 mM glutamine, and antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin) and analyzed 8–10 d after isolation. Human embryonic kidney (HEK)293 cells were grown in DMEM (Invitrogen) supplemented with 10% FCS and antibiotics as described above and transfected by the calcium phosphate technique.
Electrophysiology
Whole-cell currents were recorded from Schwann cells by using the patch-clamp technique as described previously (Sobko et al., 1998a). Signals were amplified using an Axopatch 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA), sampled at 2 kHz, and filtered below 0.8 kHz via a four-pole Bessel low-pass filter. Data acquisition was done using the pClamp 8.1 software (Molecular Devices). The patch pipettes were filled with 164 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 11 mM EGTA, 10 mM HEPES, and 11 mM glucose at pH 7.4 and had a tip resistance of 4–8 MΩ. The external solution contained 140 mM NaCl, 5 mM KCl, 5 mM CaCl2, 2 mM MgCl2, 11 mM glucose, and 10 mM HEPES at pH 7.4. Series resistances (3–13 MΩ) were compensated (75–90%) and periodically monitored.
Single-channel recordings were performed in Schwann cells using the cell-attached configuration of the patch-clamp technique. Cells were continuously perfused with a standard salt solution containing 150 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1.2 mM MgCl2, 15 mM glucose, and 10 mM HEPES (pH 7.4 with NaOH). Pipettes were pulled from borosilicate glass, coated with Sylgard, and filled with the above-mentioned standard salt solution (8–10 MΩ). Signals were sampled at 10 kHz and low-pass filtered at 2 kHz. Data were analyzed using Clampfit 9.2 (Molecular Devices).
Biochemical Fractionation of Sciatic Nerve Tissue and Cells
For analysis of Kv2.1 phosphorylation in sciatic nerves, nerve tissue was dissected from ∼3-d-old mice (30–40 mice per experimental point) and stored in liquid nitrogen until use. Tissue was homogenized in buffer containing 50 mM Tris, pH 7.4, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 50 mM sodium fluoride, 0.5 mM sodium pervanadate, and protease inhibitors [1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 40 μM bestatin, 15 μM E-64, 20 μM leupeptin, and 15 μM pepstatin; Sigma-Aldrich], and centrifuged at 21,000 × g for 30 min at 4°C. The pellet (crude membranal fraction) was resuspended and sonicated in solubilization buffer (10% glycerol, 50 mM HEPES, pH 7.4, 10 mM EDTA, 150 mM NaCl, 1.5 mM MgCl2, 1% Triton X-100, 1 mM PMSF, 50 mM sodium fluoride, 0.5 mM sodium pervanadate, and protease inhibitors). The sonicate was incubated with shaking in solubilization buffer for 1 h at 4°C, spun at 21,000 × g, and the supernatant was collected. Membranal extracts (1.5–3 mg) were incubated with shaking with anti-Kv2.1 monoclonal antibodies (Trimmer, 1991) at 4°C overnight. Immune complexes were collected by adding protein A/G-Sepharose (Santa Cruz Biotechnology, Santa Cruz, CA) and incubating in solubilization buffer for 2 h at 4°C with shaking, followed by three washes in solubilization buffer. In other studies, cultured cells were fractionated into membranal and nonmembranal (i.e., combined cytosolic and nuclear) fractions by homogenization in hypotonic buffer as described previously (Elson and Leder, 1995a).
Immunoprecipitation and Protein Blotting
Cells were lysed in buffer A (50 mM Tris-Cl, pH 7.5, 100 mM NaCl, and 1% NP-40), supplemented with protease inhibitors and either 0.5 mM sodium pervanadate (for phosphorylation studies) or 5 mM sodium iodoacetate (for substrate-trapping studies). In phosphorylation studies crude cellular proteins (1 mg) were reacted with anti-phosphotyrosine antibodies (clone PY20; BD Biosciences Transduction Laboratories, Lexington, KY) and protein A-Sepharose beads (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) for 2 h at 4°C, spun down, and washed three times in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS). In substrate-trapping studies cellular proteins (1 mg) were reacted with anti-FLAG M2 affinity beads (Sigma-Aldrich) for 4–6 h, followed by two washes in buffer A and one wash in RIPA buffer. SDS gel electrophoresis, blotting, and antibody hybridization were done as described previously (Gil-Henn et al., 2000). Primary antibodies used included rabbit polyclonal anti-PTPε, which cross reacts with RPTPα (Elson and Leder, 1995b), monoclonal [clone D4/11, generous gift of Drs. E. Peles (Weizmann Institute of Science, Rehovot, Israel) and J. Trimmer (University of California, Davis, CA) (Trimmer, 1991)] or polyclonal (Alomone Labs, Jerusalem, Israel) anti-Kv2.1, monoclonal anti-v-Src (clone 327; Calbiochem, San Diego, CA), and monoclonal anti-phosphotyrosine (clone 4G10; Upstate Biotechnology, Lake Placid, NY). Band signals at nonsaturating exposures were scanned and measured by image densitometry by using Image Gauge 4.0 (FujiFilm, Tokyo, Japan) and Adobe Photoshop 7.0 software (Adobe Systems, Mountain View, CA).
Src Activity Assay
Sciatic nerve tissue was dissected from 3- to 5-d-old mice and stored frozen in liquid nitrogen until used. Tissue was homogenized in buffer A supplemented with protease inhibitors and sodium pervanadate as described above, sonicated, and spun to remove debris. Crude lysates (0.3–0.5 mg) were used to determine activity of immune-precipitated c-Src by using [γ-32P]ATP and enolase as described previously (Gil-Henn and Elson, 2003).
In Vitro Dephosphorylation of pKv2.1
HEK293 cells expressing FLAG-tagged RPTPα or cyt-PTPε were lysed in buffer A supplemented with protease inhibitors but without pervanadate. PTPs were immunoprecipitated with monoclonal anti-FLAG M2 antibodies (Sigma-Aldrich). Precipitated material was washed three times in buffer A, twice in buffer B (20 mM HEPES, pH 7.6, 100 mM KCl, 0.5 mM EDTA, 0.4% NP-40, and 20% glycerol), followed by two short (1.5-min) washes in buffer 54K (50 mM Tris, pH 7.9, 150 mM NaCl, and 0.5% Triton X-100). Proteins were eluted by two incubations in equal volumes of Tris-buffered saline buffer (20 mM Tris-Cl, pH 7.35, and 150 mM NaCl) containing 1 mg/ml FLAG peptide (Sigma-Aldrich) and 0.1 mM EGTA, at 32°C for 3 min. Eluted material was pooled. FLAG-tagged phosphorylated Kv2.1 was purified in a similar manner from HEK293 cells expressing Kv2.1 and active (Y527F) Src, except that the lysis buffer A contained 0.5 mM sodium pervanadate. Protein purity and amounts were determined by gel electrophoresis and silver staining. pKv2.1 (15 ng) was incubated either alone or with 100 ng of purified PTP in PTP activity buffer [50 mM 2-(N-morpholino)ethanesulfonic acid, pH 7.0, 0.5 mg/ml bovine serum albumin, and 0.5 mM dithiothreitol] at 32°C for 6 h, followed by adding SDS-PAGE sample buffer and boiling. Phosphorylation of Kv2.1 was analyzed by SDS-PAGE and protein blotting with anti-pTyr antibodies as described above.
RESULTS
Mice Lacking Both PTPε and PTPα Are Viable
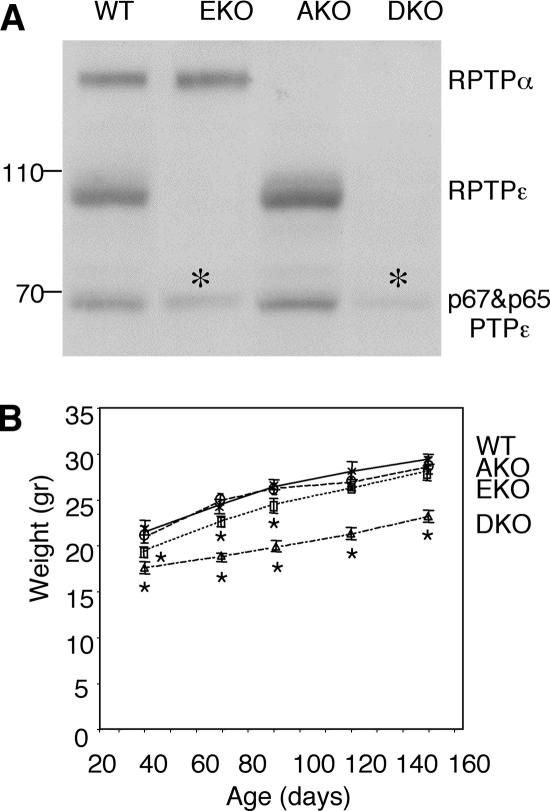
The effects of absence of PTPε or PTPα on Kv channels were compared in mice lacking either PTPε (EKO mice; Peretz et al., 2000) or PTPα (AKO mice; Su et al., 1999). To obtain additional information on the functional interactions between these PTPs, EKO and AKO mice were crossed to generate mice lacking both PTPs (DKO mice). DKO mice were viable and produced litters of normal size. Analysis of protein expression from various tissues confirmed that DKO mice lacked RPTPα and the various PTPε proteins (Figures 1A and 3A). Interestingly, DKO mice of both genders weighed 15–20% less than WT, AKO, or EKO mice (Figure 1B). Reduced DKO body weight was detected before weaning and persisted throughout adulthood, indicating that DKO mice gain weight proportionally at the same rate as WT mice and consequently remain smaller throughout life. This result indicates that PTPα and PTPε perform a redundant role in regulation of mouse body weight. Altered body weight is a complex phenotype that has been observed in mice lacking various unrelated genes, among them PTPs (Klaman et al., 2000; Cheng et al., 2002; Zhang et al., 2004). Further studies will therefore be needed to uncover its cellular and molecular bases in this particular case. Histological examination of several tissues of DKO mice did not reveal major gross structural abnormalities beyond those described for AKO mice (Su et al., 1999; Petrone et al., 2003); the tissues examined are listed in Materials and Methods. We conclude that abnormalities in these tissues, if they exist, are subtle; their detection may require use of more sensitive techniques, as shown below in the case of sciatic nerves.
Figure 1.
PTP expression and body weight of mice used in this study. (A) Expression of RPTPε and RPTPα in brain lysates. Blots containing crude extracts of brain from adult WT, AKO, EKO, and DKO mice were reacted with anti-PTPε antibodies, which also cross-react with RPTPα, to assess expression of PTPε and PTPα in this tissue. Asterisks mark nonspecific bands; molecular mass standards are in kilodaltons. (B) Weights of mice of the indicated genotypes are shown between 40 and 150 d of age. The weight of DKO mice was significantly (15–25%) lower than that of WT, AKO, or EKO mice (Mann–Whitney U-test, p < 0.0001). EKO males weighed slightly less than WT males up to 90 d (Mann–Whitney U-test, p < 0.01). Each data point represents the average weight ± SEM with n = 20–51. Asterisks mark data points significantly different from WT. Similar results were obtained with female mice (our unpublished data).
Figure 3.
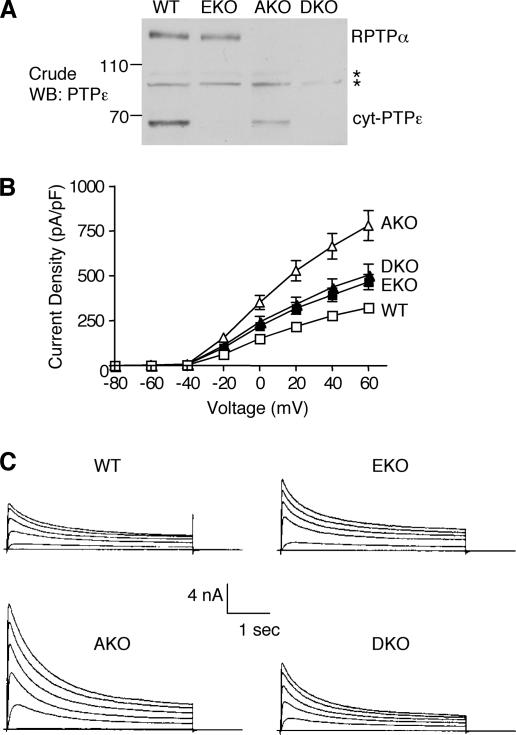
Increased Kv channel currents in primary Schwann cells of EKO, AKO, or DKO mice. (A) Blots containing crude extracts from Schwann cells from 3- to 5-d-old WT, AKO, EKO, and DKO mice were reacted with anti-PTPε antibodies to assess expression of cyt-PTPε and PTPα. Asterisks mark nonspecific bands; molecular mass markers are in kilodaltons. (B) The K+ current density (picoamperes/picofarads; mean ± SEM) of WT (n = 12), AKO (n = 11), EKO (n = 14), and DKO (n = 9) Schwann cells plotted against voltage steps (millivolts). At +60 mV, EKO cells exhibit a 47% increase in current density compared with WT cells; analogous values for AKO and DKO cells are +150 and +62%, respectively. (C) Whole-cell K+ currents recorded from primary Schwann cells of the four genotypes. Cells were stepped from a holding potential of −80 to +60 mV in + 10-mV increments for a 400-ms pulse duration.
Reduced Myelination of Sciatic Nerve Axons in AKO Mice
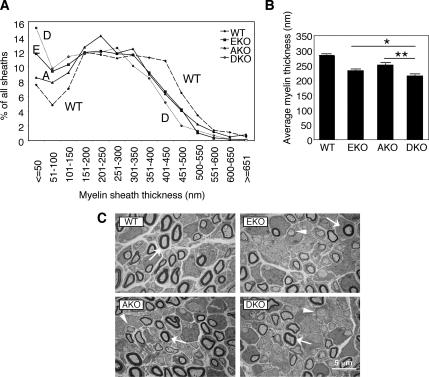
Mice lacking PTPε exhibit transient but severe hypomyelination of sciatic nerve axons at an early postnatal age (Peretz et al., 2000); the role of PTPα, the most closely related paralogue gene to PTPε, in this system is unknown. To examine sciatic nerve myelination in the absence of RPTPα and/or PTPε, we used transmission electron microscopy to quantify myelin sheath thickness in transverse cross-sections of sciatic nerves obtained from WT, AKO, and EKO, and DKO mice. Analysis of sciatic nerve axons of 5-d-old mice revealed significant reductions in the average myelin sheath thickness in AKO (down to 89% of WT control), EKO (to 82%), and DKO (to 76%) mice compared with WT mice (Figure 2). The effect was most evident in thinly myelinated axons (<150 nm), where increases of up to twofold in the number of axons in this category were seen in AKO, EKO, and DKO mice. Major changes were also observed in the most heavily myelinated sheaths (>350 nm), where a marked decrease in the number of axons in this category was observed in the various KO mice. The findings in EKO mice agree with previous studies (Peretz et al., 2000). Importantly, lack of PTPα and PTPε seem to have additive effects, because changes in myelin sheath thickness were more pronounced in the DKO mice than in AKO and EKO mice (Figure 2). The average diameter of sciatic nerve axons was increased from 1099 ± 10 nm in WT mice to 1172 ± 11 and 1164 ± 12 nm in EKO and AKO mice, respectively, and it was decreased to 1046 ± 11 nm in DKO mice (mean ± SE, n = 700–849, p ≤ 0.00056 for all KO genotypes versus WT). This result suggests that sciatic nerve abnormalities are not limited to the myelin sheath alone. However, because thicker axons are usually associated with increased myelination, increased axon diameter in EKO and AKO mice most likely does not account for the reduced myelination observed in these mice. These results indicate that both PTPα and PTPε support sciatic nerve myelination in vivo but that their roles in this process are at least in part nonredundant.
Figure 2.
Reduced myelination of axons in sciatic nerves of 5-d-old AKO, EKO, and DKO mice. (A) Distribution of thicknesses of myelin sheaths in wild-type, AKO, EKO, and DKO sciatic nerves (WT, A, E, and D, respectively). n = 1370–2320 individual axons per genotype, as indicated below. (B) Bar diagram comparing the average sheath thickness (in nanometers, ± SEM). WT, 284.2 ± 3.0, n = 2320 sheaths; EKO, 232.6 ± 3.3, n = 1652; AKO, 251.7 ± 3.8, n = 1370; and DKO, 214.9 ± 5.4, n = 1601. All knockout means are distinct from WT mean (p < 0.0001); EKO and AKO means are distinct from DKO mean (EKO, *p = 0.0054); AKO, **p < 0.0001). Statistical significance was determined by Welch's t test. (C) Representative electron microscope pictures of transverse cross-sections through sciatic nerves of 5 d-old WT, AKO, EKO, and DKO mice. Arrows mark several cross-sections of myelinated axons, evident as closed dark figures. Arrowheads mark several unmyelinated axons.
Adult mice did not display obvious difficulties indicative of neural dysfunction (e.g., in locomotion), indicating that myelination disruption was transient. This was confirmed by direct examination of sciatic nerves of mice 120 d old, which did not reveal myelination abnormalities (our unpublished data). Normal myelination in adult mice was also observed previously when EKO mice were examined (Peretz et al., 2000). We conclude therefore that although loss of both PTPε and RPTPα results in a more severe myelination phenotype than loss of either PTP alone, this phenotype is transient.
Increased Activation of Kv Channels in Schwann Cells of AKO Mice
Previous analyses revealed that Kv channel activity was increased in primary Schwann cells of EKO mice (Peretz et al., 2000). To examine the effect of lack of PTPα on regulation of Kv channels, we examined the activity of Kv channels in Schwann cells of AKO mice. Primary Schwann cells express both RPTPα and the nonreceptor form of PTPε, cyt-PTPε; as expected, either PTP was absent from Schwann cells of the corresponding single knockout mice (Figure 3A). Primary Schwann cell cultures from 3- to 5-d-old mice of the various genotypes were prepared and used for recording voltage-gated potassium currents by using the whole-cell patch-clamp technique. Schwann cells of EKO and of AKO mice exhibited a marked increase in K+ current densities (Figure 3, B and C), with the effect in AKO mice much stronger than in EKO mice (47% increase in EKO Schwann cells compared with WT (at +60 mV), versus an increase of 150% in AKO Schwann cells). This result indicates that RPTPα and cyt-PTPε each inhibit Kv channel activity in Schwann cells, but they do so most likely by distinct mechanisms. To determine the effects of simultaneous inactivation of both PTPs on current density we analyzed Schwann cells from DKO mice, which are devoid of both cyt-PTPε and RPTPα proteins (Figure 3A). Kv channel activity in DKO Schwann cells was lower than in AKO cells and was slightly higher than in EKO cells (62% increase compared with WT (Figure 3, B and C). Thus, absence of both PTPs did not increase Kv currents in an additive manner, possibly suggesting that cyt-PTPε may also promote Kv channel activity in the absence of RPTPα as discussed further below.
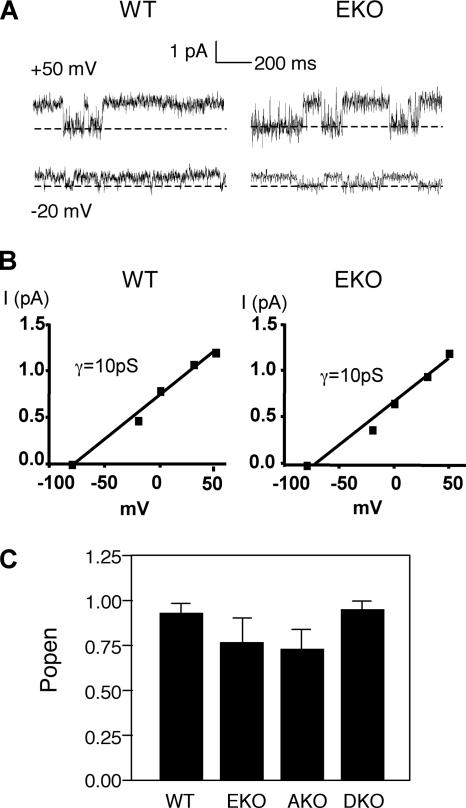
Lack of either or both PTPs affected only the amplitude of Kv currents; other biophysical parameters, such as the voltage dependence of activation and the kinetics of activation and inactivation, were not affected (Figure 3, B and C). Thus, absence of RPTPα and/or cyt-PTPε could in principle increase the number of functional channels available in the membrane; alternatively, it might increase either the channel open probability Po and/or the unitary conductance. To address this issue we performed single-channel recordings of K+ currents from Schwann cells using the cell-attached configuration of the patch-clamp technique. The resting membrane potential (Em) of Schwann cells was determined at a very short time after establishing the whole-cell configuration before dialysis of the cell. We used this value (Em = −50 ± 12 mV; n = 21) to determine the membrane potential of the intact patches. Single-channel currents could be detected easily at potentials 20 mV more positive than the resting membrane potential, i.e., above −30 mV, with current amplitudes increasing with further depolarization (Figure 4A). The unitary current–voltage relations indicated a mean single-channel conductance of 10 pS for the four mouse genotypes (Figure 4B; our unpublished data), a value very close to that obtained previously in Schwann cells from normal NMRI mice (Verkhratsky et al., 1991). Analysis of the open probability, Po, indicated that Po was very high and not significantly different among the four mouse genotypes (Figure 4C). We conclude that the significant increase in Schwann cell K+ current density observed in PTP knockout mice relative to WT mice cannot be accounted for by differences in Po or in unitary conductances; it most likely involves an increase in the number of functional channels available at the plasma membrane as discussed further below.
Figure 4.
Single-channel properties of Schwann cell K+ currents measured in cell-attached patches. (A) Single channel current traces of WT and EKO Schwann cells are displayed at two different potentials evoked from the cell resting potential (−50 mV). The closed channel state is indicated by the dashed lines. The K+ concentration in the pipette and the bath was 2.5 mM. (B) Representative unitary current–voltage relations measured from WT and EKO Schwann cells (of 21 similar cells). The straight lines correspond to the fit of data points and indicate unitary conductance of 10 pS. (C) Open probability (mean ± SEM) determined from the analysis of 17–21 cells of each the four genotypes. Differences between the four genotypes are not statistically significant.
Hyperphosphorylation of Kv2.1 and Deregulation of c-Src in the Absence of Either or Both PTPε and PTPα
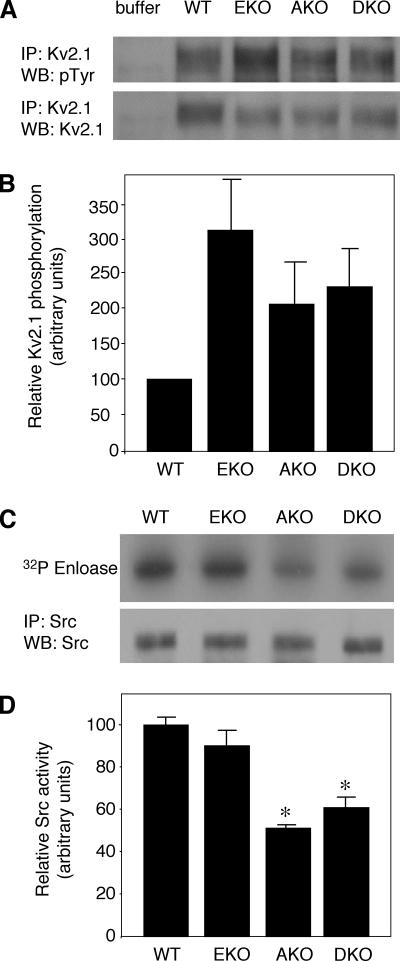
To better understand the different effects of lack of PTPε and PTPα on Kv currents, we examined the molecular mechanisms by which these PTPs may affect Kv channels. Kv2.1 is a substrate of cyt-PTPε and is hyperphosphorylated in sciatic nerve tissue and in primary Schwann cells from PTPε-deficient mice (Peretz et al., 2000). Because cyt-PTPε, RPTPε, and RPTPα can dephosphorylate Kv2.1 in cultured cells (Gil-Henn et al., 2000; Peretz et al., 2000), we examined the phosphorylation state of Kv2.1 in sciatic nerves of AKO and DKO mice. For this purpose Kv2.1 was immunoprecipitated from membranal fractions obtained from sciatic nerve tissue of 3-d-old mice of all four genotypes. Phosphorylation of Kv2.1 was assessed by blotting with anti-phosphotyrosine antibodies, followed by stripping and reprobing with anti-Kv2.1 antibodies to normalize to Kv2.1 protein levels. As seen in Figure 5, A and B, Kv2.1 phosphorylation was elevated in AKO, EKO, and DKO compared with WT, supporting a general trend for increased phosphorylation and activation occurring together.
Figure 5.
Phosphorylation of Kv2.1 and Src activity in sciatic nerves of WT, EKO, AKO, and DKO mice. (A) Crude membrane fractions of sciatic nerves from 3-d-old WT, EKO, AKO, and DKO mice were prepared and solubilized. After immunoprecipitation with anti-Kv2.1 antibodies, precipitates were blotted with anti-pTyr antibodies (top) followed by stripping and blotting with anti-Kv2.1 antibodies (bottom). Blots are from a representative experiment of four performed. (B) Bar diagram showing Kv2.1 phosphorylation intensity (± SEM) normalized to Kv2.1 protein content and presented relative to WT (= 100): EKO = 320.4 ± 105.8, AKO = 216.0 ± 58.3, and DKO = 231.8 ± 57.4; n = 4 repeats. (C) Src protein was immunoprecipitated from sciatic nerve extracts of 3- to 4-d-old mice and allowed to catalyze incorporation of 32P into enolase. Reaction mixture was subject to SDS-PAGE and blotting. Blot was exposed to document enolase-associated radioactivity (top) and then probed with anti-Src antibody to document amount of Src protein present in the precipitate (bottom). (D) Bar diagram showing relative Src activity (± SEM) in the four genotypes studied: WT = 1.00 ± 0.04; EKO = 0.89 ± 0.09; AKO = 0.50 ± 0.02; and DKO = 0.61 ± 0.05. n = 3–5 experiments per genotype. AKO and DKO are statistically different (asterisks) by Student's t test from WT (p ≤ 0.0006) and EKO (p ≤ 0.018). WT versus EKO and AKO versus DKO are not statistically distinct.
To provide further insight into the different ways by which both PTPs may regulate channel function, we examined their ability to regulate Src. It is well established that PTPε and RPTPα activate Src and related kinases in several cell types by dephosphorylating their C-terminal inhibitory tyrosine (Ponniah et al., 1999; Su et al., 1999; Gil-Henn and Elson, 2003; Pallen, 2003; Granot-Attas and Elson, 2004). In turn, Src and Fyn can phosphorylate and up-regulate Kv channels in Schwann cells (Fadool et al., 1997; Sobko et al., 1998a; Levitan, 1999; Peretz et al., 1999; MacFarlane and Sontheimer, 2000), raising the possibility that that these PTPs might affect Kv channel activation in Schwann cells also indirectly by regulating kinase activity. To examine this possibility, we immunoprecipitated Src from cells of mice of the four genotypes and measured its kinase activity in vitro by using enolase as a substrate (Figure 5, C and D). Loss of cyt-PTPε alone resulted in a small and statistically insignificant reduction in c-Src activity, whereas loss of RPTPα reduced c-Src activity by ∼50%. Interestingly, absence of both PTPs did not reduce c-Src kinase activity beyond the effect of ablation of RPTPα alone (Figure 5D), a finding compatible with a simple additive mode of regulation of Src by either PTP. These results establish RPTPα as a significant regulator of Src in this system and agree with previous results that indicate a much smaller role, if any, for cyt-PTPε in this respect (Peretz et al., 2000).
Kv2.1 Interacts Constitutively with RPTPα, but Not with cyt-PTPε
The above-mentioned results suggest that RPTPα, but not cyt-PTPε, may regulate Kv channels in Schwann cells also indirectly by affecting Src activity. However, this parameter is not sufficient to explain in full molecular detail the stronger inhibition of Kv channel activity by RPTPα. To further address this issue, we examined the abilities of either PTP to interact with and to dephosphorylate Kv channels.
Experiments using isolated cyt-PTPε, RPTPα, and Src-phosphorylated Kv2.1 indicated that either PTP can dephosphorylate Kv2.1 in vitro (Figure 6A). However, their respective activities toward Kv2.1 may differ in a cellular context. RPTPα may have better access to Kv channel proteins because it is an integral membrane protein; this is in contrast to cyt-PTPε, which is predominantly cytosolic (Elson and Leder, 1995a). Alternatively, the membrane-spanning and extracellular domains of RPTPα, which have no counterparts in cyt-PTPε, might interact with Kv2.1 and contribute to its regulation. Last, slight differences between the sequences of the active sites and regulatory regions of these highly related PTPs may make RPTPα a more effective down-regulator of Kv2.1.
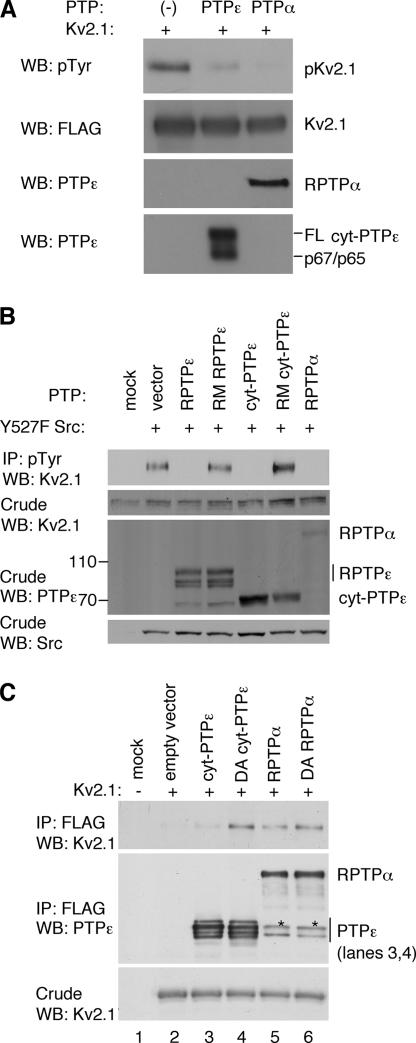
Figure 6.
PTPε and PTPα regulate tyrosine phosphorylation of Kv2.1. (A) RPTPα and cyt-PTPε can dephosphorylate tyrosine-phosphorylated Kv2.1 in vitro. Top, phosphorylated Kv2.1 remaining after incubation without added PTP or after addition of purified cyt-PTPε or RPTPα as indicated. Remaining panels document addition of purified pKv2.1, cyt-PTPε, and RPTPα to the various reactions. FL, full-length cyt-PTPε. Anti-PTPε antibodies cross-react with RPTPα. (B) RPTPα and cyt-PTPε reduce Src-mediated phosphorylation of Kv2.1 in cells. HEK293 cells stably expressing Kv2.1 were transiently transfected with activated (Y527F) Src, together with RPTPε, cyt-PTPε, RPTPα, or the inactive mutants R340M RPTPε and R283M cyt-PTPε. Cells were lysed and immunoprecipitated with anti-phosphotyrosine antibodies followed by blotting with anti-Kv2.1 antibodies. Blots containing crude extracts were reacted with anti-Kv2.1, anti-PTPε, and anti-Src antibodies to assess expression of these proteins. Blots are from a representative experiment of three performed. (C) Constitutive association of Kv2.1 with RPTPα. HEK293 cells were transiently transfected with Kv2.1 together with FLAG-tagged cyt-PTPε, RPTPα, or their substrate-trapping mutants (D245A cyt-PTPε and D437A RPTPα), as indicated. Following cell lysis and anti-FLAG immunoprecipitation, coprecipitating Kv2.1 was detected by blotting with anti-Kv2.1 antibodies (top). Second and third panels depict amounts of precipitating PTPs and expression of Kv2.1 in the crude lysates, respectively. Asterisks denote p66 PTPα, a cytosolic cleaved form of RPTPα (Gil-Henn et al., 2001).
To tell apart these possibilities, we examined PTP-Kv2.1 interactions in HEK293 cells. Preliminary experiments indicated that RPTPα, RPTPε, and cyt-PTPε can dephosphorylate Kv2.1 after its phosphorylation by activated (Y527F) Src; as expected, the catalytically inactive mutants R283M cyt-PTPε and R340M RPTPε did not affect Kv2.1 phosphorylation (Figure 6B). Src phosphorylates Kv2.1 mainly at Y124 (Tiran et al., 2003), indicating that all three PTP molecules examined efficiently dephosphorylate this site in Kv2.1. Activated Src was used here in order to prevent the PTPs from dephosphorylating and activating the kinase, thereby complicating interpretation of the results.
We next compared the ability of substrate-trapping mutants of cyt-PTPε and RPTPα to bind WT Kv2.1. Mutants of this type (D245A cyt-PTPε and D437A RPTPα) are almost entirely catalytically inactive, but their catalytic sites can still bind phosphorylated substrates (Flint et al., 1997; Peretz et al., 2000; Tiran et al., 2003; Blanchetot et al., 2005). Severalfold more Kv2.1 coprecipitated with the substrate-trapping D245A mutant of cyt-PTPε than with WT cyt-PTPε (Figure 6C), indicating that a significant part of the interactions between Kv2.1 and cyt-PTPε is mediated by the active site of PTPε. In contrast, similar amounts of Kv2.1 associated with the WT RPTPα and with its substrate-trapping mutant D437A RPTPα (Figure 6C). The interactions between Kv2.1 and RPTPα are therefore more stable and are likely to involve other regions in addition to the active site, in agreement with the stronger effect of RPTPα on Kv channel activity documented above.
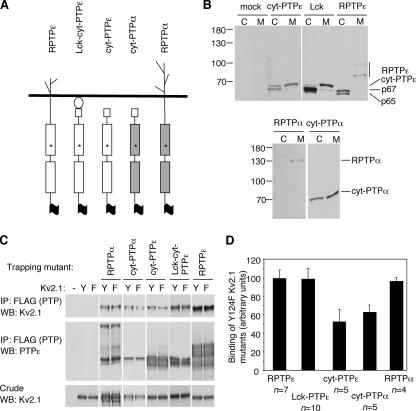
RPTPα is an integral membrane protein with membrane-spanning and extracellular domains. cyt-PTPε lacks such domains; although a fraction of cyt-PTPε molecules are membrane associated, most are cytosolic. To examine the effects of membrane localization on the ability to interact with Kv2.1, we turned to a series of D-to-A substrate-trapping mutants shown in Figure 7A. This series included cyt-PTPα, an artificial cytoplasmic derivative of RPTPα in which the transmembranal and extracellular regions of RPTPα were replaced with the first 12 amino acids of cyt-PTPε. These 12 residues are unique to cyt-PTPε and regulate membranal localization of this isoform (Gil-Henn et al., 2000); access of cyt-PTPα to the cell membrane is similar to that of cyt-PTPε (Figure 7B). In a second construct (Lck-cyt-PTPε), cyt-PTPε was targeted to the plasma membrane by an N-terminal Lck dual myristoylation motif (Zlatkine et al., 1997). This construct is predominantly membrane-associated, but it lacks membrane-spanning and extracellular domains. The series also included RPTPε, the receptor-type isoform of PTPε, as well as cyt-PTPε and RPTPα. All molecules were expressed in HEK293 cells, and their expected subcellular localization was verified by biochemical fractionation (Figure 7B). Full-length cyt-PTPε was mostly localized in the cytoplasmic fraction with a portion present in the membranal fraction, as shown previously (Elson and Leder, 1995a). Adding the Lck motif in Lck-cyt-PTPε resulted in a significant shift of full-length cyt-PTPε to the membrane fraction; the receptor-type RPTPε was found exclusively in the membrane fraction. In all three cases, p67 and p65, which are coexpressed with the full-length PTPε molecules but which lack membrane-targeting sequences, were found in the cytosol. RPTPα was found exclusively at the cell membrane, and cyt-PTPα was expressed at similar levels in the cytoplasmic and membranal fractions. The cellular expression patterns of these two PTPα proteins are in good agreement with their PTPε counterparts—RPTPε and cyt-PTPε, respectively.
Figure 7.
Membranal localization of PTPs α and ε drives their constitutive interaction with Kv2.1. (A) Schematic representation of the various FLAG-tagged substrate-trapping D-to-A mutants of PTPε and PTPα used in this study: D437A RPTPα, D437A cyt-PTPα, D245A cyt-PTPε, D245A Lck-cyt-PTPε, and D302A RPTPε. The mutated aspartic residue (asterisk) is the same in all cases, although its exact numbering differs among the various molecules. Residue numbering in cyt-PTPα and Lck-cyt-PTPε are as in RPTPα and cyt-PTPε, respectively, for clarity. (B) The subcellular localization of molecules shown in A. After transfection into HEK293 cells, cells were fractionated and the cytoplasmic (C) and membranal (M) fractions were analyzed on 7% SDS-PAGE gels and blotted with anti-PTPε/α antibodies. (C) Constitutive association with Kv2.1 is dependent on membrane localization. HEK293 cells were transiently transfected with WT Kv2.1 (Y) or Y124F Kv2.1 (F) together with the indicated FLAG-tagged substrate-trapping D-to-A mutants. After cell lysis and anti-FLAG immunoprecipitation, amount of coprecipitating Kv2.1 was assessed by blotting. Shown is a representative set of blots documenting coimmunoprecipitation of WT versus Y124F Kv2.1 with the various trapping mutants used. Also shown are amounts of precipitating PTP and Kv2.1 expression in the crude lysates (second and third panels, respectively). (D) Bar diagram showing Y124F Kv2.1 coimmunoprecipitated with substrate-trapping D-to-A mutants normalized to protein content. Values (mean ± SEM) are relative to association of WT Kv2.1 with each phosphatase. D437A cyt-PTPα and D245A cyt-PTPε exhibit reduced binding to Y124F Kv2.1 compared with WT Kv2.1 (63.3 ± 10.4 and 55.5 ± 13.8%, respectively; p = 0.03–0.05; Welch's t test). In contrast, the membrane-bound mutants D437A RPTPα, D245A Lck-cyt-PTPε, and D302A RPTPε bind WT and Y124F Kv2.1 similarly.
We next examined the interactions between the molecules shown in Figure 7A and Kv2.1. We had shown previously that Y124 of Kv2.1 is a major site targeted by cyt-PTPε and RPTPα; accordingly, tyrosine phosphorylation of Y124F Kv2.1 is decreased by 70%, and binding of D245A cyt-PTPε to this mutant is decreased by ∼50% compared with WT Kv2.1 (Tiran et al., 2003) (Tiran and Elson, unpublished data). We reasoned therefore that if binding of a particular D-to-A PTP mutant to Kv2.1 relies mainly on interactions mediated by the PTP catalytic domain, significantly less PTP would bind Y124F Kv2.1. In contrast, PTP-Kv2.1 binding in which interactions outside of the PTP catalytic domain are prominent would not be affected as strongly by the Y124F mutation in Kv2.1. The set of molecules of Figure 7A was therefore constructed as D-to-A substrate traps with a FLAG tag at their C termini. Each PTP was coexpressed with WT or Y124F Kv2.1 in HEK 293 cells. After PTP immunoprecipitation, levels of coprecipitated Kv2.1 were assessed by blotting the precipitated material with anti-Kv2.1 antibodies (Figure 7, C and D); the amount of precipitated Kv2.1 was normalized for expression levels of Kv2.1 protein. Care was taken to use data only from experiments in which for a given PTP expression levels of the PTP trapping mutant and of WT and Y124F Kv2.1 in the cells compared were similar.
Examination of binding of the various PTP substrate-trapping mutants to WT versus Y124F Kv2.1 revealed a clear distinction between receptor-type and nonreceptor-type forms of the PTPs (Figure 7D). Approximately 45% less Y124F Kv2.1 coprecipitated with D245A cyt-PTPε compared with WT Kv2.1, despite similar expression of both forms of Kv2.1 and similar precipitation of D245A cyt-PTPε in these cells (Figure 7C). Less Y124F Kv2.1 also coprecipitated with D437A cyt-PTPα compared with WT Kv2.1; in both cases the reductions in association were statistically significant. In contrast, associations of D302A RPTPε with WT and Y124F Kv2.1 were comparable; similar results were obtained using D437A RPTPα (Figure 7D). Together, these results indicate strongly that the receptor-type forms of both PTPs can bind Kv2.1 constitutively, whereas their cytosolic forms do so much less efficiently; strong binding segregates with cellular localization and not with PTP identity. The differences observed between RPTPα and cyt-PTPε in binding Kv2.1 are therefore not the result of sequence differences between these two distinct PTPs or in the phosphotyrosine residues they target in Kv2.1, but rather they are the result of one of them being a receptor-type molecule, whereas the other is not.
A question left unresolved was whether RPTPα and RPTPε bind Kv2.1 constitutively because their membranal localization makes Kv2.1 more readily accessible or because their membrane-spanning and extracellular domains mediate additional interactions with Kv2.1. To resolve this issue, binding of D245A Lck-cyt-PTPε to WT and to Y124F Kv2.1 was compared. This membrane-associated protein behaved similarly to the two receptor-type forms and bound similar amounts of WT and of Y124F Kv2.1, despite lacking membrane spanning and extracellular domains (Figure 7, C and D). We therefore conclude that the membrane-spanning and extracellular domains of RPTPε and RPTPα do not play a significant role in binding Kv2.1. Localization at the cell membrane is sufficient to drive constitutive association between PTPs ε and α and Kv2.1. The inherent differences between the effects of either PTP on this channel protein are most likely the result of their differing patterns of subcellular localization in Schwann cells.
DISCUSSION
Comparison of the phenotypes of EKO and AKO mice indicates that the functional overlaps and interactions between PTPs ε and α in Schwann cells are complex and context dependent. Analyses of sciatic nerve axons of 5-d-old mice revealed significant reductions in myelin sheath thickness in AKO, EKO, and DKO mice compared with WT mice. The effects of lack of PTPα and PTPε were additive, with DKO mice most severely affected. These results indicate that both PTPα and PTPε support sciatic nerve myelination in a manner that is at least partially nonredundant and that the time period when this role is most significant is in early postnatal life. The transient nature of the myelination phenotype implies that as mice age, the need for PTPε and PTPα in regulating myelination decreases or that other PTPs compensate for lack of these two PTPs. Possible candidates for such compensation include CD45 (Nakahara et al., 2005), SHP-1 (Massa et al., 2004), PTPζ (Harroch et al., 2002), and PTPς (Wallace et al., 1999), because mice separately lacking these PTPs exhibit defects in myelination and/or in recovery of myelination after damage to neurons. Nonetheless, existence of myelination defects in postnatal EKO, AKO, and DKO mice indicates that PTPε and PTPα perform a unique function in Schwann cells and that other gene products cannot replace these enzymes at this developmental stage.
Previous reports have established a correlation between decreased Kv channel activation on the one hand, and exit of Schwann cells from the cell cycle and onset of myelination on the other hand (Sobko et al., 1998a; MacFarlane and Sontheimer, 2000). Increased Kv channel activity observed in Schwann cells of AKO and EKO mice can suggest that loss of cyt-PTPε or RPTPα reduces myelination by preventing the normal decrease in Kv channel activation that occurs as Schwann cells mature in early postnatal mice. However, this possibility does not hold up well in the more complex setting of DKO mice, where the largest decreases in myelination occur together with less-than-maximal increases in Kv channel activation. Together, our observations suggest that RPTPα and cyt-PTPε can affect sciatic nerve myelination by additional mechanisms beyond regulation of Kv channels. For example, a possible additional mechanism for linking PTP activation and Schwann cell-mediated myelination is cell adhesion, which is necessary for proper axonal myelination. The involvement of RPTPα in cell adhesion is well established, evident in its ability to control integrin-mediated responses through activation of Src (Su et al., 1999), to form a complex with contactin (Zeng et al., 1999), to regulate integrin-stimulated focal adhesion kinase autophosphorylation and cytoskeletal rearrangement in cell spreading and migration (Zeng et al., 2003), and to participate in neural cell adhesion molecule-mediated signaling (Bodrikov et al., 2005). cyt-PTPε is also involved in cell adhesion, because osteoclasts from EKO mice do not adhere well to matrix and display significantly disorganized adhesion structures (Chiusaroli et al., 2004). Further studies will indicate whether these effects participate in regulating myelination in this system. Finally, we note that axons from AKO and EKO mice are slightly thicker than their WT counterparts. Thicker axons are often more heavily myelinated; hence, reduced myelination in AKO and EKO axons is probably not caused by this change in axon diameter. Conversely, absence of both PTPs results in a slight reduction in axon diameter in DKO mice, which may possibly contribute to the reduced myelination of these axons. In all, these results indicate that absence of either or both PTPs can affect the axons themselves as well. Further studies are required to better understand this issue.
The experimental system studied here allows better understanding of the molecular mechanisms by which PTPs ε and α regulate Kv channels. Loss of either cyt-PTPε or RPTPα in primary Schwann cells resulted in marked increases in maximal K+ current density and in increased tyrosine phosphorylation of Kv2.1. Importantly, these results indicate that the ultimate physiological effect of either PTP in Schwann cells in vivo is to inhibit phosphorylation and activation of Kv channel proteins. In principle, increased K+ current density may be caused by an increase in the number of functional channels available at the plasma membrane, a rise in Po, and/or elevated channel unitary conductance. Our single-channel analyses indicate that the increase in Schwann cell K+ current density observed in the PTP knockout mice cannot be accounted for by differences in Po or in unitary conductances and most likely results from increased numbers of functional channels available at the plasma membrane. The gross expression levels of Kv2.1 and Kv1.5 in primary Schwann cells from all four genotypes are similar (Figure 5A; our unpublished data). However, channel availability may be regulated by more subtle means such as interactions with other proteins or subcellular localization of channel molecules. The latter phenomenon has been described recently in studies of hippocampal neurons, which showed that neuronal activity and ischemia cause rapid dephosphorylation of Kv2.1 channels and translocation from clusters to a more uniform localization (Misonou et al., 2004, 2005). In this regard, we previously showed that Kv2.1 channels are among other delayed rectifier channel subunits, which prominently encode IK currents in Schwann cells (Sobko et al., 1998a, b). Hence, our findings prompt the need to consider a possible role for phosphorylation and dephosphorylation (by PTPs α and ε) in dynamic modulation of IK/Kv2.1 localization.
By which molecular mechanisms do RPTPα and cyt-PTPε regulate Kv channels? We have shown that both PTPs can dephosphorylate Kv2.1 in vitro and in vivo. However, the observation that absence of RPTPα increased Kv channel activity in vivo significantly more than absence of cyt-PTPε indicates that there are additional molecular parameters that differ between both PTPs. One such likely parameter that was examined is Src activation. The data indicate that in the system examined here, there is clear distinction between both PTPs in this respect. The role of cyt-PTPε seems to be direct: cyt-PTPε dephosphorylates and down-regulates Kv channels without affecting Src. Loss of this PTP increases channel protein phosphorylation and current amplitude but does not change Src activity. In contrast, the role of RPTPα seems more complex and contains both direct and indirect components. Absence of RPTPα inhibits Src activity strongly but also activates Kv channels strongly. Src activates Kv channels in Schwann cells; hence, reduced Src activity in the absence of RPTPα would be expected to reduce Kv channel activity. Activation of Src by RPTPα is therefore not the only mechanism by which this PTP affects Kv channels in Schwann cells. We conclude that although cyt-PTPε and RPTPα differ significantly in their abilities to regulate Src in this system, this factor is not sufficient to explain the full extent of their different abilities to regulate Kv channel activity.
The strong constitutive association that exists between RPTPα and Kv2.1 may provide an additional mechanism to account for the stronger effect of RPTPα on Kv channel activity. Interestingly, strong constitutive interactions do not exist between cyt-PTPε and Kv2.1, with the main cause of this difference being the distinct cellular localizations of RPTPα and cyt-PTPε. Constitutive binding between RPTPα and Kv2.1 may affect Kv2.1 in several ways. Although Y124 of Kv2.1 is a major target of both RPTPα and cyt-PTPε (Figure 6) (Tiran et al., 2003), it is formally possible that its greater access allows RPTPα to target additional tyrosines besides Y124 in Kv2.1. Alternatively, constitutive interactions may influence channel activity by regulating access of regulatory molecules to channel proteins or by affecting channel protein conformation. Support for these latter possibilities comes from our demonstration in this study that WT RPTPα binds Kv2.1 constitutively at locations in addition to Y124. This notion is also consistent with interactions documented to exist between RPTPα and the N and C termini of the related channel protein Kv1.2, regions known to be important in regulating the activities of Shaker-type potassium channels (Tsai et al., 1999).
Of particular interest is the observation that Kv channel activity in DKO Schwann cells is reduced relative to that measured in AKO cells (Figure 3B). Because results from the single-knockout cells indicate that removal of either PTP on its own activates the channels, this result is counterintuitive and suggests that cyt-PTPε may also play a role in promoting Kv channel activity specifically in the absence of RPTPα. This role is most likely not mediated by c-Src, because cyt-PTPε does not affect c-Src activity in these cells (Figure 5, C and D). One of several alternative possibilities is that cyt-PTPε interacts with a negative regulator of Kv channels and prevents this regulator from affecting the channels. According to this model, removal of cyt-PTPε in the absence of RPTPα prevents cyt-PTPε-mediated dephosphorylation but also releases the negative regulator, which inhibits Kv channel molecules and decreases overall Kv channel activity in DKO mice relative to AKO mice. Presumably, removal of cyt-PTPε in the presence of RPTPα does not cause this effect due to the already existing significant inhibition of Kv channels by RPTPα. This model may be challenged by identification of molecules that interact with cyt-PTPε in Schwann cells.
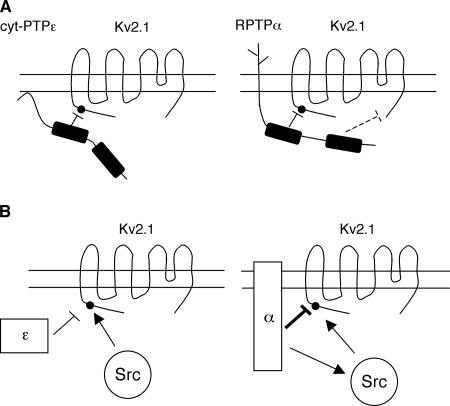
A molecular scheme that summarizes the known differences between the functions of cyt-PTPε and RPTPα versus Kv2.1 in this system is presented in Figure 8. Both PTPs down-regulate Kv2.1 activity by dephosphorylating the channel molecule (Figure 8A). This interaction between the active site of the PTP and Y124 of Kv2.1 is the major interaction between Kv2.1 and cyt-PTPε, in agreement with its nonconstitutive nature and strong dependence on Y124 phosphorylation. RPTPα performs the same function, but in addition it interacts with Kv2.1 also at other sites. These latter interactions are constitutive and are not dependent upon phosphorylation of Kv2.1 at Y124. Further studies are required to identify specific domains of RPTPα that are involved, but the C-terminal PTP domain, which has been suggested to fulfill regulatory roles in several PTPs, is a possibility. Several such interactions can be hypothesized, and one is illustrated in Figure 8A. Introduction of Src into this model reveals that both PTPs can antagonize Src-mediated activation of Kv2.1 (Figure 8B). In agreement with our previous studies of EKO mice (Peretz et al., 2000), a PTP–PTK–common substrate triangle exists therefore in Schwann cells, in which the PTP and PTK antagonize each other's function toward their common substrate, the Kv channel protein. The data suggest that this is a complete account of the events that occur between cyt-PTPε, Src, and Kv2.1 in Schwann cells (Figure 8B, left). RPTPα, in contrast, can also strongly activate Src in this system (Figure 8B, right). RPTPα therefore fulfills two roles that can antagonize each other: direct inhibition of Kv2.1 by its dephosphorylation and indirect activation of Kv2.1 by dephosphorylation and activation of Src. Data presented in this study show that the overall effect of RPTPα is to inhibit Kv channel activity strongly under the conditions in which the Schwann cell system was studied here. These results raise the need to postulate a regulatory mechanism that can coordinate between these opposing effects of RPTPα. Recent studies have suggested that phosphorylation of RPTPα at its C-terminal Y789 may preferentially direct RPTPα to activate Src by exposing Y527 of the kinase to dephosphorylation by RPTPα (Zheng et al., 2000). It is therefore tempting to speculate that the balance between the two opposing roles of RPTPα toward Kv2.1 may be regulated by phosphorylation of RPTPα by upstream kinases in response to physiological signals.
Figure 8.
Schematic model illustrating the distinct mechanisms by which cyt-PTPε and RPTPα regulate Src and Kv2.1 in Schwann cells. (A) Distinct modes of interaction with Kv2.1. Left, cyt-PTPε down-regulates Kv2.1 by dephosphorylating the channel protein at Y124 (dark dot). Right, in addition to dephosphorylating Kv2.1 at Y124, RPTPα inhibits Kv2.1 activity by interacting with the channel protein at other locations. One such hypothetical interaction is marked by the dashed line. (B) Differences in the PTP-Kv-Src triangle. Left, cyt-PTPε does not interact with Src in Schwann cells and acts only to counteractivation of Kv2.1 by Src-mediated phosphorylation at Y124. Right, RPTPα regulates Kv2.1 also, but not exclusively, by antagonizing Src activity at Y124 of Kv2.1. The overall inhibitory effect of RPTPα is stronger than that of cyt-PTPε. RPTPα can also dephosphorylate and activate Src, thereby contributing indirectly to activation of Kv2.1. Y124 is noted by the dark dot near the N terminus of Kv2.1. Dark rectangles represent the PTP domains of cyt-PTPε and RPTPα; in both cases, activity is associated mainly with the N terminal PTP domain, whereas the C terminal PTP domain performs mainly regulatory roles.
An additional conclusion from our studies is that the ability of PTPε to activate c-Src is context dependent. Although cyt-PTPε can activate c-Src in other systems (Gil-Henn and Elson, 2003), it does not do so in the system examined here, indicating somewhat unexpectedly that PTPε and PTPα do not always regulate Src redundantly in vivo. Furthermore, the ability to bind Kv2.1 stably is linked with membranal localization, and the receptor form of PTPε, RPTPε, binds Kv2.1 stably as RPTPα. Because expression of cyt-PTPε and RPTPε differs among cell and tissue types, the manner in which PTPε proteins regulate Kv channels and their redundancy with RPTPα may be tissue and isoform specific. We conclude that similarities between PTP activities at the molecular level may not always lead to full functional redundancy between them in vivo. Finally, we note that DKO mice are viable and seem to tolerate well loss of both PTPs α and ε. It is therefore possible that some of the physiological functions of these PTPs are redundant with those of other PTPs, at least within a laboratory environment. Because genomic analyses have made it highly unlikely that additional members of the type IV subfamily of receptor-type PTPs exist (Alonso et al., 2004; Andersen et al., 2004), these functions may be shared with other, more distantly related PTPs.
ACKNOWLEDGMENTS
We thank colleagues mentioned in the text for generously providing valuable reagents. We also thank Ms. Dalia Berman-Golan for help in figure preparation, Dr. Ori Brenner for performing histological analyses of mouse tissues, and Ms. Judith Hermesh and colleagues at the Weizmann Institute Genetically Manipulated Animals Facility for expert animal care. This study was supported by Grant 2000045 from the United States-Israel Binational Science Foundation (to A.E. and J.S.) and by Israel Science Foundation (672/05), Wolfson Family, and Recanati grants (to B.A.).
Abbreviations used:
- Kv channel, delayed rectifier, voltage-gated K+ channel
- PTP
protein tyrosine phosphatase.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-02-0151) on July 26, 2006.
REFERENCES
- Alonso A., Sasin J., Bottini N., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Andersen J. N., Elson A., Lammers R., Romer J., Clausen J. T., Moller K. B., Moller N. P. Comparative study of protein tyrosine phosphatase-epsilon isoforms: membrane localization confers specificity in cellular signalling. Biochem. J. 2001;354:581–590. doi: 10.1042/0264-6021:3540581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. N., Jansen P. G., Echwald S. M., Mortensen O. H., Fukada T., Del Vecchio R., Tonks N. K., Moller N. P. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 2004;18:8–30. doi: 10.1096/fj.02-1212rev. [DOI] [PubMed] [Google Scholar]
- Bjorge J. D., Pang A., Fujita D. J. Identification of protein-tyrosine phosphatase 1B as the major tyrosine phosphatase activity capable of dephosphorylating and activating c-Src in several human breast cancer cell lines. J. Biol. Chem. 2000;275:41439–41446. doi: 10.1074/jbc.M004852200. [DOI] [PubMed] [Google Scholar]
- Blanchetot C., Chagnon M., Dube N., Halle M., Tremblay M. L. Substrate-trapping techniques in the identification of cellular PTP targets. Methods. 2005;35:44–53. doi: 10.1016/j.ymeth.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bodrikov V., Leshchyns'ka I., Sytnyk V., Overvoorde J., den Hertog J., Schachner M. RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation. J. Cell Biol. 2005;168:127–139. doi: 10.1083/jcb.200405073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Uetani N., Simoncic P. D., Chaubey V. P., Lee-Loy A., McGlade C. J., Kennedy B. P., Tremblay M. L. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev. Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Chiusaroli R., Knobler H., Luxenburg C., Sanjay A., Granot-Attas S., Tiran Z., Miyazaki T., Harmelin A., Baron R., Elson A. Tyrosine phosphatase epsilon is a positive regulator of osteoclast function in vitro and in vivo. Mol. Biol. Cell. 2004;15:234–244. doi: 10.1091/mbc.E03-04-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C. J., Krueger N. X., Saito H., Zinn K. Competition and cooperation among receptor tyrosine phosphatases control motoneuron growth cone guidance in Drosophila. Development. 1997;124:1941–1952. doi: 10.1242/dev.124.10.1941. [DOI] [PubMed] [Google Scholar]
- Elchebly M., et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Elson A., Leder P. Identification of a cytoplasmic, phorbol ester-inducible isoform of protein tyrosine phosphatase epsilon. Proc. Natl. Acad. Sci. USA. 1995a;92:12235–12239. doi: 10.1073/pnas.92.26.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson A., Leder P. Protein-tyrosine phosphatase epsilon. An isoform specifically expressed in mouse mammary tumors initiated by v-Ha-ras OR neu. J. Biol. Chem. 1995b;270:26116–26122. doi: 10.1074/jbc.270.44.26116. [DOI] [PubMed] [Google Scholar]
- Fadool D. A., Holmes T. C., Berman K., Dagan D., Levitan I. B. Tyrosine phosphorylation modulates current amplitude and kinetics of a neuronal voltage-gated potassium channel. J. Neurophysiol. 1997;78:1563–1573. doi: 10.1152/jn.1997.78.3.1563. [DOI] [PubMed] [Google Scholar]
- Flint A. J., Tiganis T., Barford D., Tonks N. K. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA. 1997;94:1680–1685. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Henn H., Elson A. Tyrosine phosphatase-epsilon activates Src and supports the transformed phenotype of Neu-induced mammary tumor cells. J. Biol. Chem. 2003;278:15579–15586. doi: 10.1074/jbc.M210273200. [DOI] [PubMed] [Google Scholar]
- Gil-Henn H., Volohonsky G., Elson A. Regulation of protein-tyrosine phosphatases alpha and epsilon by calpain-mediated proteolytic cleavage. J. Biol. Chem. 2001;276:31772–31779. doi: 10.1074/jbc.M103395200. [DOI] [PubMed] [Google Scholar]
- Gil-Henn H., Volohonsky G., Toledano-Katchalski H., Gandre S., Elson A. Generation of novel cytoplasmic forms of protein tyrosine phosphatase epsilon by proteolytic processing and translational control. Oncogene. 2000;19:4375–4384. doi: 10.1038/sj.onc.1203790. [DOI] [PubMed] [Google Scholar]
- Granot-Attas S., Elson A. Protein tyrosine phosphatase epsilon activates Yes and Fyn in Neu-induced mammary tumor cells. Exp. Cell Res. 2004;294:236–243. doi: 10.1016/j.yexcr.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Harroch S., Furtado G. C., Brueck W., Rosenbluth J., Lafaille J., Chao M., Buxbaum J. D., Schlessinger J. A critical role for the protein tyrosine phosphatase receptor type Z in functional recovery from demyelinating lesions. Nat. Genet. 2002;32:411–414. doi: 10.1038/ng1004. [DOI] [PubMed] [Google Scholar]
- Imbrici P., Tucker S. J., D'Adamo M. C., Pessia M. Role of receptor protein tyrosine phosphatase alpha (RPTPalpha) and tyrosine phosphorylation in the serotonergic inhibition of voltage-dependent potassium channels. Pflueg. Arch. Eur. J. Physiol. 2000;441:257–262. doi: 10.1007/s004240000406. [DOI] [PubMed] [Google Scholar]
- Klaman L. D., et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990;9:3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacasa D., Boute N., Issad T. Interaction of the insulin receptor with the receptor-like protein tyrosine phosphatases PTPalpha and PTPepsilon in living cells. Mol. Pharmacol. 2005;67:1206–1213. doi: 10.1124/mol.104.009514. [DOI] [PubMed] [Google Scholar]
- Lammers R., Moller N. P., Ullrich A. The transmembrane protein tyrosine phosphatase alpha dephosphorylates the insulin receptor in intact cells. FEBS Lett. 1997;404:37–40. doi: 10.1016/s0014-5793(97)00080-x. [DOI] [PubMed] [Google Scholar]
- Le H. T., Ponniah S., Pallen C. J. Insulin signaling and glucose homeostasis in mice lacking protein tyrosine phosphatase alpha. Biochem. Biophys. Res. Commun. 2004;314:321–329. doi: 10.1016/j.bbrc.2003.12.087. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Modulation of ion channels by protein phosphorylation. How the brain works. Adv. Second Messenger Phosphoprotein Res. 1999;33:3–22. doi: 10.1016/s1040-7952(99)80003-2. [DOI] [PubMed] [Google Scholar]
- MacFarlane S. N., Sontheimer H. Modulation of Kv1.5 currents by Src tyrosine phosphorylation: potential role in the differentiation of astrocytes. J. Neurosci. 2000;20:5245–5253. doi: 10.1523/JNEUROSCI.20-14-05245.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., Whyte D. B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Martens J. R., Kwak Y. G., Tamkun M. M. Modulation of Kv channel alpha/beta subunit interactions. Trends Cardiovasc. Med. 1999;9:253–258. doi: 10.1016/s1050-1738(00)00037-2. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Wu C., Fecenko-Tacka K. Dysmyelination and reduced myelin basic protein gene expression by oligodendrocytes of SHP-1-deficient mice. J. Neurosci. Res. 2004;77:15–25. doi: 10.1002/jnr.20155. [DOI] [PubMed] [Google Scholar]
- Misonou H., Mohapatra D. P., Menegola M., Trimmer J. S. Calcium- and metabolic state-dependent modulation of the voltage-dependent Kv2.1 channel regulates neuronal excitability in response to ischemia. J. Neurosci. 2005;25:11184–11193. doi: 10.1523/JNEUROSCI.3370-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H., Mohapatra D. P., Park E. W., Leung V., Zhen D., Misonou K., Anderson A. E., Trimmer J. S. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat. Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Moller N. P., Moller K. B., Lammers R., Kharitonenkov A., Hoppe E., Wiberg F. C., Sures I., Ullrich A. Selective down-regulation of the insulin receptor signal by protein-tyrosine phosphatases alpha and epsilon. J. Biol. Chem. 1995;270:23126–23131. doi: 10.1074/jbc.270.39.23126. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Aoki N., Aoyama K., Shimizu H., Shimano H., Yamada N., Miyazaki H. Receptor-type protein tyrosine phosphatase epsilon (PTPepsilonM) is a negative regulator of insulin signaling in primary hepatocytes and liver. Zoolog. Sci. 2005;22:169–175. doi: 10.2108/zsj.22.169. [DOI] [PubMed] [Google Scholar]
- Nakahara J., Seiwa C., Tan-Takeuchi K., Gotoh M., Kishihara K., Ogawa M., Asou H., Aiso S. Involvement of CD45 in central nervous system myelination. Neurosci. Lett. 2005;379:116–121. doi: 10.1016/j.neulet.2004.12.066. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizuno Y., Kikuchi K. Molecular cloning of a novel cytoplasmic protein tyrosine phosphatase PTP epsilon. Biochem. Biophys. Res. Commun. 1996;218:726–732. doi: 10.1006/bbrc.1996.0129. [DOI] [PubMed] [Google Scholar]
- Pallen C. J. Protein tyrosine phosphatase alpha (PTPalpha): a Src family kinase activator and mediator of multiple biological effects. Curr. Top. Med. Chem. 2003;3:821–835. doi: 10.2174/1568026033452320. [DOI] [PubMed] [Google Scholar]
- Pani G., Siminovitch K. A., Paige C. J. The motheaten mutation rescues B cell signaling and development in CD45-deficient mice. J. Exp. Med. 1997;186:581–588. doi: 10.1084/jem.186.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A., Gil-Henn H., Sobko A., Shinder V., Attali B., Elson A. Hypomyelination and increased activity of voltage-gated K(+) channels in mice lacking protein tyrosine phosphatase epsilon. EMBO J. 2000;19:4036–4045. doi: 10.1093/emboj/19.15.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A., Sobko A., Attali B. Tyrosine kinases modulate K+ channel gating in mouse Schwann cells. J. Physiol. 1999;519:373–384. doi: 10.1111/j.1469-7793.1999.0373m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone A., Battaglia F., Wang C., Dusa A., Su J., Zagzag D., Bianchi R., Casaccia-Bonnefil P., Arancio O., Sap J. Receptor protein tyrosine phosphatase alpha is essential for hippocampal neuronal migration and long-term potentiation. EMBO J. 2003;22:4121–4131. doi: 10.1093/emboj/cdg399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O. Regulation of the activity of voltage-gated potassium channels by beta subunits. Semin. Neurosci. 1995;7:137–146. [Google Scholar]
- Ponniah S., Wang D. Z., Lim K. L., Pallen C. J. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- Schaapveld R. Q., Schepens J. T., Robinson G. W., Attema J., Oerlemans F. T., Fransen J. A., Streuli M., Wieringa B., Hennighausen L., Hendriks W. J. Impaired mammary gland development and function in mice lacking LAR receptor-like tyrosine phosphatase activity. Dev. Biol. 1997;188:134–146. doi: 10.1006/dbio.1997.8630. [DOI] [PubMed] [Google Scholar]
- Schindelholz B., Knirr M., Warrior R., Zinn K. Regulation of CNS and motor axon guidance in Drosophila by the receptor tyrosine phosphatase DPTP52F. Development. 2001;128:4371–4382. doi: 10.1242/dev.128.21.4371. [DOI] [PubMed] [Google Scholar]
- Skelton M. R., Ponniah S., Wang D. Z., Doetschman T., Vorhees C. V., Pallen C. J. Protein tyrosine phosphatase alpha (PTP alpha) knockout mice show deficits in Morris water maze learning, decreased locomotor activity, and decreases in anxiety. Brain Res. 2003;984:1–10. doi: 10.1016/s0006-8993(03)02839-7. [DOI] [PubMed] [Google Scholar]
- Sobko A., Peretz A., Attali B. Constitutive activation of delayed-rectifier potassium channels by a src family tyrosine kinase in Schwann cells. EMBO J. 1998a;17:4723–4734. doi: 10.1093/emboj/17.16.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobko A., Peretz A., Shirihai O., Etkin S., Cherepanova V., Dagan D., Attali B. Heteromultimeric delayed-rectifier K+ channels in sSchwann cells: developmental expression and role in cell proliferation. J. Neurosci. 1998b;18:10398–10408. doi: 10.1523/JNEUROSCI.18-24-10398.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somani A. K., Bignon J. S., Mills G. B., Siminovitch K. A., Branch D. R. Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J. Biol. Chem. 1997;272:21113–21119. doi: 10.1074/jbc.272.34.21113. [DOI] [PubMed] [Google Scholar]
- Stepanek L., Stoker A. W., Stoeckli E., Bixby J. L. Receptor tyrosine phosphatases guide vertebrate motor axons during development. J. Neurosci. 2005;25:3813–3823. doi: 10.1523/JNEUROSCI.4531-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J., Muranjan M., Sap J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- Sully V., Pownall S., Vincan E., Bassal S., Borowski A. H., Hart P. H., Rockman S. P., Phillips W. A. Functional abnormalities in protein tyrosine phosphatase epsilon-deficient macrophages. Biochem. Biophys. Res. Commun. 2001;286:184–188. doi: 10.1006/bbrc.2001.5360. [DOI] [PubMed] [Google Scholar]
- Sun Q., Schindelholz B., Knirr M., Schmid A., Zinn K. Complex genetic interactions among four receptor tyrosine phosphatases regulate axon guidance in Drosophila. Mol. Cell. Neurosci. 2001;17:274–291. doi: 10.1006/mcne.2000.0939. [DOI] [PubMed] [Google Scholar]
- Tanuma N., Nakamura K., Kikuchi K. Distinct promoters control transmembrane and cytosolic protein tyrosine phosphatase epsilon expression during macrophage differentiation. Eur. J. Biochem. 1999;259:46–54. doi: 10.1046/j.1432-1327.1999.00004.x. [DOI] [PubMed] [Google Scholar]
- Tanuma N., Nakamura K., Shima H., Kikuchi K. Protein-tyrosine phosphatase PTPepsilon C inhibits Jak-STAT signaling and differentiation induced by interleukin-6 and leukemia inhibitory factor in M1 leukemia cells. J. Biol. Chem. 2000;275:28216–28221. doi: 10.1074/jbc.M003661200. [DOI] [PubMed] [Google Scholar]
- Tanuma N., Shima H., Nakamura K., Kikuchi K. Protein tyrosine phosphatase epsilonC selectively inhibits interleukin-6- and interleukin-10-induced JAK-STAT signaling. Blood. 2001;98:3030–3034. doi: 10.1182/blood.v98.10.3030. [DOI] [PubMed] [Google Scholar]
- Tanuma N., Shima H., Shimada S., Kikuchi K. Reduced tumorigenicity of murine leukemia cells expressing protein-tyrosine phosphatase, PTPepsilon C. Oncogene. 2003;22:1758–1762. doi: 10.1038/sj.onc.1206267. [DOI] [PubMed] [Google Scholar]
- Tiran Z., Peretz A., Attali B., Elson A. Phosphorylation-dependent regulation of Kv2.1 Channel activity at tyrosine 124 by Src and by protein-tyrosine phosphatase epsilon. J. Biol. Chem. 2003;278:17509–17514. doi: 10.1074/jbc.M212766200. [DOI] [PubMed] [Google Scholar]
- Toledano-Katchalski H., Kraut J., Sines T., Granot-Attas S., Shohat G., Gil-Henn H., Yung Y., Elson A. Protein tyrosine phosphatase epsilon inhibits signaling by mitogen-activated protein kinases. Mol. Cancer Res. 2003;1:541–550. [PubMed] [Google Scholar]
- Trimmer J. S. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc. Natl. Acad. Sci. USA. 1991;88:10764–10768. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai W., Morielli A. D., Cachero T. G., Peralta E. G. Receptor protein tyrosine phosphatase alpha participates in the m1 muscarinic acetylcholine receptor-dependent regulation of Kv1.2 channel activity. EMBO J. 1999;18:109–118. doi: 10.1093/emboj/18.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Hoppe D., Kettenmann H. Single K+ channel properties in cultured mouse Schwann cells: conductance and kinetics. J. Neurosci. Res. 1991;28:200–209. doi: 10.1002/jnr.490280207. [DOI] [PubMed] [Google Scholar]
- von Wichert G., Jiang G., Kostic A., De Vos K., Sap J., Sheetz M. P. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 2003;161:143–153. doi: 10.1083/jcb.200211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wabakken T., Hauge H., Finne E. F., Wiedlocha A., Aasheim H. Expression of human protein tyrosine phosphatase epsilon in leucocytes: a potential ERK pathway-regulating phosphatase. Scand. J. Immunol. 2002;56:195–203. doi: 10.1046/j.1365-3083.2002.01126.x. [DOI] [PubMed] [Google Scholar]
- Wallace M. J., Batt J., Fladd C. A., Henderson J. T., Skarnes W., Rotin D. Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPsigma. Nat. Genet. 1999;21:334–338. doi: 10.1038/6866. [DOI] [PubMed] [Google Scholar]
- Yi B. A., Minor D. L., Jr, Lin Y. F., Jan Y. N., Jan L. Y. Controlling potassium channel activities: interplay between the membrane and intracellular factors. Proc. Natl. Acad. Sci. USA. 2001;98:11016–11023. doi: 10.1073/pnas.191351798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., D'Alessandri L., Kalousek M. B., Vaughan L., Pallen C. J. Protein tyrosine phosphatase alpha (PTPalpha) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J. Cell Biol. 1999;147:707–714. doi: 10.1083/jcb.147.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Si X., Yu W. P., Le H. T., Ng K. P., Teng R. M., Ryan K., Wang D. Z., Ponniah S., Pallen C. J. PTP alpha regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J. Cell Biol. 2003;160:137–146. doi: 10.1083/jcb.200206049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E. E., Chapeau E., Hagihara K., Feng G. S. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc. Natl. Acad. Sci. USA. 2004;101:16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. M., Resnick R. J., Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatkine P., Mehul B., Magee A. I. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J. Cell Sci. 1997;110:673–679. doi: 10.1242/jcs.110.5.673. [DOI] [PubMed] [Google Scholar]