Abstract
The role of migration in the Anglo-Saxon transition in England remains controversial. Archaeological and historical evidence is inconclusive, but current estimates of the contribution of migrants to the English population range from less than 10 000 to as many as 200 000. In contrast, recent studies based on Y-chromosome variation posit a considerably higher contribution to the modern English gene pool (50–100%). Historical evidence suggests that following the Anglo-Saxon transition, people of indigenous ethnicity were at an economic and legal disadvantage compared to those having Anglo-Saxon ethnicity. It is likely that such a disadvantage would lead to differential reproductive success. We examine the effect of differential reproductive success, coupled with limited intermarriage between distinct ethnic groups, on the spread of genetic variants. Computer simulations indicate that a social structure limiting intermarriage between indigenous Britons and an initially small Anglo-Saxon immigrant population provide a plausible explanation of the high degree of Continental male-line ancestry in England.
Keywords: computer simulation, migration, population, ethnicity, Y-chromosome
1. Introduction
The traditional model of the Anglo-Saxon immigration into fifth-century Britain was based on scanty written sources (Gildas, Bede, Anglo-Saxon Chronicle) and envisaged mass ‘invasion’ from the Continent and large-scale replacement of the natives (Stenton 1947). This model, with minor variations, remained largely undisputed until the late 1980s (Myres 1985). At that time, many historians and archaeologists began to favour the elite replacement model: the immigration of a small elite, which achieved military, political and social ascendancy (Hodges 1989; Higham 1992). Within a decade or so, this had become the majority opinion among Anglo-Saxonist scholars (Hills 2003), with a critical discussion of the new model only emerging in the late 1990s (Härke 1998, 2002). More recently, studies of Y-chromosome variation have indicated substantial migration of Anglo-Saxon men into Central England. Weale et al. (2002) found a striking similarity in the distribution of Y-chromosome haplotypes in Central England and Friesland, but a dissimilarity between Central England and North Wales. Using population-genetic models that incorporated both continuous gene flow and mass migration, they concluded that their data is best explained by a massive migration of Anglo-Saxon men into Central England, but not into North Wales, contributing 50–100% to the male population at that time. Capelli et al. (2003) examined the distribution of Y-chromosomes throughout the British Isles as well as in southern Denmark, northern Germany and Norway. Using an likelihood-based admixture approach (Chikhi et al. 2001) they found a more heterogeneous pattern of Continental input into the English gene pool. However, using southern Danish and northern German populations as the descendants of putative Anglo-Saxon source populations, their median estimates for Continental introgression into England ranged between 24.4 and 72.5% (mean 54.1%).
Explaining such a high proportion of Continental genetic input with immigration alone would require migration on a massive scale (approx. 500 000+), well above documented population movements of the early middle ages (see, for example, Heather (1991)). An alternative explanation would be provided by an apartheid-like situation (Woolf 2004) in which elevated social and economic status grant higher reproductive success to the immigrants when compared to the native population and a degree of post-migration reproductive isolation is maintained among ethnic groups for several generations (Hughes 1986; Mulder 1987; Mace 1996). Under a model of cross-generation ethnic apartheid it is expected that the chromosomes of the advantaged ethnic group would increase in overall frequency. However, intermarriage between ethnic groups would serve to homogenize the genetic makeup of those groups. Once homogeneity is attained, the reproductive advantage of an ethnic group would not systematically affect the overall frequency of any chromosome type.
Reproductive isolation and differential social status along ethnic lines is a frequent, temporary consequence of conquest and settlement, the best-known modern case being the Apartheid system in South Africa. In the post-Roman period, intermarriage between dominant immigrants and subject natives was banned in Visigothic France and Spain in the late fifth and early sixth century (King 1972). The Normans in eleventh- and twelfth-century England operated a conquest society in which the native English and Welsh had a lower legal status than Normans (Garnett 1985), and intermarriage, where it happened, was predominantly unidirectional, i.e. Norman men marrying English women. In Anglo-Saxon England, elements of an apartheid-like society can also be perceived in a Wessex law code of the seventh century which distinguishes clearly between Saxons and ‘Welsh’ (Britons) and gives the former a significantly higher legal status, some two centuries after the initial immigration (Whitelock 1979). Archaeological and skeletal data (Härke 1990, 1992), as well as textual evidence (Woolf 2004), have been used to suggest a situation of limited intermarriage between immigrant Anglo-Saxons and native Britons until the seventh century when this distinction began to break down.
Here we perform computer simulations to examine the rate of increase in overall frequency of chromosomes that are found initially only among the advantaged group, in the period leading up to genetic homogenization. The use of theoretical models enables us to formulate hypotheses and follow them through rigorously to see if the outcome of a model is commensurate with the observed data. While such models are necessarily simpler than reality, they differ from the popular ‘verbal’ models/hypotheses referred to above in the consistent and logical manner in which assumptions can be tested.
2. Material and methods
We apply a model that assumes two distinct ethnic groups (A and B). We let NT be the total population size and NA and NB be the population sizes of the respective ethnic groups. We let S be the additional reproductive advantage of belonging to the economically privileged ethnic group per generation, such that the ratio of reproductive success in A and B are 1+S and 1, respectively. We let D be the proportion of individuals in A that are available to move to a less reproductively advantaged ethnic category per generation, and we let U be the proportion of individuals in B that are available to move to a reproductively advantaged ethnic group, per generation. The total number of individuals that move from one ethnic group to another per generation is picked randomly from a binomial distribution, Binomial(n, p), where the number of trials, n, is equal to the number of individuals in the losing ethnic group, and the probability p is given by either U or D scaled by the proportion of individuals in the total population that belong to the gaining ethnic group. Finally, we reset NA and NB to sum to NT by picking from a random multinomial with probabilities (1+S)NA and NB. Note that it is possible to specify NT for all times T and we can thus examine the effect of variable population size on the genetic/social makeup of the mixed population. The chromosomes (one per individual) are ‘painted’ either ‘incoming’ or ‘indigenous’ at the start of simulations such that all chromosomes in A are ‘incoming’ and all chromosomes in B are ‘indigenous’. The initial size of A is given by NTp (where p represents the proportion of the total population that is made up of incomers that establish the privileged ethnic group, A). The distribution of chromosome types in each ethnic group does not contribute to the reproductive advantage of that group. Simulations are run over a range of values of U, D, S (in most simulations U and D were set to be equal as we consider this to be the most parsimonious assumption). We tested values of p=0.05, 0.1 and 0.2. Note that these are considerably smaller and in better agreement with known historical and archaeological information than the estimates obtained from the analysis of Y-chromosome data (Weale et al. 2002; Capelli et al. 2003).
All simulations are carried out using the statistical package ‘R’ (see http://www.R-project.org/). Simulation code is available from the authors.
3. Results
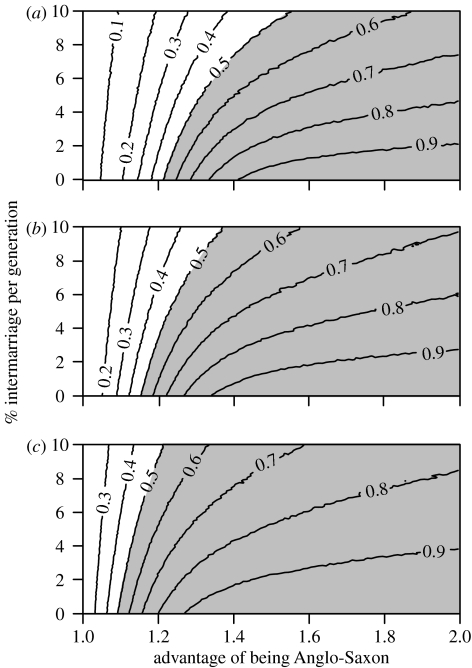
Initially we explored the effects of different combinations of selective advantage and intermarriage rate (U=D; see §2) on the proportion of ‘incoming’ Y-chromosomes in the total population after 15 generations. Values for the proportion of individuals available to marry out of their ethnic group ranged from 0 to 10% per generation in steps of 0.1%. Values of selective advantage ranged from 1 to 2 per generation in steps of 0.01. Figure 1 shows the results assuming the population was made up of (a) 5%; (b) 10% and (c) 20% immigrants immediately following migration.
Figure 1.
The simulated proportion of ‘Anglo-Saxon’ Y-chromosomes in the total population, after 15 generations, under different combinations of selective advantage and intermarriage rate (U=D; see §2), assuming the population was made up of (a) 5%, (b) 10% and (c) 20% immigrants immediately following migration. Shaded area of graph indicates parameter combinations that lead to greater than 50% ‘Anglo-Saxon’ Y-chromosomes (Weale et al. 2002; Capelli et al. 2003).
Next we examined the rate of increase in the proportion of ‘incoming’ Y-chromosomes under different values of selective advantage and intermarriage (U=D; see §2), starting with 10% immigrants immediately following migration. Figure 2 shows this increase assuming selective advantage values of (a) 1.2, (b) 1.5 and (c) 1.8. For each value of selective advantage, values of intermarriage of 0.02, 0.04, 0.06, 0.08 and 0.10 were used.
Figure 2.
Increase in the proportion of ‘Anglo-Saxon’ Y-chromosomes through time under different combinations of selective advantage and intermarriage (U=D; see §2), starting with 10% immigrants immediately following migration. Selective advantage values of (a) 1.2, (b) 1.5 and (c) 1.8 were modelled. For each value of selective advantage, values of intermarriage of 0.02, 0.04, 0.06, 0.08 and 0.10 were used, as indicated above lines.
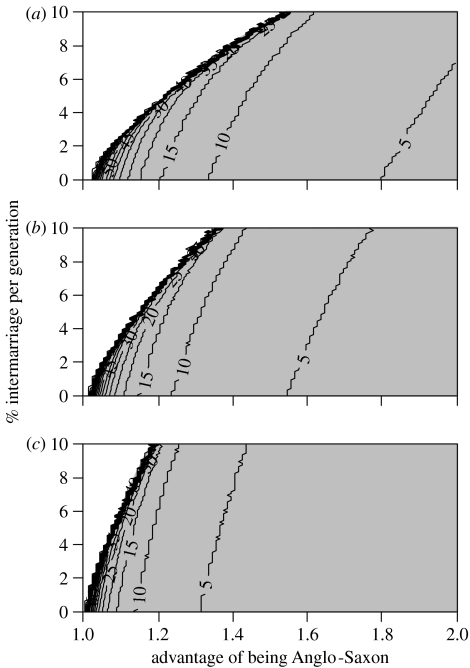
Given a conservative estimate of 50% for the proportion of Y-chromosomes in central England that originate among Anglo-Saxon migrants (Weale et al. 2002; Capelli et al. 2003), we next explored the effects of different combinations of selective advantage and intermarriage (U=D, see §2) on the number of generations that would be required to reach 50% ‘incoming’ Y-chromosomes, assuming the population was made up of (a) 5%; (b) 10% and (c) 20% immigrants immediately following migration (figure 3).
Figure 3.
Number of generations required to reach 50% ‘Anglo-Saxon’ Y-chromosomes (Weale et al. 2002; Capelli et al. 2003), under different combinations of selective advantage and intermarriage rate (U=D; see §2), assuming the population was made up of (a) 5%, (b) 10% and (c) 20% immigrants immediately following migration. Shaded area of graph indicates parameter combinations that lead to greater than 50% ‘Anglo-Saxon’ Y-chromosomes in less than 100 generations.
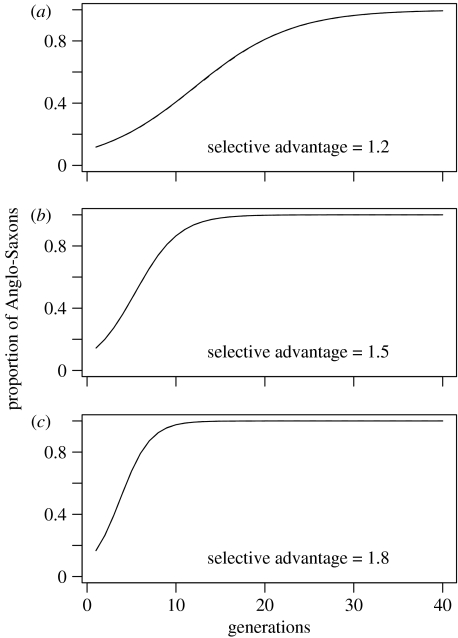
Finally, we examined the change through time in the proportion of individuals belonging to the ‘Anglo-Saxon’ ethnic group assuming a selective advantage of (a) 1.2; (b) 1.5 and (c) 1.8 to being Anglo-Saxon (figure 4). This rate is unaffected by the intermarriage rate given that U=D, but is strongly affected by the selection parameter.
Figure 4.
Change through time in the proportion of individuals belonging to the ‘Anglo-Saxon’ ethnic group assuming a selective advantage of (a) 1.2; (b) 1.5 and (c) 1.8 to being Anglo-Saxon.
4. Discussion
From figure 1 it can be seen that under a range of plausible combinations of selective advantage to being Anglo-Saxon and intermarriage rate (see below), the proportion of ‘immigrant’ Y-chromosomes rises from 20% or less immediately following migration to greater than 50% in 15 generations. We consider 50% to be a conservative estimate of the proportion of Y-chromosomes in the present-day English gene pool that originate among Anglo-Saxon migrants in the fifth century (Weale et al. 2002; Capelli et al. 2003). Fifteen generations marks the upper limit for the duration of an Anglo-Saxon/British apartheid-like social structure since, by assuming an intergenerational time of between 25 and 30 years, this is the approximate time span between the initial immigration in the middle of the fifth century and the laws of Alfred the Great (issued around AD 890), which do not contain any indications of legal status differences between Britons and Anglo-Saxons (Whitelock 1979). Although others have reported a male-specific intergeneration time of around 35 years (Tremblay & Vezina 2000), this estimate is based on genealogical records from a rapidly growing population between the seventeenth and the twentieth centuries. We reason that in Anglo-Saxon England, life expectancy, and as a consequence, intergeneration time would be shorter. However, 15 generations cannot be considered as a conservative estimate of the duration of such an apartheid-like social structure. For this reason we also examined the rate of increase in the proportion of ‘immigrant’ Y-chromosomes over time under a range of parameter values (figure 2). The proportion of ‘immigrant’ Y-chromosomes rises rapidly at first then levels off to its ceiling value. It is notable that the time taken to reach a near-ceiling value is largely determined by the reproductive parameter and is relatively unaffected by the intermarriage rate, although the ceiling value itself is strongly affected by the by the intermarriage rate. Most importantly, the proportion of ‘immigrant’ Y-chromosomes can rise from 10% to in excess of 50% in considerably fewer than 15 generations under plausible parameter values. This is best illustrated in figure 3, where we have explored the effects of different parameter values on the number of generations required to reach 50% ‘incoming’ Y-chromosomes in the whole population. Comparing figure 3a–c (representing 5, 10 and 20% Anglo-Saxon migration, respectively), it is evident that the initial size of the migration is a highly influential parameter here.
The model we present is necessarily simple but serves to illustrate some of the effects of differential reproductive success among groups, with a degree of reproductive isolation, on patterns genetic variation (Woolf 2004). We have only presented the results of simulations assuming symmetric intermarriage rates (U=D, see §2). We also tested asymmetric intermarriage rates whereby we multiplied U by 0.5 and D by 1.5 or vice versa and obtained very similar results to those presented (data not shown; figures available from the authors).
We reason that an apartheid-like social structure is a likely outcome of the Anglo-Saxon immigration into post-Roman Britain on theoretical and evidential grounds. The theoretical argument derives from the migration context and the relative sizes of the two groups. The immigration led to an encounter of two blocks of different ethnic groups with different, mutually incomprehensible languages (Celtic and some Latin on the part of the natives, Germanic languages on the part of the immigrants) and probably rather different degrees of social complexity and military mobilization (the native population having been largely unarmed and untrained in Roman times). At the same time, the natives are likely to have been in the majority: current population estimates for late third-century Roman Britain are as high as 3.7 million (Millett 1990), and allowing for a population decline in the fourth and early fifth century, one would still have to assume a native population in the region of 2 million. By contrast, estimates for migrating populations in the early middle ages are between tens and low hundreds of thousands; for example, Heather (1991) has estimated that the Ostrogoths moving from the Balkans into Italy in the late fifth century numbered more than 100 000. As a consequence, the minority immigrants faced the danger of losing their identity and their political and military control by assimilating in the much larger autochtonous population. The imposition of an apartheid-like social structure is one of the strategies open to a dominant ethnic minority, and it is one which is historically and ethnographically documented under such conditions (Thurnwald 1931).
The evidential argument rests on a textual source and some skeletal evidence. The laws of Ine, the late seventh-century ruler of the Anglo-Saxon kingdom of Wessex, distinguish clearly between Saxons and ‘Welsh’ (native Britons) and accord them different legal status even though the laws imply that the two groups live in close proximity, and often under the same roof (Whitelock 1979). Such a distinction is unlikely to have arisen in the seventh century, two centuries after the initial contact. It is much more likely to have originated in the immigration situation of the fifth and early sixth centuries. On the other hand, this ethnic distinction of two intermingling populations and its formalization in law cannot have survived for such a long period without some mechanism that perpetuated the distinction. Physical segregation could have this effect, but this is not what the laws of Ine imply; therefore an apartheid-like social structure seems to be the most obvious mechanism.
Skeletal evidence for the existence of two populations that are to an extent reproductively isolated is more circumstantial, and rests on the stature differential between men buried with, and those buried without weapons in cemeteries of Anglo-Saxon England. Men with weapons (47% of male adults) have been suggested, on a number of archaeological and skeletal indicators, to be immigrants and descendants of immigrants, while men without weapons (53%) are a more disparate group that appears to include a sizeable proportion of native Britons (Härke 1990, 1992). Most importantly, the stature differential between the two groups persists from the later fifth until the end of the sixth century, but begins to break down in the seventh century. The even distribution of stress indicators, as well as recently published stature data from the undisputedly indigenous cemetery of Queenford Farm (Fuller et al. 2006), indicate that environmental factors are unlikely to be the cause of the differential; and the long persistence of the differential is consistent with a low level of intermarriage between the two groups. In turn, the absence of a change in environmental factors in the seventh century suggests that the breakdown of the differential is the consequence of increasing intermarriage.
An apartheid-like social structure may be a more widespread phenomenon in the conquest societies of post-Roman western and southern Europe. Thus, the Visigothic king Euric (AD 466–484) banned intermarriage between his followers and the natives in southern France and Spain (King 1972). And some 600 years later, the eleventh-century Norman conquerors of England created a legal framework for a society in which the native English and Welsh had inferior legal status and in which intermarriage, where it happened at all, involved Norman men marrying English women (Garnett 1985). But here, as in other early historical cases of ethnic isolation in conquest societies, the actual rates of intermarriage are impossible to determine from the surviving documentary evidence. Some data does exist on intermarriage among distinct ethnic groups. For example, the modern rate among the major ethnic groups on Mauritius has been estimated to be ca 6.6% (Nave 2000). However, eighteenth- to twentieth-century demographic data demonstrate a consistently low marriage rate across the linguistic and cultural divide created by the eleventh century settlement of Flemish immigrants in southern Pembrokeshire by the Normans; in the twentieth century this rate was around 4% of all marriages (Woolley 1986). It is likely that pre-modern rates were even lower.
In all historical cases of conquest societies, the politically and militarily dominant ethnic group is known, or can be assumed, to have had a substantial social and economic advantage, but the quantification of this advantage is difficult. In the Anglo-Saxon case, the best evidence may be found in the rates of wergild in seventh century laws. Wergild is the ‘blood money’ payable to the family of any victim of killing in order to prevent a blood feud; this is graded according to the social and ethnic status of the victim. The late seventh century laws of King Ine of Wessex, which differentiate between natives and Saxons, stipulate wergild for the latter which is between two and five times the money payable for a ‘Welshman’ (native Briton) of comparable status (Whitelock 1979). The early seventh century laws of King Ethelbert of Kent mention a distinct social group, the læti, who have been suggested to be native Britons (Whitelock 1979); their wergild is consistently lower than that payable for a free man, which is between 1.25 and 2.5 times that of the blood money for a læt (Whitelock 1979). Similar wergild differences between immigrants and natives are found elsewhere in early medieval Europe, for example in the Frankish kingdom (Ward-Perkins 2005).
An additional, but less reliable type of evidence may be the relative wealth expressed in the number of grave-goods. Adult men buried with weapons, suggested to be Germanic immigrants or their descendants (cf. above), have an average of four artefacts in their graves while men buried without weapons have an average of 2.3 artefacts (not counting male graves without any finds; Härke 1990, 1992). This is strongly suggestive of a wealth differential between these two groups, and thus of a marked economic advantage of the immigrant group. However, the burial rite itself may have affected the deposition of artefacts in graves; and the fact that some of the artefacts (the weapons) have been used in the identification of ethnic affiliations (cf. above) means that their further use here to identify an economic differential between Britons and Anglo-Saxons may be close to a circular argument. It is, however, noteworthy that the wealth differential between the two groups is within the bracket of wergild differentials between Saxons and Britons found in the laws.
There is a considerable literature on a positive correlation between wealth and reproductive success, based both on empirical evidence (Hull & Hull 1977; Boone 1986; Hughes 1986; Mulder 1987; Mace 1996) and evolutionary theory (Fisher 1958; Beauchamp 1994; Mace 1998). In modern Gabbra pastoralists in Kenya, residual fertility (a measure of the number of children a person has had relative to others in that population of the same age and sex) was found to correlate significantly with wealth: from ca −0.5 for the poorest to ca 1 for the wealthiest. Furthermore, this differential is greater in men than in women (Mace 1996). A similar correlation has been seen late Medieval–early Modern Portugal. A significant, approximate twofold difference in reproductive performance was seen between the highest and lowest status males, whereas no significant difference was seen among women grouped by natal status (Boone 1986).
We have only considered the effects of differences in ethnic reproductive advantage and inter-ethnic marriage rate on patterns of genetic variation. If there were no sex bias in the intermarriage rate, then we would expect these effects to be equal for the different genetic systems (mitochondrial DNA, Y-chromosome, X-chromosome, autosomes). However, part of the motivation for this study was to seek an explanation for the discrepancy between archaeological estimates of the size of the Anglo-Saxon migration (Härke 1998, 2002; Hills 2003) and estimates based on Y-chromosome data (Weale et al. 2002; Capelli et al. 2003). There are three further factors that could exacerbate replacement of indigenous Y-chromosomes. The first is that when intermarriage does occur the offspring may be more likely to assume the identity of the father, thus reducing the effective intermarriage rate, as it would affect patterns of Y-chromosome diversity. The second is that forced extra-marital matings are more likely to occur between Anglo-Saxon men and native British women than the reverse since, as the law codes of Ine indicate, the degree of punishment was determined by the social status of the victim. The third is based on the theory that relatively ‘good condition’ males tend to out-reproduce females of a similar condition, whereas relatively ‘poor condition’ females tend to out-reproduce their male counterparts (Trivers & Willard 1973). From this, a strategy of sex-biased parental investment, whereby relatively wealthy parents favour wealth transfer to their sons, should emerge (Hartung 1976). Such a phenomenon is supported by genealogical data (Boone 1986) and should lead to an asymmetric increase in the population frequency of Y-chromosomes carried by wealthy men, when compared to the other genetic systems.
The motivation for this study was to reconcile the discrepancy between, on the one hand, archaeological and historical ideas about the scale of the Anglo-Saxon immigration (Hills 2003), and on the other, estimates of the genetic contribution of the Anglo-Saxon immigrants to the modern English gene pool (Weale et al. 2002; Capelli et al. 2003). We have shown that this discrepancy can be resolved by the assumption of an apartheid-like social structure within a range of plausible values for interethnic marriage and socially driven reproductive advantage following immigration (Woolf 2004). Perhaps most strikingly, our model indicates that, by using plausible parameter values, the genetic contribution of an immigrant population can rise from less than 10% to more than 50% in as little as five generations, and certainly less than fifteen generations. Similar processes are likely to have shaped patterns of genetic variation in other ‘conquest societies’ of the period, and perhaps more recently (Carvajal-Carmona et al. 2000). The social structures described here may have been of wider significance in processes of language replacement and the interactions of hunter-gatherers and early farmers. This is of particular relevance in cases where genetic data indicate a demographic expansion of farmers, such as the Bantu (Passarino et al. 1998; Scozzari et al. 1999; Thomas et al. 2000; Cruciani et al. 2002; Salas et al. 2002; Luis et al. 2004; Beleza et al. 2005) and Austronesian expansions (Melton et al. 1998; Hagelberg et al. 1999; Hurles et al. 2002; Lum et al. 2002). Future work might include incorporating the model presented here into larger, demic models in order to improve our understanding of the process of colonization in geographic space (Currat & Excoffier 2005).
Acknowledgments
We thank A. Woolf, S. Shennan, R. Mace, M. E. Weale, C. Hills, C. Renfrew and N. Bradman for valuable discussion and the two anonymous referees for their comments.
References
- Beauchamp G. The functional-analysis of human-fertility decisions. Ethol. Sociobiol. 1994;15:31–53. doi:10.1016/0162-3095(94)90026-4 [Google Scholar]
- Beleza S, Gusmao L, Amorim A, Carracedo A, Salas A. The genetic legacy of western Bantu migrations. Hum. Genet. 2005;117:366–375. doi: 10.1007/s00439-005-1290-3. doi:10.1007/s00439-005-1290-3 [DOI] [PubMed] [Google Scholar]
- Boone J.L. Parental investment and elite family structure in preindustrial states: a case study of late Medieval–Early Modern Portuguese genealogies. Am. Anthropol. 1986;88:859–878. doi:10.1525/aa.1986.88.4.02a00050 [Google Scholar]
- Capelli C, et al. A Y chromosome census of the British Isles. Curr. Biol. 2003;13:979–984. doi: 10.1016/s0960-9822(03)00373-7. doi:10.1016/S0960-9822(03)00373-7 [DOI] [PubMed] [Google Scholar]
- Carvajal-Carmona L.G, et al. Strong Amerind/white sex bias and a possible Sephardic contribution among the founders of a population in Northwest Colombia. Am. J. Hum. Genet. 2000;67:1287–1295. doi: 10.1016/s0002-9297(07)62956-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhi L, Bruford M.W, Beaumont M.A. Estimation of admixture proportions: a likelihood-based approach using Markov chain Monte Carlo. Genetics. 2001;158:1347–1362. doi: 10.1093/genetics/158.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani F, et al. A back migration from Asia to sub-Saharan Africa is supported by high-resolution analysis of human Y-chromosome haplotypes. Am. J. Hum. Genet. 2002;70:1197–1214. doi: 10.1086/340257. doi:10.1086/340257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currat M, Excoffier L. The effect of the Neolithic expansion on European molecular diversity. Proc. R. Soc. B. 2005;272:679–688. doi: 10.1098/rspb.2004.2999. doi:10.1098/rspb.2004.2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. Dover; New York, NY: 1958. The genetical theory of natural selection. [Google Scholar]
- Fuller B.T, Molleson T.I, Harris D.A, Gilmour L.T, Hedges R.E. Isotopic evidence for breastfeeding and possible adult dietary differences from Late/Sub-Roman Britain. Am. J. Phys. Anthropol. 2006;129:45–54. doi: 10.1002/ajpa.20244. doi:10.1002/ajpa.20244 [DOI] [PubMed] [Google Scholar]
- Garnett G. Franci et Angli: the legal distinctions between peoples after the conquest. Anglo-Norman Stud. 1985;8:109–137. [Google Scholar]
- Hagelberg E, Kayser M, Nagy M, Roewer L, Zimdahl H, Krawczak M, Lio P, Schiefenhovel W. Molecular genetic evidence for the human settlement of the Pacific: analysis of mitochondrial DNA, Y chromosome and HLA markers. Phil. Trans. R. Soc. B. 1999;354:141–152. doi: 10.1098/rstb.1999.0367. doi:10.1098/rstb.1999.0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härke H. Warrior graves? The background of the Anglo-Saxon weapon burial rite. Past Present. 1990;126:22–43. [Google Scholar]
- Härke H. Rheinland-Verlag and Habelt; Cologne and Bonn, Germany: 1992. Angelsächsische Waffengräber des 5. bis 7. Jahrhunderts. [Google Scholar]
- Härke H. Archaeologists and migrations: a problem of attitude? Curr. Anthropol. 1998;39:19–45. doi:10.1086/204697 [Google Scholar]
- Härke H. Kings and warriors: population and landscape from post-Roman to Norman Britain. In: Slack P, Ward R, editors. The peopling of Britain: the shaping of a human landscape (the Linacre lectures 1999) Oxford University Press; Oxford, UK: 2002. pp. 145–175. [Google Scholar]
- Hartung J. Natural-selection and inheritance of wealth. Curr. Anthropol. 1976;17:607–622. doi:10.1086/201799 [Google Scholar]
- Heather, P. J. 1991 Goths and Romans 332–489 Oxford historical monographs. Oxford, UK: Clarendon Press.
- Higham N.J. Seaby; London, UK: 1992. Rome, Britain and the Anglo-Saxons. [Google Scholar]
- Hills C. Duckworth; London, UK: 2003. Origins of the English. [Google Scholar]
- Hodges R. Cornell University Press; Ithaca, NY: 1989. The Anglo-Saxon achievement: archaeology and the beginnings of English society. [Google Scholar]
- Hughes A.L. Reproductive success and occupational class in eighteenth-century Lancashire, England. Soc. Biol. 1986;33:109–115. doi: 10.1080/19485565.1986.9988627. [DOI] [PubMed] [Google Scholar]
- Hull T.H, Hull V.J. The relation of economic class and fertility: an analysis of some Indonesian data. Popul. Stud. 1977;31:43–57. doi: 10.1080/00324728.1977.10412746. [DOI] [PubMed] [Google Scholar]
- Hurles M.E, Nicholson J, Bosch E, Renfrew C, Sykes B.C, Jobling M.A. Y chromosomal evidence for the origins of oceanic-speaking peoples. Genetics. 2002;160:289–303. doi: 10.1093/genetics/160.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P.D. Cambridge studies in medieval life and thought. vol. 5. Cambridge University Press; Cambridge, UK: 1972. Law and society in the visigothic kingdom. [Google Scholar]
- Luis J.R, Rowold D.J, Regueiro M, Caeiro B, Cinnioglu C, Roseman C, Underhill P.A, Cavalli-Sforza L.L, Herrera R.J. The Levant versus the Horn of Africa: evidence for bidirectional corridors of human migrations. Am. J. Hum. Genet. 2004;74:532–544. doi: 10.1086/382286. doi:10.1086/382286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J.K, Jorde L.B, Schiefenhovel W. Affinities among Melanesians, Micronesians, and Polynesians: a neutral biparental genetic perspective. Hum. Biol. 2002;74:413–430. doi: 10.1353/hub.2002.0031. [DOI] [PubMed] [Google Scholar]
- Mace R. Biased parental investment and reproductive success in Gabbra pastoralists. Behav. Ecol. Sociobiol. 1996;38:75–81. doi: 10.1007/s002650050219. doi:10.1007/s002650050219 [DOI] [PubMed] [Google Scholar]
- Mace R. The coevolution of human fertility and wealth inheritance strategies. Phil. Trans. R. Soc. B. 1998;353:389–397. doi: 10.1098/rstb.1998.0217. doi:10.1098/rstb.1998.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton T, Clifford S, Martinson J, Batzer M, Stoneking M. Genetic evidence for the proto-Austronesian homeland in Asia: mtDNA and nuclear DNA variation in Taiwanese aboriginal tribes. Am. J. Hum. Genet. 1998;63:1807–1823. doi: 10.1086/302131. doi:10.1086/302131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett M. Cambridge University Press; New York, NY: 1990. The Romanization of Britain: an essay in archaeological interpretation. [Google Scholar]
- Mulder M.B. Resources and reproductive success in women with an example from the Kipsigis of Kenya. J. Zool. 1987;213:489–505. doi: 10.1111/j.1469-7998.1987.tb03722.x. [DOI] [PubMed] [Google Scholar]
- Myres J.N.L. Clarendon; Oxford, UK: 1985. The English settlements. [Google Scholar]
- Nave A. Marriage and the maintenance of ethnic group boundaries: the case of Mauritius. Ethnic Racial Stud. 2000;23:329–352. doi:10.1080/014198700329079 [Google Scholar]
- Passarino G, Semino O, Quintana-Murci L, Excoffier L, Hammer M, Santachiara-Benerecetti A.S. Different genetic components in the Ethiopian population, identified by mtDNA and Y-chromosome polymorphisms. Am. J. Hum. Genet. 1998;62:420–434. doi: 10.1086/301702. doi:10.1086/301702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas A, Richards M, De la Fe T, Lareu M.V, Sobrino B, Sanchez-Diz P, Macaulay V, Carracedo A. The making of the African mtDNA landscape. Am. J. Hum. Genet. 2002;71:1082–1111. doi: 10.1086/344348. doi:10.1086/344348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scozzari R, et al. Combined use of biallelic and microsatellite Y-chromosome polymorphisms to infer affinities among African populations. Am. J. Hum. Genet. 1999;65:829–846. doi: 10.1086/302538. doi:10.1086/302538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenton F.M. The oxford history of England. vol. 2. Clarendon Press; Oxford, UK: 1947. Anglo-Saxon England. [Google Scholar]
- Thomas M.G, Parfitt T, Weiss D.A, Skorecki K, Wilson J.F, le Roux M, Bradman N, Goldstein D.B. Y chromosomes traveling south: the cohen modal haplotype and the origins of the Lemba—the ‘Black Jews of Southern Africa’. Am. J. Hum. Genet. 2000;66:674–686. doi: 10.1086/302749. doi:10.1086/302749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnwald R. W. de Gruyter; Berlin, Germany: 1931. Die menschliche Gesellschaft in ihren ethno-soziologischen Grundlagen. [Google Scholar]
- Tremblay M, Vezina H. New estimates of intergenerational time intervals for the calculation of age and origins of mutations. Am. J. Hum. Genet. 2000;66:651–658. doi: 10.1086/302770. doi:10.1086/302770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L, Willard D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Ward-Perkins B. Oxford University Press; Oxford, UK: 2005. The fall of Rome and the end of civilization. [Google Scholar]
- Weale M.E, Weiss D.A, Jager R.F, Bradman N, Thomas M.G. Y chromosome evidence for Anglo-Saxon mass migration. Mol. Biol. Evol. 2002;19:1008–1021. doi: 10.1093/oxfordjournals.molbev.a004160. [DOI] [PubMed] [Google Scholar]
- Whitelock D. English historical documents. vol. 1. Eyre Methuen; London, UK: 1979. English historical documents, c. 500–1042. [Google Scholar]
- Woolf A.Apartheid and Genocide: legal theory and economic realityPresentation given at conferenceBritons in Anglo-Saxon England 2004Manchester, UK, 14–16 April 2004. [Google Scholar]
- Woolley V. Demographic studies in Pembrokeshire. In: Harper P.S, Sunderland E, editors. Genetic and population studies in Wales. University of Wales Press; Cardiff, UK: 1986. pp. 236–250. [Google Scholar]