Abstract
As fitness returns during a breeding attempt are context-dependent, parents are predicted to bias their food allocation within a brood from poor towards good condition nestlings when environmental conditions deteriorate. We tested this prediction in the Alpine swift and the European starling, two migratory bird species, by modifying an ultraviolet (UV) visual signal of condition in nestlings and exploring how parents allocate food to their young as the season progresses. We show in both species that: (i) UV light reflected by the body skin of offspring positively correlates with their stature (i.e. body mass and skeletal size) and (ii) parental favouritism towards young with more UV reflective skin gradually increases as the season progresses. Early-breeding parents supplied food preferentially to UV pale (i.e. small stature) nestlings, whereas late-breeding parents favoured UV bright offspring (i.e. large stature). These results emphasize that parents use UV signals of offspring condition to adjust their feeding strategies depending on the ecological context.
Keywords: parent–offspring interactions, honest signalling theory, laying date, skin reflectance, ultraviolet
1. Introduction
When delivering food to dependent offspring, parents are expected to decide which young to feed in a way that maximizes their own reproductive success by supplying food preferentially to young with the largest fitness return per amount of food invested (Godfray 1991, 1995; Mock & Parker 1997). Honest signalling theory assumes that offspring communicate their need to parents by soliciting them with costly signals, and predicts that, since young in poorer condition benefit more from receiving extra food, parents should supply food preferentially to needy offspring (Godfray 1995; Mock & Parker 1997). Although this prediction has received some empirical support (Kilner 1995; Price & Ydenberg 1995; Mock & Parker 1997; Leonard & Horn 2001), this parental strategy is expected to become unstable when food is limited because fixed investment in young in poorer conditions can result in complete brood failure (Royle et al. 2002). Hence, it is expected that parental decision strategies depend on the context, and that parental food allocation decisions should change from feeding young in poorer to better body condition when environmental conditions deteriorate and resources are becoming limited (Davis et al. 1999; Royle et al. 2002). In agreement with this prediction, computer simulations demonstrated that fitness returns of various food allocation strategies vary with the level of resources, the strategy with the highest payoff being to supply food preferentially to the ‘smallest’ young in rich environments and to the ‘largest’ ones when resources are scarce (Davis et al. 1999). Alternatively, conditional responses could be associated with differences in parental quality (Arnold et al. 2004). However, to our knowledge, context-dependent parental favouritism has never been experimentally demonstrated in any species (but see Kölliker et al. (1998) and Kilner (2001, 2002) for context-dependent modifications of offspring solicitations).
In an experimental test of this hypothesis, we first examined whether skin reflectance of nestlings in two distantly related migratory and insectivorous bird species, the Alpine swift (Apus melba; Apodiformes) and the European starling (Sturnus vulgaris; Passeriformes), varies according to offspring condition. It has recently been shown that ultraviolet (UV) skin reflectance is involved in parental food allocation decisions (Jourdie et al. 2004), but evidence for a causal link between UV skin reflectance and condition in offspring is still lacking. As in the starling (Jourdie et al. 2004), the skin of swift nestlings substantially reflects light in the UV range (figure 1), and we tested for a causal relationship between UV skin reflectance and body condition in nestling swifts by manipulating brood sizes 2 days after hatching and by measuring 8 days later the effect of brood manipulation on body condition (Martins & Wright 1993) and skin reflectance. In a separate study with starlings, we compared the relationship between UV skin reflectance and body mass in first and second broods. If skin UV reflectance acts as a signal of nestling quality, we predicted that nestlings in better body condition have skin that is more brightly coloured in the UV part of the spectrum (i.e. higher UV chroma) than nestlings in poorer body conditions.
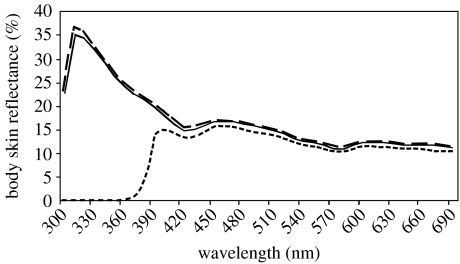
Figure 1.
Reflectance spectra by the skin of Alpine swift nestlings before (solid line) and after they were treated with control petroleum jelly (long-dashed line) or petroleum jelly containing UV-light blocker (short-dashed line). Reflectance spectra by the skin of European starlings are provided in Jourdie et al. (2004).
In many birds, breeding conditions deteriorate with time, and late-breeding parents are therefore under greater time and energy constraints when rearing their brood before migrating (Martin 1987; Brinkhof & Cave 1997; Arnold et al. 2004). This can be further exacerbated by the fact that late-breeding parents are frequently young and inexperienced breeders, which may have difficulties foraging food for the whole brood (Daunt et al. 1999; Arnold et al. 2004). Hence, independent of the exact mechanisms mediating the seasonal change in resource levels available for offspring (i.e. variations in food quantity, available time remaining to rear the brood and/or parental quality), theory predicts that parental favouritism should change during the season from young in poor condition towards young in good condition. Although both parents and offspring have some control over food allocation (Mock & Parker 1997; Kölliker et al. 2000; Kilner 2002; Parker et al. 2002; Royle et al. 2002), we here describe two experiments that we carried out on blind nestlings where we controlled for hunger level and offspring competitiveness. By applying UV-light blockers on the bodies of randomly chosen young within the broods, while applying a control petroleum jelly on other young (Jourdie et al. 2004), we tested the prediction that parental favouritism for UV-blocked nestlings and control siblings should change with time during the breeding season.
2. Material and methods
(a) Study sites and general protocols
The study was performed in Switzerland in 2003 and 2004 in a population of European starlings breeding in nest-boxes installed in the surroundings of the University of Lausanne (Jourdie et al. 2004), and in 2004 in a colony of Alpine swifts breeding under the roof of a clock-tower in Bienne. In Switzerland, starlings produced two broods per year, and our study was carried out on second broods in 2003 and on first and second broods in 2004. In both species, for each breeding pair we recorded laying date, clutch size, date when the first egg hatched (defined as day 0) and brood size at hatching. When starling and swift nestlings were 6 and 10 days old, respectively, we weighed them to the nearest 0.1 g, measured their skeletal size (i.e. tarsus and sternum length in starling and swift nestlings, respectively) to the nearest 0.5 mm, and assessed skin reflectance. For each nestling, we computed two indices of body condition. First, as an index of offspring stature, we calculated the first principal component (PC1) of a principal component analysis, with body mass and skeletal size as loading factors. PC1 index explained 96.6 and 95.6% of the total variance in starling and swift nestlings, respectively. High PC1 index indicates that nestlings were large and heavy for their age (loading factors in the starling: body mass=0.71, tarsus length=0.71; swift: body mass=0.71, sternum length=0.71), and in turn that they will require less food up to fledging relative to nestlings with low PC1 index. Nestling stature can thus be interpreted as an indicator of nestling long-term needs (sensu Price et al. 1996). Second, as an index of nestling body reserves, we calculated the residuals of a linear regression of body mass on skeletal size (starling: body mass=−47.5+3.3×tarsus length, r2=0.88, F1,70=506.3, p<0.0001; swift: body mass=−29.5+4.6×sternum length, r2=0.83, F1,61=297.7, p<0.0001). High residual body mass indicates that nestlings were heavy for their size, and in turn that they had greater energy reserves relative to siblings with low residual body mass (Ardia 2005). Nestling reserves can thus be interpreted as an indicator of nestling short-term needs (sensu Price et al. 1996). There was no significant relationship between our two indices of condition (starling: Pearson correlation between PC1 index and residual body mass, r=0.18, p=0.14, n=72 nestlings; swift: r=0.20, p=0.11, n=63 nestlings), which suggests that large nestlings did not have larger energy reserves (see also Ardia 2005). Young were individually identified at hatching by using different combinations of down feather plucking on the head and wings in the starling and by marking them under the wings with non-toxic permanent colour markers in the swift. In both species, feathers become noticeable at 10 days onward.
(b) Calculation of the UV reflectance by the skin of nestlings
Reflectance spectra (300–700 nm) were recorded using an Ocean Optics S2000 spectrometer and a DH-2000 deuterium–halogen lamp in starling nestlings, and a PX-2 pulsed xenon lamp in swift nestlings (Ocean Optics Inc., Duiven, The Netherlands). Reflectance is expressed as the proportion of reflectance from a spectralon white standard disk (type WS). Reflectance spectra in starling and swift nestlings were collected in 2004, and in both species for each individual we measured skin reflectance on four body regions, namely the head, throat, back and chest. In both species, we summarized skin reflectance data by calculating three colour variables: total reflectance (RT), reflectance in UV light and UV chroma. Total reflectance is calculated as the mean of reflectance values, in 10 nm intervals, from 300 to 700 nm, reflectance in UV light (RUV) as the mean of reflectance values, in 10 nm intervals, from 300 to 400 nm, and UV chroma as the ratio RUV/RT (Andersson et al. 1998). Ultraviolet chromas were moderately correlated between body regions (swift: range in Pearson r coefficients=0.43–0.60, all p-values<0.001; starling: r=0.27–0.37, all p-values<0.05), and hence for each individual, we calculated a mean body skin UV chroma. Statistical analyses performed separately for each body region do not alter the content of our results (data not presented).
(c) Brood size manipulation
To experimentally test for a link between body condition and the UV chroma of the swift nestlings' skin, we reduced or enlarged brood sizes by one nestling by exchanging at random an unbalanced number of 2-day-old young between 28 nests matched in pairs by hatching date. Nests included in the experiment hatched 2–3 nestlings, and thus brood size after the manipulation remained within the natural range (1–4 nestlings) of this species. Brood size at 10 days after hatching was significantly different between enlarged and reduced broods (mean±s.e.=3.4±0.1 versus 1.4±0.1 nestlings, respectively; U14,14=196, p<0.0001). We could not quantify skin reflectance in one reduced swift brood, reducing our sample size to 13 reduced broods and 14 enlarged broods. Due to fieldwork and sample size constraints this experiment was not carried out with starlings.
(d) Manipulation of UV reflectance by the skin of nestlings
To experimentally assess how parents allocate food in relation to skin UV reflectance of their offspring, we applied a petroleum jelly with UV-light blockers on the body of randomly chosen nestlings (referred to hereafter as ‘UV-blocked nestlings’) and compared their body mass gain with siblings treated with a control petroleum jelly (‘control nestlings’; figure 1; Jourdie et al. 2004). Petroleum jelly with UV-light blockers contained 79.95% petroleum jelly, 6% Cetiol B, 0.05% butylated hydroxytoluene (BHT), 3% Parsol 1789, 6% Parsol 2-ethylhexyl p-methoxycinnamate (MCX) and 5% Eusolex OS, and control petroleum jelly contained 93.95% petroleum jelly, 6% Cetiol B and 0.05% BHT (Roche, Switzerland). At day 6 and 10 after hatching in the starling and in the swift, respectively, nestlings were removed from the nest, their body and mouth flanges were covered either with control petroleum jelly or with UV-blocking petroleum jelly, and their body mass was measured after defecation. We applied petroleum jelly later in the development of swift nestlings because they have a slower development and in turn become able to thermoregulate on their own at an older age than starling nestlings (Bize et al. 2004). In both species, there was no significant difference in body mass between UV-blocked and control nestlings before the experiment (starling: mean±s.e.=26.4±1.2 g versus 26.7±1.2 g; paired t-test: t=0.12, p=0.62, d.f.=56; swift: 36.6±1.8 g versus 37.8±1.8 g; t=0.60, p=0.56, d.f.=19), and the difference in body mass between UV-blocked and control nestlings did not change through the breeding season (linear regression with the difference in body mass between UV-blocked and control nestlings as a dependent variable, and the date of test as explanatory variable: starling: F1,55=0.33, p=0.57; swift: F1,18=0.19, p=0.67). After two and four hours of experiment in the starling and in the swift, respectively, we measured the body mass of nestlings again and calculated the mean body mass gain per UV-blocked and control sibling. We used a longer interval of time in the swift to calculate the mean body mass gain per sibling because swift parents provision their brood at slower rates than starling parents (Bize et al. 2004). In swift broods, we separated pairs of siblings within a nest with a Plexiglas partition, allowing us to exclude jostling by nestlings and increase parental control over food allocation (Kilner 1995). We controlled for nestlings' position by switching their positions in the nest after two hours of experiment. An examination of spectra for several swift and starling nestlings showed that the difference between treatments persisted until the end of the experiment. However, the starling control nestlings tend to have lower reflectance in the UV part of their spectra presumably due to close body contact with UV-blocked nestlings (Jourdie et al. 2004). Thus, our results are conservative since reduction in the efficiency of our treatment over the test hours reduces the probability of detecting significant parental favouritism towards either UV-blocked or control nestlings. Nestling swifts used in the brood size manipulation and in the UV reflectance manipulation were issued from different broods. Although body mass gain by offspring is an indirect measure of parental care, starling and swift nestlings entirely depend on parents for their food. Nestlings significantly gained mass during the time interval of the experiment (starling: paired t-test: tpaired=16.85, p<0.0001, d.f.=225; swift: tpaired=2,33, p=0.025, d.f.=39), and the use in 2005 of neck-collars in the swift to measure parental feeding rate of 15-day-old nestlings showed that nestling body mass gain is strongly correlated with the amount of food provided by parents (r=0.79, p=0.0005, n=15 nestlings). Thus, variation in body mass gain between siblings can be interpreted as a valuable measure of parental favouritism (e.g. Heeb et al. 2003).
(e) Ethical note
Experiments in the starling and in the swift were carried out under the legal authorization of the veterinary services of the Canton Vaud (licence no. 1704) and Berne (licence no. 51/04), respectively. Although the brood size manipulation experiment increased nestling swift mortality in enlarged broods, the overall mean numbers of fledglings per experimental nest (i.e. enlarged and reduced broods) did not differ from the mean numbers of fledglings per non-manipulated nest, indicating that at the population level negative effects of brood size enlargement on subsequent nestling survival were counterbalanced by positive effects of experimental brood size reduction at hatching on subsequent nestling survival (Bize & Roulin in press). Growth and survival were similar in swift and starling nestlings treated with petroleum jelly compared to unmanipulated nestlings (all p-values>0.34; Jourdie et al. 2004).
(f) Statistical analyses
Statistical analyses were performed using JMP IN v. 5.1. Throughout the paper mean values are quoted ±1 s.e., statistical tests are two tailed and p-values less than 0.05 considered significant.
3. Results
(a) UV skin reflectance as a signal of offspring condition
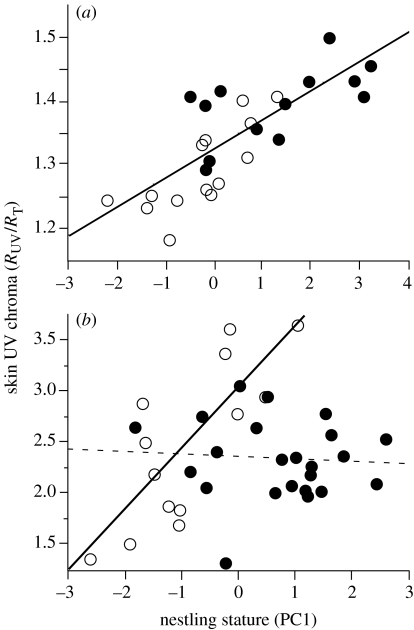
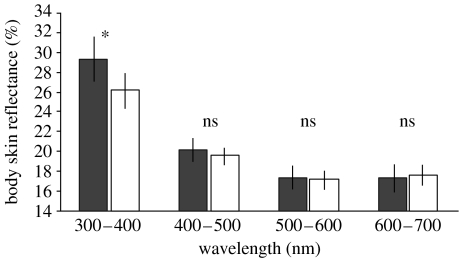
Ultraviolet light reflected from the bodies of swift nestlings was a good indicator of their stature, and by extension of their long-term needs, since young from enlarged broods had lower PC1 scores and UV chroma than young from reduced broods (mean±s.e. PC1 scores of nestlings raised in enlarged and reduced broods were −0.33±0.26 and 1.25±0.35, respectively; t-test, t=3.65, p=0.0012, d.f.=26; mean±s.e. UV chromas of nestlings raised in enlarged and reduced broods were 1.30±0.02 and 1.40±0.02, respectively; t=4.21, p=0.0003, d.f.=25). There was a strong positive correlation between PC1 score and UV chroma (r=0.78, p<0.0001, n=27; figure 2a). Difference in UV chroma between treatments was due to the higher reflectance by the skin of nestlings raised in reduced than enlarged broods in the UV part of the spectra only (repeated-measures ANOVA with brood size manipulation as a factor and mean reflectance between 300–400, 400–500, 500–600 and 600–700 nm as repeats: treatment: F1,25=1.44, p=0.24; interaction term: F3,23=5.14, p=0.007; figure 3). Brood size manipulation had no significant effect on nestling reserves, and by extension on short-term needs (mean±s.e. residual body mass of nestlings raised in reduced and enlarged broods was 1.59±1.00 and −0.43±0.64, respectively; t=1.70, p=0.10, d.f.=25), and there was no significant relationship between nestling residual body mass and UV skin chroma (r=0.17, p=0.40, n=27). Ultraviolet light reflected by the skin of swift nestlings did not vary significantly with the time of breeding (r=0.01, p=0.95, n=27).
Figure 2.
Relationship between nestling PC1 index and body skin UV chroma. PC1 index is the first principal component of a principal component analysis, with body mass and skeletal size as loading factors. High PC1 index indicates that offspring were large and heavy for their age. (a) Alpine swift nestlings issued from reduced and enlarged broods (closed and open symbols, respectively). (b) European starling nestlings issued from first broods (closed symbols and dashed line) and second broods (open symbols and solid line).
Figure 3.
Mean±95% CIs skin reflectance in swift nestlings raised in reduced (filled bars) and enlarged broods (open bars). Contrasts indicate that skin reflectance is significantly higher (p=0.026) in nestlings raised in reduced than enlarged broods in the UV part of the spectra only.
In the starling, there was a significant interaction between brood and PC1 score on UV skin chroma (ANOVA with brood as a factor and PC1 score as a covariate: brood effect: F1,32=12.71, p=0.0012; PC1 score: F1,32=14.01, p=0.0007; interaction: F1,32=17.22, p=0.0002), with UV skin chroma of 6-day-old nestlings being significantly and positively correlated with PC1 score in second broods (r=0.78, p=0.0018, n=13; figure 2b) but not in first broods (r=−0.09, p=0.70, n=23; figure 2b). Interestingly, although nestlings had a lower PC1 score in second broods compared to first broods (−0.94±0.29 versus 0.65±0.23; t=4.26, p=0.0002, d.f.=34) after controlling for stature, UV skin chroma was higher in second broods compared to first broods (least square means after controlling for PC1 score: 3.10±0.18 versus 2.36±0.11). Altogether, these results indicate that UV skin chroma is not an absolute measure of nestling stature, and thus information content of UV skin colour can change with the season. Offspring raised in first and second broods had similar residual body mass (t=0.64, p=0.53, d.f.=34), and there was no significant relationship between residual body mass and UV skin chroma in first (r=0.36, p=0.09, n=23) and second broods (r=−0.04, p=0.89, n=13; ANOVA with brood as a factor and residual body mass as a covariate: brood effect: F1,33=0.32, p=0.58; residual body mass: F1,33=2.71, p=0.11; interaction was not significant (p=0.36) and dropped from the final model).
(b) Parental favouritism towards UV-blocked and control nestlings
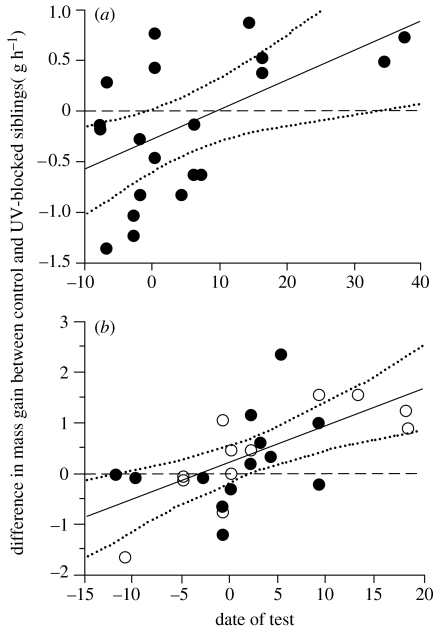
In agreement with our prediction, we found that early-breeding swifts supplied food preferentially to UV-blocked nestlings, whereas late-breeding parents favoured control siblings (figure 4a). In the starling, parental favouritism towards UV-blocked and control nestlings over time differed between first and second broods (ANOVA with brood as a factor and date of test as a covariate: brood effect: F1,53=0.05, p=0.82; date of test: F1,53=0.04, p=0.85; interaction: F1,53=6.53, p=0.014). Starlings raising their second broods showed a similar seasonal change in food allocation towards control offspring (figure 4b). Parental favouritism towards UV-blocked and control nestlings did not differ between years (p=0.75), and thus the effect of year was dropped from our final model (presented above). Interestingly, we did not detect parental favouritism towards skin UV signals in starlings raising their first broods (F1,30=1.43; p=0.24). The fit of polynomial regressions to swift and starling data provides similar results (statistics not presented).
Figure 4.
Difference in body mass gain (g h−1) between siblings that were treated with a petroleum jelly containing or not containing ultraviolet-light blocker (UV-blocked and control nestlings, respectively) in relation to breeding time. Negative values on the y-axis indicate that UV-blocked nestlings gained more body mass than control nestlings during the experimental period, and vice versa for positive values. (a) Alpine swift broods: linear regression: F1,18=7.68, p=0.013, r2=0.30. (b) Second broods of European starling in 2003 and 2004 (open and closed symbols, respectively): F1,23=14.47, p=0.0009, r2=0.39. Date of test is the number of days deviating from the yearly median. Linear regressions are shown with 95% CIs.
4. Discussion
We found that the body skin of swift and starling nestlings, two distantly related species, strongly reflects light in the UV range suggesting that UV reflectance by the bodies of altricial nestlings might be found in numerous species (Jourdie et al. 2004). In addition, as far as we are aware, our findings show for the first time that brightness of UV skin reflectance by swift and starling nestlings is related to their stature (and by extension their long-term needs), and that biases in food allocation towards nestlings presenting signal intensities revealing large (control nestlings) or small (UV-blocked nestlings) stature changes as the season progresses. This suggests that parents may use contextdependent food allocation strategies to maximize their yearly reproductive success (Davis et al. 1999). By feeding disproportionably small offspring, early breeders may take advantage of time and food availability to maximize the number of offspring at fledging. Conversely, late-breeders may minimize the risks of complete brood failure by feeding large nestlings, and thus trading-off offspring number against condition. Our results imply that higher mortality in latehatching broods reported in numerous studies might not only reflect differences in parental quality (Daunt et al. 1999; Arnold et al. 2004) and/or food availability (Brinkhof & Cave 1997), but also alternative parental decision strategies. Although scramble competition in nestlings can play an important role in food allocation within the brood (Mock & Parker 1997; Parker et al. 2002), seasonal bias in parental favouritism from small (UV-blocked) towards large (control) nestlings was detected both in the swift, where jostling for position was prevented by separating offspring with a Plexiglas barrier, and in the starling, where nestlings were allowed to jostle. This suggests that parents can control resource allocation early in nestling development. Finally, it is interesting to note that, in first broods in the starling, we found no significant relationship between nestling stature and UV skin reflectance, as well as no significant change in parental allocation decisions in relation to nestling UV skin reflectance. This result points out that the signalling contents in skin coloration changed with the season, and that parents were cueing on nestling UV skin reflectance only when it honestly reflected offspring stature.
The mechanisms by which nestling stature affects UV skin reflectance are yet unknown. Prum & Torres (2003) have demonstrated that in adult birds UV skin reflectance is produced by organized arrays of parallel collagen fibres in the dermis, with UV reflectance being determined by collagen fibre size and inter-fibre spacing. They point out that although structural coloration in avian skin is apparently permanent once developed, subtle changes in UV reflectance can result from derma shrinkage caused by dehydration and derma growth due to expansion in collagen array size. This mechanism suggests that in nestlings UV reflectance can vary with age and development, and that climatic (seasonal) factors may disrupt/enhance the signalling content of skin UV reflectance. Future work should be focused upon effects of age, origin and environment on development of collagen arrays in nestling dermis.
Current models of parent–offspring interactions assume (Parker & Macnair 1979; Parker 1985) or predict (Godfray 1995) fixed parental responses to offspring solicitation signals. Our study reveals that parent–offspring interactions are shaped by a visual signal in a context-dependent manner. It may provide a mechanism accounting for difficulties faced in demonstrating handicaps associated with honest signalling (Kilner & Johnstone 1997; Kilner 2001; Royle et al. 2002). The study of parental decision making in relation to condition-dependent signals provides a promising avenue for exploring the evolution of multiple signals used in parent–offspring interactions (Kilner 2002; Royle et al. 2002), and should enhance our understanding of the various food allocation strategies reported in nature (reviewed in Wright & Leonard 2002).
Acknowledgments
We thank Violaine Jourdie for her help in the field in 2003, Philippe Maillan for providing the petroleum jelly, Heinz Richner for kindly lending us his spectrometer, the late Theo Mabot for admittance to the Alpine swift colony and Etienne Danchin, Innes Cuthill and two anonymous referees for comments on the manuscript. This research was financed by a professorship grant from the ‘Fondation de famille Sandoz’ to P.H., and grants from the Swiss National Science Foundation to P.H. (grants 31-102104/1) and P.B. (PP00A-109009).
References
- Andersson S, Õrnborg J, Andersson H. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc. R. Soc. B. 1998;265:445–450. 10.1098/rspb.1998.0315 [Google Scholar]
- Ardia D.R. Super size me: an experimental test of the factors affecting lipid content and the ability of residual body mass to predict lipid stores in nestling European starlings. Funct. Ecol. 2005;19:414–420. 10.1111/j.1365-2435.2005.00997.x [Google Scholar]
- Arnold J.M, Hatch J.J, Nisbet I.C.T. Seasonal declines in reproductive success of the common tern Sterna hirundo: timing or parental quality? J. Avian Biol. 2004;35:33–45. 10.1111/j.0908-8857.2004.03059.x [Google Scholar]
- Bize, P. & Roulin, A. In press. No experimental evidence that sibling competition induces young to switch nest in the colonial Alpine swift Apus melba Anim. Behav
- Bize P, Roulin A, Tella J.L, Bersier L.F, Richner H. Additive effects of ectoparasites over the reproductive attempts in the long-lived alpine swifts. J. Anim. Ecol. 2004;73:1080–1088. 10.1111/j.0021-8790.2004.00880.x [Google Scholar]
- Brinkhof M.W.G, Cave A.J. Food supply and seasonal variation in breeding success: an experiment in the European coot. Proc. R. Soc. B. 1997;264:291–296. 10.1098/rspb.1997.0041 [Google Scholar]
- Daunt F, Wanless S, Harris M.P, Monaghan P. Experimental evidence that age-specific reproductive success is independent of environmental effects. Proc. R. Soc. B. 1999;266:1489–1493. 10.1098/rspb.1999.0805 [Google Scholar]
- Davis J.N, Todd P.M, Bullock S. Environment quality predicts parental provisioning decisions. Proc. R. Soc. B. 1999;266:1791–1797. 10.1098/rspb.1999.0848 [Google Scholar]
- Godfray H.C.J. Signalling of need by offspring to their parents. Nature. 1991;352:328–330. 10.1038/352328a0 [Google Scholar]
- Godfray H.C.J. Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 1995;146:1–24. 10.1086/285784 [Google Scholar]
- Heeb P, Schwander T, Faoro S. Nestling detectability affects parental feeding preferences in a cavity nesting bird. Anim. Behav. 2003;66:637–642. 10.1006/anbe.2003.2238 [Google Scholar]
- Jourdie V, Moureau B, Bennett A.T.D, Heeb P. Ultraviolet reflectance by the skin of nestlings. Nature. 2004;431:262. doi: 10.1038/431262a. 10.1038/431262a [DOI] [PubMed] [Google Scholar]
- Kilner R. When do canary parents respond to nestling signals of need? Proc. R. Soc. B. 1995;260:343–348. [Google Scholar]
- Kilner R. A growth cost of begging in captive canary chicks. Proc. Natl Acad. Sci. USA. 2001;98:11 394–11 398. doi: 10.1073/pnas.191221798. 10.1073/pnas.191221798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner R. The evolution of complex begging displays. In: Wright J, Leonard M.L, editors. The evolution of begging. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. pp. 87–106. [Google Scholar]
- Kilner R, Johnstone R.A. Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol. Evol. 1997;12:11–15. doi: 10.1016/s0169-5347(96)10061-6. 10.1016/S0169-5347(96)10061-6 [DOI] [PubMed] [Google Scholar]
- Kölliker M, Richner H, Werner I, Heeb P. Begging signals and biparental care: nestling choice between parental feeding locations. Anim. Behav. 1998;55:215–222. doi: 10.1006/anbe.1997.0571. 10.1006/anbe.1997.0571 [DOI] [PubMed] [Google Scholar]
- Kölliker M, Brinkhof M.W.G, Heeb P, Fitze P.S, Richner H. The quantitative genetic basis of offspring solicitation and parental response in a passerine bird with biparental care. Proc. R. Soc. B. 2000;267:2127–2132. doi: 10.1098/rspb.2000.1259. 10.1098/rspb.2000.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard M.L, Horn A.G. Begging calls and parental feeding decisions in tree swallows (Tachycineta bicolor) Behav. Ecol. Sociobiol. 2001;49:170–175. 10.1007/s002650000290 [Google Scholar]
- Martin T.E. Food as a limit on breeding birds: a lifehistory perspective. Annu. Rev. Ecol. Syst. 1987;18:453–487. 10.1146/annurev.es.18.110187.002321 [Google Scholar]
- Martins T.L.F, Wright J. Brood reduction in response to manipulated brood sizes in the common swift (Apus apus) Behav. Ecol. Sociobiol. 1993;32:61–70. 10.1007/BF00172224 [Google Scholar]
- Mock D.W, Parker G.A. Oxford University Press; Oxford, UK: 1997. The evolution of sibling rivalry. [Google Scholar]
- Parker G.A. Models of parent–offspring conflict. V. Effects of the behaviour of the two parents. Anim. Behav. 1985;33:519–533. [Google Scholar]
- Parker G.A, Macnair M.R. Models of parent offspring conflict. IV. Suppression: evolutionary retaliation by the parent. Anim. Behav. 1979;27:1210–1235. 10.1016/0003-3472(79)90068-X [Google Scholar]
- Parker G.A, Royle N.J, Hartley I.R. Begging scrambles with unequal chicks: interactions between need and competitive ability. Ecol. Lett. 2002;5:206–215. 10.1046/j.1461-0248.2002.00301.x [Google Scholar]
- Price K, Ydenberg R. Begging and provisioning in broods of asynchronously-hatched yellow-headed blackbird nestlings. Behav. Ecol. Sociobiol. 1995;37:201–208. 10.1007/s002650050182 [Google Scholar]
- Price K, Harvey H, Ydenberg R. Begging tactics of nestling yellow-headed blackbirds Xanthocephalus xanthocephalus, in relation to need. Anim. Behav. 1996;51:421–435. 10.1006/anbe.1996.0039 [Google Scholar]
- Prum R.O, Torres R. Structural colouration of avian skin: convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 2003;206:2409–2429. doi: 10.1242/jeb.00431. 10.1242/jeb.00431 [DOI] [PubMed] [Google Scholar]
- Royle N.J, Hartley I.R, Parker G.A. Begging for control: when are offspring solicitation behaviours honest? Trends Ecol. Evol. 2002;17:434–440. 10.1016/S0169-5347(02)02565-X [Google Scholar]
- Wright J, Leonard M.L. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2002. The evolution of begging. [Google Scholar]