Abstract
Mechanisms of chronic pain, including neuropathic pain, are poorly understood. Upregulation of voltage-gated calcium channel (VGCC) α2δ1 subunit (Cavα2δ1) in sensory neurons and dorsal spinal cord by peripheral nerve injury has been suggested to contribute to neuropathic pain. To investigate the mechanisms without the influence of other injury factors, we have created transgenic mice that constitutively overexpress Cavα2δ1 in neuronal tissues. Cavα2δ1 overexpression resulted in enhanced currents, altered kinetics and voltage-dependence of VGCC activation in sensory neurons; exaggerated and prolonged dorsal horn neuronal responses to mechanical and thermal stimulations at the periphery; and pain behaviors. However, the transgenic mice showed normal dorsal horn neuronal responses to windup stimulation, and behavioral responses to tissue-injury/inflammatory stimuli. The pain behaviors in the transgenic mice had a pharmacological profile suggesting a selective contribution of elevated Cavα2δ1 to the abnormal sensations, at least at the spinal cord level. In addition, gabapentin blocked VGCC currents concentration-dependently in transgenic, but not wild-type, sensory neurons. Thus, elevated neuronal Cavα2δ1 contributes to specific pain states through a mechanism mediated at least partially by enhanced VGCC activity in sensory neurons and hyperexcitability in dorsal horn neurons in response to peripheral stimulation. Modulation of enhanced VGCC activity by gabapentin may underlie at least partially its antihyperalgesic actions.
Keywords: Calcium channels, Neuropathic pain, Transgenic mice, Spinal hyperexcitability
1. Introduction
Nerve injury-induced pain, or neuropathic pain, is a debilitating disorder affecting millions of people per year. Clinically, it is difficult to manage neuropathic pain, reflecting both a limited understanding of the disorder, and a lack of selective agents with which to target putative mechanisms. Symptoms of neuropathic pain can persist beyond the disappearance of its originating clinical pathologies indicating long-term changes in processes underlying sensory perception. These changes may include sprouting of uninjured fibers in the periphery and the central nervous system, changes in the distribution and activation states of ion channels and receptors within both injured and uninjured afferent neurons, and changes in the expression pattern of different proteins, including neurotransmitters, receptors and ion channels in neuronal and non-neuronal tissues. However, a causal link of these changes to the initiation and maintenance of neuropathic pain is not clear. This is particularly true for the α2δ-1 subunit (Cavα2δ1) of the voltage-gated calcium channel (VGCC). We and others have demonstrated that this subunit is dramatically upregulated in dorsal root ganglion (DRG) neurons and spinal dorsal horn following nerve injury (Luo et al., 2001; Newton et al., 2001; Costigan et al., 2002; Wang et al., 2002; Valder et al., 2003), and this increase is highly correlated with the genesis and maintenance of neuropathic pain behaviors (Luo et al., 2001, 2002; Li et al., 2004).
The Cavα2δ1 is a heavily glycosylated structural subunit important for functional assembly of the VGCC in the cell membrane (Scott et al., 1991; Williams et al., 1992; Dolphin et al., 1999; Kang et al., 2002). Altered Cavα2δ1 expression can lead to changes in calcium channel current density, thereby affecting sensory information processing. However, nerve injury may also alter expression of other genes and the activation states of other functional proteins, which may also contribute to neuropathic pain development and maintenance. Thus, a causal link between the nerve-injury-induced increase in the Cavα2δ1 subunit and neuropathic pain remains elusive.
To define the contribution of VGCC Cavα2δ1 subunit to pain processing without the complication from other factors associated with injury, we have generated transgenic (TG) mice over-expressing the Cavα2δ1 subunit under the control of the thy-1 promoter (Vidal et al., 1990; Feng et al., 2000). We examined the behavioral responses of the TG mice and their wild-type (WT) littermates to different types of stimuli and their sensitivity to different classes of drugs with analgesic and antihyperalgesic properties. In addition, we characterized the effects of Cavα2δ1 overexpression in VGCC properties on isolated DRG neurons, and evoked dorsal horn neuronal responses to mechanical and thermal stimulation from the periphery.
2. Materials and methods
2.1. Transgenic mice
The TG mice over-expressing the Cavα2δ1 gene were generated as described previously (Feng et al., 2000). The mouse brain Cavα2δ1 cDNA (Genbank Accession No. U73484) was cloned into the transgene vector down-stream of a 6.5 kb murinethy-1.2 gene extending from the promoter region to the intron after exon 4 without exon 3 and its flanking introns. Thy-1 is a member of the immunoglobulin superfamily that is expressed in both neuronal and non-neuronal tissues, including thymocytes (Gordon et al., 1987). Deletion of exon 3 and its flanking introns has been shown to abolish expression in nonneuronal cells (Vidal et al., 1990). Only adult male TG mice and WT littermates were used for the experiments. All the mice appeared normal with respect to grooming, social interactions and feeding, and showed no signs of abnormality or any obvious motor defects, tremor, seizure, or ataxia. All animal care and experiments were performed according to protocols approved by the Institutional Animal Care Committees of the University of California, Irvine, the University of Maryland, Baltimore, and the University College London.
2.2. Western blot
Pulverized frozen tissues were extracted with lysis buffer (50 mM Tris-HCl buffer, pH 7.5, containing 0.5% Triton X-100, 150 mM NaCl, 1 mM EDTA) containing protease inhibitors. Equal amounts of total proteins from each sample were applied to electrophoresis in NuPAGE Tris-acetate gels (Invitrogen, Carlsbad, CA) under reducing conditions, then electrophoretically transferred to the nitrocellulose membranes. After blocking nonspecific binding with 5% non-fat milk for 1 h at room temperature, the membranes were incubated with antibodies against proteins of interest in PBS containing 0.1% Tween 20 for 1 h at room temperature or overnight at 4 °C. The dilutions of the antibodies were: anti-Cavα2δ1 antibody, 1:2000 (monoclonal, Sigma, St. Louis, MO); anti-Cava1B antibody, 1:200 (polyclonal, Exalpha Biologicals, Inc., Boston, MA); anti-Cavb3 antibody, 1:300 (polyclonal, Sigma, St. Louis, MO). The antigen-antibody complexes were detected by incubating the membranes with horseradish peroxidase labeled secondary antibodies followed by substrate addition. The blots were stripped and re-blotted with primary antibodies against different proteins or glyceraldehyde-3-phosphate dehydrogenase for loading control (GAPDH, monoclonal, 1:10,000. Ambion, Austin, TX), which was not changed due to Cavα2δ1 overexpression and other manipulations on the mice. Under reducing conditions, the δ-peptide separates from the α2 subunit so the positive bands detected by the primary antibody for Cavα2δ1 reflect the α2 subunit only. Data quantification was performed with luminescent detection (Kodak Image Station 2000MM) or densitometry within the linear range of the CCD camera or X-ray films, respectively. Changes in band densities between WT and TG samples were calculated after taking the ratio of protein bands of interest over GAPDH bands for normalization of loading.
2.3. Behavioral assays
After 1 h acclimation, mouse behavioral response to mechanical stimulation was tested as described previously (Luo et al., 2001). The 50% paw withdrawal thresholds (PWT) to calibrated von Frey filament (Stoelting, Wood Dale, IL) stimulation were determined using a modified up-down method of Dixon (1980). A series of filaments with buckling weight between 0.04 and 2.0 g were applied with a bending force to plantar surface of the hindpaw, starting with a 0.4 g filament. Paw lifting within 5 s was considered a positive response and led to the use of the next weaker filament. Absence of a paw lifting after 5 s led to the use of the next filament with increasing weight. The 50% paw withdrawal threshold was calculated from the resulting scores of six measurements starting from the one prior to the first change in response; or until four consecutive positive (assigned a score of 0.01 g) or three consecutive negative (assigned a score of 3.0 g) responses had occurred. Except for mice with ipsilateral spinal nerve ligation in which PWT was compared between the ligation side and the contralateral side, averaged paw withdrawal threshold from both sides of hindpaws was used for comparing behavioral sensitivities between WT and TG mice, and before and after drug treatments.
The mouse hindpaw withdrawal latencies (PWL) from a thermal stimulation in mice were measured in a modified Hargreaves-type thermal testing device (Hargreaves et al., 1988). Briefly, after acclimation for at least 30 min in individual boxes on the glass surface of a hot box (University of California, San Diego) maintained at 30 °C, the radiant light source underneath the glass surface was aligned to the plantar surface of the hindpaw. Activation of the light source activated a timer, and paw withdrawal from the light source or 20 s of light stimulation turned off the light bulb and timer. Unless indicated, averaged escape latency from both hindpaws was used for comparing sensitivities between WT and TG mice, and before and after drug treatments.
For the formalin test (Dubuisson and Dennis, 1977), 20 ll of 2% formalin was injected subcutaneously into the plantar surface of right hindpaw of WT and TG mice after at least 1 h acclimation in individual transparent cylinders. This induced two phases of spontaneous behaviors: flinching and lifting/licking. The flinching responses were recorded automatically from 0 to 60 min post formalin injection in an automated nociception analyzer (University of California, San Diego) and lifting/licking was recorded by a digital camcorder and counted manually. The numbers of flinching/min or lifting/licking per 5 min were compared.
For carrageenan-induced inflammation and mechanical hyperalgesia (Tao et al., 2004), the paw withdrawal thresholds to mechanical stimulation were measured before and at designated time points post intraplantar injections of 20 ll carrageenan (1% w/v) into the right hindpaw of WT and TG mice after at least 1 h of acclimation. The degree of inflammation induced by carrageenan was determined by measuring the thickness of right hindpaws before and at designated time points post injection with a caliper. The same amount of sterile saline was injected similarly into control WT and TG mice as control.
2.4. Drug administration
Working solutions of gabapentin, ketorolac and morphine were prepared in sterile saline before intrathecal injection between lumbar regions 5 and 6 (in 5 ll) (Inoue et al., 2004) or intraperitoneal injection. Behavioral testing started 1 h post drug treatment unless indicated.
2.5. Immunohistostaining
Lumbar spinal cord and DRG were dissected from deeply anesthetized mice and fixed in 4% paraformaldehyde. Paraffin-embedded spinal cord and DRG sections (5 μm, Pathology core of the University of California, Irvine) were treated with a citrate buffer (pH 6.0) followed by 3% H2O2 in PBS, washed and blocked with 1% bovine serum albumin and 10% normal goat serum, then incubated with the monoclonal antibodies against the Cavα2δ1 subunit (Sigma, Saint Louis, MO) overnight at 4 °C. After incubation with biotinylated secondary antibody conjugated to horseradish peroxidase and then avidin-biotin complex solution (Vectastain Elite ABC kit, Vector Laboratories), the sections were developed in diaminobenzidine-H2O2 solution, washed, and mounted on slides, air-dried, dehydrated and coverslipped with Permount. Omission of primary antibodies from some sections was used as control. The positive staining in sections was examined and images were taken under a microscope.
2.6. Electrophysiological recordings in isolated DRG neurons
Lumbar DRG of adult mice were enzymatically treated and mechanically dispersed as described (Jiang et al., 1998), except that the ganglia were bubbled in carbogen (5% CO2, 95% O2) during the 20 min collagenase treatment. DRG neurons were plated onto laminin/ornithine-coated glass coverslips and incubated for 2 h in MEM containing 50 lM cadmium, 10% fetal bovine serum at 37 °C, 90% humidity, and 3% CO2. Neurons were then transferred to an L-15 based medium containing 10% fetal bovine serum, and stored at room temperature before recording. All recording was performed within 8 h of harvesting ganglia.
Whole-cell patch voltage-clamp recordings were performed using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Data were filtered with a 4-pole Bessel filter and digitized. Series resistance <12 MΩ was compensated (>80%) by using amplifier circuitry. Only data obtained from neurons in which uncompensated series resistance resulted in voltage-clamp errors of less than 5 mV were used. A P/4 protocol was used for leak subtraction. Electrode and bath solutions were formulated so as to eliminate voltage-gated sodium, potassium currents as well as calcium activated potassium and chloride currents. Activation and deactivation rates were determined with a 40 ms voltage step while that of inactivation was determined with a 400 ms voltage step (data not shown). The current-voltage relationship was used to assess peak inward current for each cell, which was evoked between -20 and 0 mV. To validate conductance measurements based on estimates of current reversal potential (i.e., conductance , where I is membrane current, Vtest, is the potential at which current was evoked and Vrev is the current reversal potential), we compared derived conductance-voltage relationship to that based on tail currents (i.e., an instantaneous G-V); both methods yielded similar results (data not shown).
Barium (5 mM) was used as a charge carrier in place of calcium to limit run-down associated with the use of calcium as a charge carrier (Jiang et al., 1998). The bath solution contained 130 mM choline chloride, 5 mM BaCl2, 0.6 mM MgCl2, 10 mM Hepes, and 10 mM glucose (pH 7.4 adjusted with Tris base and osmolarity 325 milliosmolar adjusted with sucrose). The electrode solution contained 110 mM Cs-methansulfonate, 30 mM TEA-Cl, 1 mM CaCl2, 5 mM MgCl2, 11mM EGTA, 10 mM Hepes, 2 mM Mg-ATP, and 1 mM Li-GTP (pH 7.2 adjusted with Tris base and osmolarity 310 milliosmolar adjusted with sucrose). Patch pipettes filled with electrode solution had resistances of 1.5-3 MX.
Ba2+ currents were evoked from a holding potential of 70 mV. Conductance-voltage curves were constructed for each neuron from I to V curves generated by assessing peak current evoked with 40-ms voltage steps between -80 and +60 mV taken at every 5 mV. Reversal potential was determined by interpolating between inward and outward currents. Conductance was determined by dividing peak current by driving force. To estimate maximal conductance, conductance voltage-curves were fitted with a modified Boltzmann equation: Conductance ; where gmax is maximal conductance, Vm is membrane potential, V½ is the potential at which conductance is half of maximal and k is a slope factor. Current activation was fitted with a single exponential equation to determine the activation time constant. Tail currents, evoked following membrane repolarization to 70 mV, following 40 ms depolarizing voltage-steps to potentials ranging between -25 and +40 mV, were fitted with a single exponential equation to determine the deactivation time constant. Concentration-response data were fitted with a modified Hill equation: Percent inhibition = maximal inhibition/; where, drug, gabapentin concentration; EC50, gabapentin concentration leading to 50% maximal inhibition; N, Hill coefficient.
2.7. Electrophysiological recordings in dorsal horn neurons
Animals under anesthesia were placed in a stereotaxic frame and a laminectomy was performed to expose the L3-L6 spinal segments. Extracellular recordings were made from single wide dynamic range neurons using parylene coated tungsten electrodes (A-M Systems, USA). Neurons, visualized on an oscilloscope, were isolated and discriminated on a spike amplitude and waveform basis. All neurons had receptive fields over the hindpaw. Depth of the neuron was measured from the surface of the spinal cord. On isolation of a neuron any spontaneous activity was quantified over a period of at least 100 s in the absence of any evoked stimulus.
Electrical stimulation was given via two needles inserted into the receptive field and the evoked activity was characterized and quantified as follows. A train of 16 stimuli was given (2 ms pulse duration, 0.5 Hz at three times the C-fiber threshold), and the evoked neuronal responses were quantified. The ‘input’ (non-potentiated response), and the ‘calculated windup’ (potentiated response, evident by increased neuronal excitability to repeated stimulation) were determined. Input = [action potentials (50-800 ms) evoked by first pulse at 3 times C-fiber threshold] × total number of pulses (16). Wind-up = [total action potentials (50-800 ms) after 16 train stimulus at 3 time C-fiber threshold] -Input. Windup is also represented in curve form, displayed as spikes per stimulus of the electrical train.
A wide range of natural stimuli, including von Frey filaments (0.6, 1, 2, 5, 9, 15, 30, 75 g), heat (27, 32, 35, 40, 45, 48 and 50 °C water jet), were applied to the receptive field for 10 s per stimulus. Evoked neuronal firing was quantified and expressed as evoked firing over the 10 s stimulation, the total evoked firing, evoked firing after cessation of stimulus and total time spent firing. Data were captured and analyzed by a CED 1401 interface coupled to a Pentium computer with Spike 2 software (Cambridge Electronic Design; PSTH and rate functions). Unfortunately the effect of gabapentin on these responses could not be assessed as the stability of mouse in vivo electrophysiology recordings is not sufficient for repeated test stimuli and dose-responses.
The receptive field area was mapped on standard diagrams of the projected area of the plantar surface of the paw to a pinch stimulus. Diagrams were copied to plain copier paper (80 g/m2) and marked areas were cut out and weighed. Receptive field sizes were measured as the weight of the particular area and expressed as a percentage of the mean weight of 10 control diagrams of the whole paw (79.0 ± 0.5 mg).
2.8. Statistical analyses
Data were reported as means ± SEM. Unpaired Student’s t tests were performed where significance was indicated by two-tailed p values:*p <0.05.
3. Results
3.1. Neuronal Cavα2δ1 overexpression induces abnormal sensations
Data from Western blot analysis indicated that Cavα2δ1 protein levels were elevated in forebrain, cortex, hippocampus, cerebellum, spinal cord, and DRG of the TG mice compared with WT littermates (Fig. 1a and b). Similar increases in neuronal Cavα2δ1 expression were observed in a different mouse line (data not shown) showing similar behavioral sensitivity (see results of Fig. 2), but not in non-neuronal tissues (Fig. 1c). Relative Cavα2δ1 expression levels varied among different tissues from the transgenic mice. Endogenous Cavα2δ1 proteins from non-DRG neuronal tissues of the WT mice differed from that in the DRG as indicated by the faster migration of the former in Western blot, consistent with findings in rat tissues (Luo, 2000). Cavα2δ1 species with different migration rates were also observed in different non-neuronal tissues (Fig. 1c). Interestingly, heterogeneous Cavα2δ1 expression was observed in some TG tissues, and most prominently in DRG where two bands were observed. Since the upper band can be eliminated by dorsal rhizotomy (Li et al., 2004), it is likely representative of Cavα2δ1 proteins at the pre-synaptic central terminals of DRG neurons. Since the transgene did not contain introns for alternative splicing, these Cavα2δ1 species were likely derived from post-translational modifications, including different glycosylation as indicated in previous studies (Luo, 2000). In TG spinal cord samples, the increases in Cavα2δ1 proteins were more dramatic in the faster migrating band than that in the slower migrating band, which has a similar migration rate as the endogenous DRG Cavα2δ1 proteins. Expression of other neuronal-type VGCC sub-units, such as the channel forming Cava1B and intracellular Cavβ3 subunits, was not changed in the TG mice.
Fig. 1.
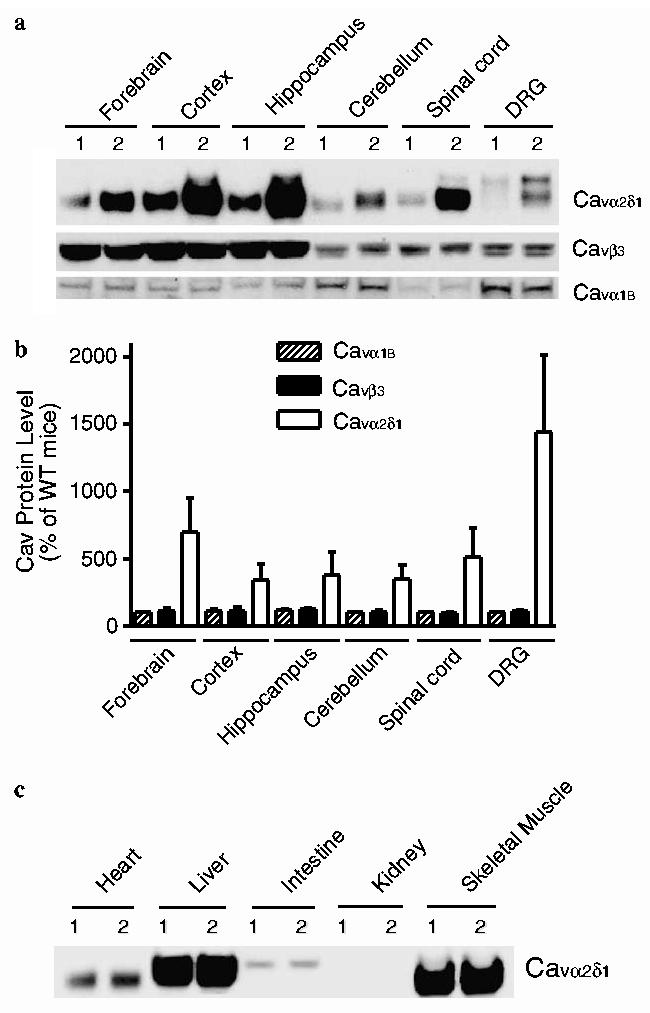
Enhanced neuronal Cavα2δ1 expression in the TG mice. Western blot analyses were performed on neuronal and non-neuronal tissues isolated from adult WT and TG mice as described in Section 2. (a) Representative Western blot data of three independent determinations showing selective increases of Cavα2δ1 subunit expression in neuronal tissue samples of adult TG mice. Cavα1B-VGCC alpha-1B subunit. Cavβ3-VGCC beta-3 subunit. (b) Summarized Western blot data presented as means ± SEM from three independent determinations shown in (a). (c) Representative Western blot data of two independent determinations showing Cavα2δ1 subunit expression in non-neuronal tissue samples of adult TG mice were not changed compared with those in the WT mice. 1, WT mice; 2, TG mice.
Fig. 2.
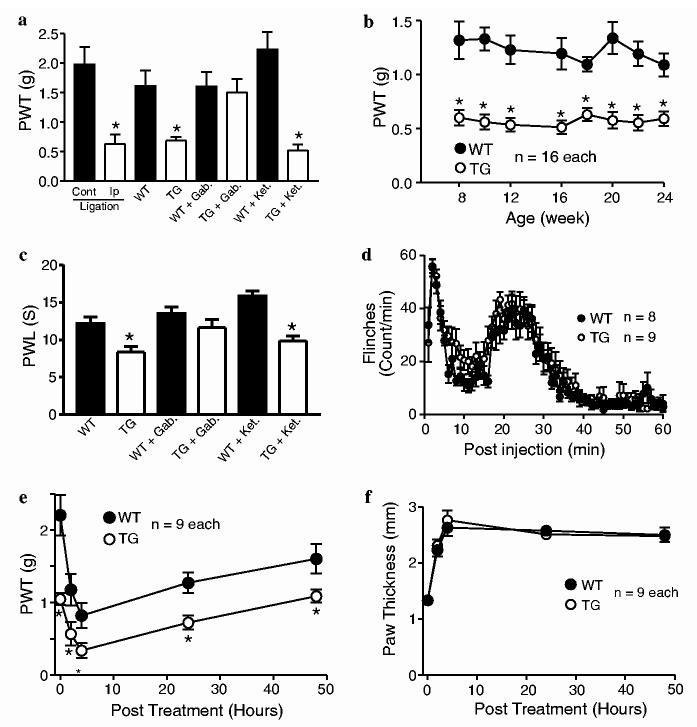
Specific pain behaviors in the TG mice. Responses to mechanical, thermal and inflammatory stimuli in male adult WT and TG mice were examined as described in Section 2. (a) Comparisons of paw withdrawal thresholds (PWT) to mechanical stimulation from mice with one-week (left) L5 spinal nerve ligation, TG, and WT mice with or without drug treatments. TG mice showed reduced PWT to mechanical stimulation compared with WT mice to a level similar to that seen after one-week L5 spinal nerve ligation in wild-type mice (left two columns). Gabapentin (Gab., 50 mg/kg, i.p.), but not ketorolac (Ket., 7.5 mg/kg, i.p.), could reverse the allodynic state in the TG mice. N = 8-10 in each group. (b) Durations of tactile allodynia in the TG mice. The tactile allodynia state in the TG mice lasted for at least six months, the longest duration tested. (c) Comparisons of paw withdrawal latency (PWL) to thermal stimulation in WT and TG mice with or without drug treatments. TG mice showed reduced PWL to thermal stimulation (thermal hyperalgesia) that could be blocked by gabapentin (Gab, 50 mg/kg, i.p.), but not ketorolac (Ket, 7.5 mg/kg, i.p.), treatment. N = 16 in each group. (d) Comparisons of formalin-induced responses between WT and TG mice. TG and WT mice showed similar flinching responses in the formalin test. (e) Comparisons of PWT to mechanical stimulation between WT and TG mice after carrageenan-induced inflammation. TG and WT littermates showed similar onset and duration of tactile allodynia after carrageenan, but not saline (data not shown), injections. (f) Comparisons of inflammatory responses to carrageenan injection between WT and TG mice. TG and WT littermates showed similar onset and duration of paw thickness (inflammatory response) after carrageenan, but not saline (data not shown), injections. All data are presented as means ± SEM. *p <0.05 compared with WT or contralateral side of spinal nerve ligated mice.
Elevated Cavα2δ1 expression in TG mice resulted in abnormal nociceptive responses. Hindpaw withdrawal thresholds to mechanical stimulation were significantly reduced (tactile allodynia), to a level similar to that seen in WT mice with one-week spinal nerve ligation (Fig. 2a). The tactile allodynia state lasted for six months, the longest time tested (Fig. 2b). Paw withdrawal latencies to noxious thermal stimulation were also significantly reduced (thermal hyperalgesia) in TG mice compared with WT littermates (Fig. 2c). A similar behavioral phenotype was observed in another TG line with similarly enhanced Cavα2δ1 expression (data not shown), suggesting that altered nociceptive processing in the TG mice did not reflect disruption of a host gene by random transgene incorporation.
While enhanced Cavα2δ1 expression resulted in dramatic changes in the response to acute stimuli, there were no detectabl changes in response to inflammatory stimuli: the onset and duration of behavioral responses to intradermal formalin and carrageenan (Fig. 2d and e) and the inflammatory response to carrageenan injection (Fig. 2f) were not significantly altered in the TG mice compared with WT littermates. Interestingly, the carrageenan-, but not formalin-, induced hyperalgesic state is additive to that induced by elevated Cavα2δ1 expression in the TG mice (Fig. 2e). Intradermal saline injections in the WT and TG mice were without effects (data not shown).
3.2. Spinal Cavα2δ1 subunit contributes to the abnormal sensations
To evaluate specifically the contribution of elevated Cavα2δ1 in spinal cord and DRG to abnormal sensations in the TG mice, we assessed the localization of Cavα2δ1 proteins in DRG and spinal cord and the influence of intrathecal gabapentin, an antihyperalgesic drug that binds to the Cavα2δ1 subunit (Gee et al., 1996; Marais et al., 2001), on tactile allodynia in the TG mice. In WT samples, Cavα2δ1 immunoreactivity was low in both spinal cord and DRG, in agreement with data in the Western blots (Fig. 1), but detectable in mainly small DRG neurons (Fig. 3a), neurons and axons in the dorsal (Fig. 3c,e,g) and ventral (not shown) spinal cord. Cavα2δ1 immunoreactivity in DRG and spinal cord of the TG mice was much higher in DRG neurons of different sizes (Fig. 3b), in superficial (Fig. 3d) and deep dorsal horn (Fig. 3h) neurons, axons entering the superficial dorsal horn (Fig. 3f), in addition to ventral horn neurons and associated structures (data not shown).
Fig. 3.
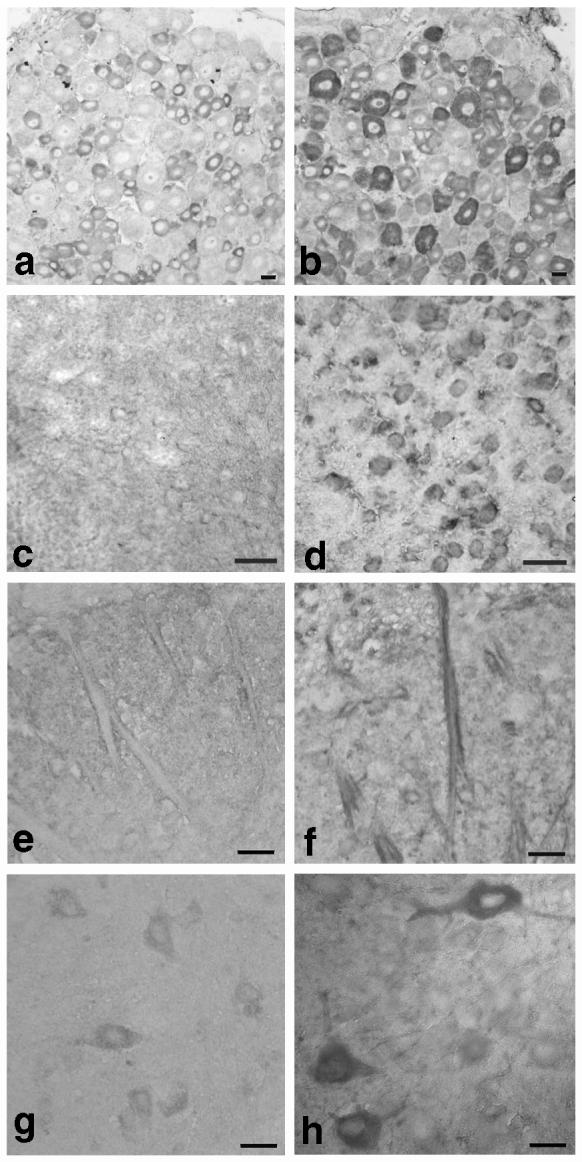
Cavα2δ1 immunohistostaining in lumbar spinal dorsal horn and DRG sections from WT and TG mice. Immunohistostaining with antibodies against the Cavα2δ1 subunit was performed in sections of lumbar spinal cord and DRG of adult WT and TG mice as described in Section 2. (a) WT DRG. (b) TG DRG. (c) WT superficial dorsal horn (outer). (d) TG superficial dorsal horn (outer). (e) WT superficial dorsal horn (inner). (f) TG superficial dorsal horn (inner). (g) WT deep dorsal horn. (h) TG deep dorsal horn. Each pair of images were representative images processed simultaneously and from at least three independent determinations. Scale bar = 20 μM.
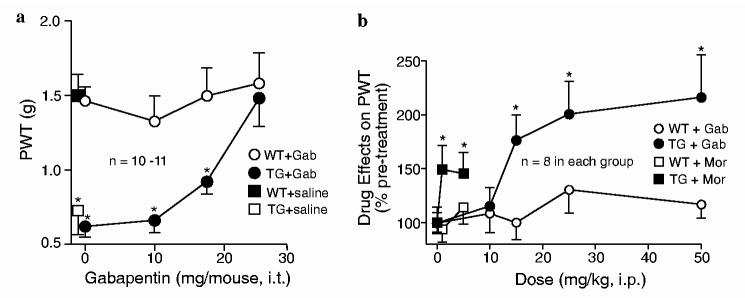
Intrathecal gabapentin dose-dependently reversed the tactile allodynia in the TG mice without significantly altering the paw sensitivity to mechanical stimulation in the WT mice (Fig. 4a). This gabapentin-sensitive allodynic state in the TG mice is similar to that observed in spinal nerve-injured animals (Hwang and Yaksh, 1997; Luo et al., 2001, 2002; Li et al., 2004), and suggests that a similar mechanism mediated at least partially by elevated Cavα2δ1 subunit at the spinal cord level underlies these hypersensitivity states.
Fig. 4.
Dose-dependent reversal of tactile allodynia by gabapentin in the TG mice. The effects of gabapentin and morphine on behavioral sensitivity of adult WT and TG mice to mechanical stimulation were analyzed before and after drug administrations as described in Section 2. (a) Intrathecal gabapentin dose-dependently reversed tactile allodynia in the TG mice. (b) Gabapentin (Gab) had better efficacy than morphine (Mor) in allodynia reversal in the TG mice. All data are presented as means ± SEM from numbers of mice indicated. *p <0.05 compared with WT littermates.
3.3. Pharmacological profiles of the abnormal sensations in the transgenic mice support the involvement of Cavα2δ1 mediated pathway(s)
To test the sensitivity of abnormal sensations in the TG animals to different classes of compounds known to have anti-hyperalgesic effects on human patients and experimental animals, we compared the efficacies of gabapentin, morphine, and ketorolac on the allodynic and thermal hyperalgesic states of the TG mice. Intra-peritoneal administration of gabapentin (50 mg/kg) reversed the tactile allodynia (Fig. 2a) and thermal hyperalgesia (Fig. 2c) in the TG mice. The effects of gabapentin were dose-dependent with an estimated ED50 value ∼13 mg/kg (Fig. 4b). Intraperitoneal ketorolac, a cyclooxygenase inhibitor used to treat inflammation and related pain conditions, at a dose that has been shown to exhibit analgesic efficacy (7.5 mg/kg) (Domer, 1990), failed to reverse the tactile allodynia and thermal hyperalgesia states in the TG mice (Fig. 2a and c). Intraperitoneal morphine could only partially reverse tactile allodynia at the dose of 1 mg/kg, and failed to further reduce allodynia at a higher dose (5 mg/kg) (Fig. 4b). All these drugs did not affect significantly the baseline tactile responses in the WT animals. Thus, the hypersensitive states in the TG mice have a pharmacological profile consistent with a mechanism mediated by pathway(s) involving elevated Cavα2δ1 subunit.
3.4. Increased Cavα2δ1 expression results in an increased current density and altered gating properties of high threshold VGCC in DRG neurons
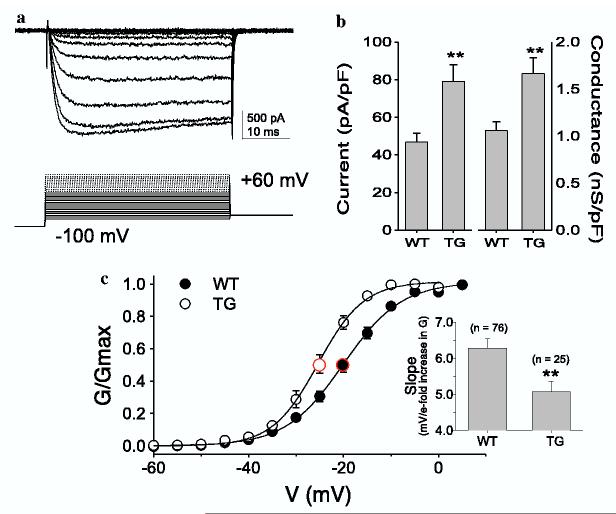
To assess the impact of increased Cavα2δ1 on current density and biophysical properties of VGCC, we employed whole-cell voltage-clamp techniques to study voltage-gated barium currents (IBa) in acutely isolated DRG neurons from the TG and WT mice (Fig. 5a). Given that nociceptive afferents generally arise from small DRG neurons, we assessed the impact of enhanced Cavα2δ1 expression on barium current in small diameter neurons (i.e., <27 μm). Both the peak inward current density (Fig. 5b, left) and maximal conductance (Fig. 5b, right) were significantly increased in small DRG neurons from TG mice compared to those in small DRG neurons from WT mice. This increase in conductance was associated with a 5 mV hyperpolarizing shift in the potential for half activation (V0.5 = -20 ± 1 mV and -25 ± 1 mV for WT and TG neurons, respectively. Fig. 5c). This shift in V0.5 was largely due to an increase in the voltage-dependence of activation as it was associated with a decrease in the slope of the conductance-voltage curve (inset Fig. 5c) with little change in thresholds.
Fig. 5.
Increased currents and shifted voltage-dependence of activation of VGCC in TG sensory neurons. Whole-cell patch voltage-clamp recordings of barium currents were performed in acutely isolated DRG neurons from WT and TG mice as described in Section 2. (a) Whole-cell current evoked from a 15.6 pF neuron obtained from a TG mouse. Only traces to -10 mV (peak inward current) are shown for clarity. (b) Both the current density (left), and maximal conductance (right) were significantly increased in TG neurons (n = 25) compared with WT neurons (n = 76). (c) The voltage-dependence of barium current activation was shifted in the hyperpolarizing direction (by ∼5 mV) in TG neurons, which was associated with a significant decrease in the slope of the G-V curve (inset). **p <0.01 compared with WT neurons. All data are presented as means ± SEM from numbers of neurons indicated.
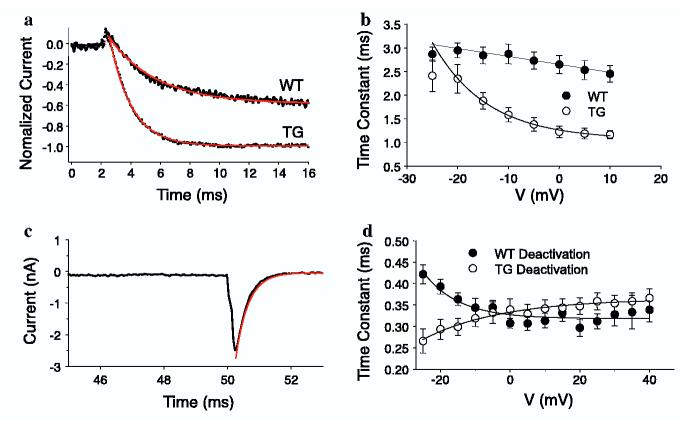
The increased Cavα2δ1 subunit in the TG mice also caused a significant increase in IBa activation rate at voltages > -20 mV compared with that observed in currents from WT neurons, as assessed with an exponential fit of the increasing phase of voltage-gated IBa following membrane depolarization (Fig. 6a). Interestingly, IBa activation rate became voltage dependent in TG neurons in contrast to the minimal voltage-dependence of IBa in WT neurons (Fig. 6a and b). While there was no dramatic difference between WT and TG neurons in deactivation rates of high threshold IBa (assessed with an exponential fit of the decay phase of the tail current,Fig. 6c), the deactivation rate of evoked IBa was slowed with increased membrane depolarization in TG neurons, but increased with membrane depolarization in WT neurons (Fig. 6d). IBa inactivation was similar in DRG neurons from TG and WT mice (data not shown). The increases in current and maximal conductance are consistent with results from heterologous expression systems where the Cavα2δ1 subunit has been shown to increase membrane density of Cava1 subunits (Gurnett et al., 1996; Walker and De Waard, 1998; Dolphin et al., 1999) as well as increase open channel probability (Shistik et al., 1995; Walker and De Waard, 1998).
Fig. 6.
Increased activation rate, development of a voltage-dependence to activation, and shifted voltage-dependence of deactivation in TG sensory neurons. Barium current activation and deactivation time constants were analyzed in DRG neurons from WT and TG mice as described in Section 2. (a) Current activation was fitted with a single exponential equation (in red) in order to determine the activation time constant. Both traces were normalized to the peak inward current obtained during a 40 ms test pulse to -10 mV. (b) The activation time constant for currents evoked at potentials between -25 and +10 mV was determined for each neuron in each group and pooled from TG (n = 11) and WT (n = 12) neurons. (c) Representative tail current from a transgenic neuron showing how tail currents, evoked following membrane repolarization to 70 mV, following 40 ms depolarizing voltage-steps to potentials ranging between -25 and +20 mV, were fitted with a single exponential equation (curve in red) in order to determine the deactivation time constant. The first 35 ms of the current trace has been omitted for clarity. (d) Deactivation time constant data pooled from TG (n = 11) and WT (n = 12) neurons. All data are presented as means ± SEM.
3.5. Increased Cavα2δ1 expression mediates gabapentin inhibition of high threshold VGCC current in DRG neurons
To assess the impact of increased Cavα2δ1 expression on the efficacy of gabapentin directly, we examined the effects of gabapentin on voltage-gated IBa in WT and TG DRG neurons (Fig. 7). While the inhibitory effects of gabapentin on high threshold voltage-gated IBa in WT DRG neurons were minimal (Fig. 7b), gabapentin significantly inhibited peak inward IBa in a concentration-dependent fashion with an EC50 2 lM in TG neurons. The inhibitory effects of gabapentin ∼ were not due to current run-down since the current amplitudes in the WT neurons were stable for the duration of the recording (Fig. 7c). The insensitivity of the acutely isolated adult WT neurons to gabapentin is in contrast to the gabapentin sensitivity seen in neonatal sensory neurons maintained in culture conditions for over four days (Sutton et al., 2002). In addition to developmental and other factors, culture conditions, which are known to influence sensory neuron morphology and gabapentin-mediated inhibition on VGCC currents (Martin et al., 2002), might have contributed to the discrepancy.
Fig. 7.
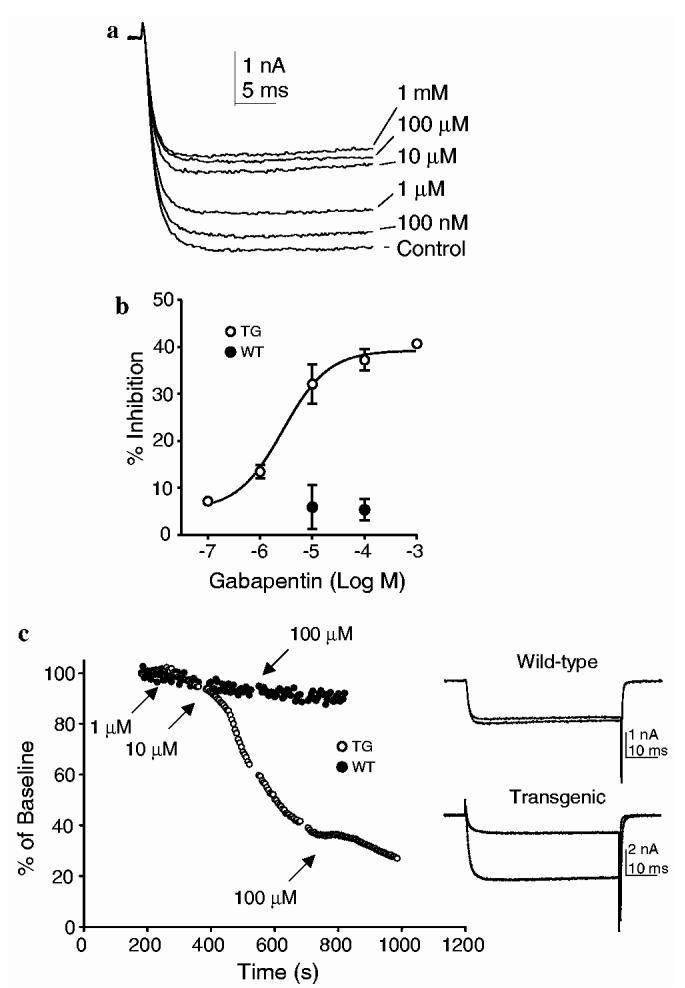
Concentration-dependent attenuation of high threshold voltage-gated barium currents in TG sensory neurons by gabapentin. The effects of gabapentin on barium currents from acutely isolated DRG neurons of WT and TG mice were analyzed as described in Section 2. (a) Raw current evoked at -5 mV from a TG neuron before (control) and after addition of increasing concentrations of gabapentin. (b) % inhibition data [(current in presence of gabapentin/current in absence of gabapentin) × 100] fitted by a modified Hill equation and plotted as function of gabapentin concentrations. EC50 = 2.0 μM. Hill coefficient = 0.8. N = 4 for TG neurons except for the group of 10 μM (n = 21). N = 6-10 for WT neurons. All data are presented as means ± SEM. (c) Representative current amplitude and time plot derived from data taken from WT (closed symbols) and TG (open symbols) neurons. Neurons were held at -70 mV. VGCC currents were evoked every 5 s with a 50 ms voltage step to -5 mV. Peak inward current versus time was plotted. Time of gabapentin application is indicated. 100 μM gabapentin was added sooner to the WT neuron, in the absence of a response to the 10 μM concentration. Current traces are examples from each neuron evoked at baseline (bottom trace in each pair) and following application of 100 μM of gabapentin (top trace in each pair).
3.6. Increased Cavα2δ1 expression results in exaggerated evoked-responses and prolonged post-stimulation firing of dorsal horn neurons to mechanical and thermal stimulation through a mechanism independent of windup
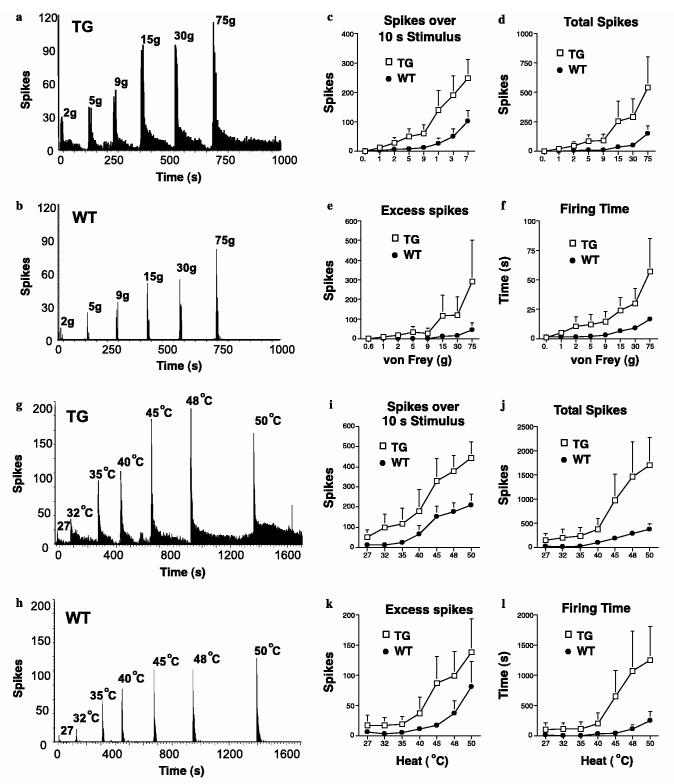
To evaluate the functional influence of elevated Cavα2δ1 expression, we compared dorsal horn neuronal responses over a wide range of electrical and natural, mechanical and thermal, peripheral stimulations in TG and WT mice. Compared with dorsal horn neurons from the WT mice, TG dorsal horn neurons exhibited exaggerated responses to non-noxious and noxious mechanical and thermal stimulation in an intensity-dependent manner, shown not only as increased responses to the applied stimulus but also as prolonged firing after cessation of the stimuli (Fig. 8). This increase in response to stimuli was reflected by increases in neuronal spikes for stimulus duration, total evoked spikes, evoked firing time and excess post-stimulation firing after mechanical (Fig. 8a-f) and thermal (Fig. 8g-l) stimulation, respectively. Interestingly, as we have reported previously (Suzuki et al., 2003), the basal responses of WT neurons to thermal stimulation were always higher than those to mechanical stimulation in almost all the measurements. Importantly, the TG neurons responded to low levels of mechanical stimulation (von Frey forces 1-5 g) with a level of excitability similar to that seen in WT neurons evoked by higher and presumed noxious stimulation. This leftward shift in the neuronal responses reflected remarkably well the reduced mechanical thresholds for behavioral withdrawal. A similar hyper-responsibility of spinal neurons to thermal stimulation with a higher excitability level over the warm and noxious range was also observed (compare Fig. 8a-f with g-l). The increase of firing varied considerably among the transgenic neurons, probably due to variations in the Cavα2δ1 expression levels.
Fig. 8.
Exaggerated and prolonged responses to mechanical and thermal stimulations in TG dorsal horn neurons. Extracellular recordings were performed in single wide dynamic neurons in the spinal dorsal horn of WT and TG mice before and after mechanical or thermal stimulations applied to the receptive fields over the hindpaw as described in Section 2. (a and b) Representative traces of evoked dorsal horn neuronal responses from TG (a) and WT (b) mice after von Frey filament stimulations. (c-f) Summarized data from evoked dorsal horn neuron responses shown in (a) and (b). (g) and (h) Representative traces of evoked dorsal horn neuronal responses from TG (g) and WT (h) mice after thermal stimulations. (i-l) Summarized data from evoked dorsal horn neuron responses as shown in (g) and (h). Data presented are means ± SEM from N = 11 in each groups.
To determine whether there is a difference in dorsal horn neuronal response to windup stimulation as well, we compared the dorsal horn neuronal responses to transcutaneous electrical stimulation after discrete activation of C-fiber afferents (windup). As indicated in Fig. 9, TG dorsal horn neurons responded to peripheral electrical stimulation to elicit windup in a similar way as the WT dorsal horn neurons as represented by the calculated wind-up and wind-up curves. This would suggest that the spinal neurons in the TG mice are not inherently more excitable and that the enhanced responses to mechanical and thermal stimulation are due to altered excitability from afferents onto the spinal neurons. Furthermore, receptive field areas were not significantly different between WT and TG mice (TG = 23%, WT = 17% of whole hindpaw) indicative of a lack of increased intrinsic spinal excitability in TG animals.
Fig. 9.
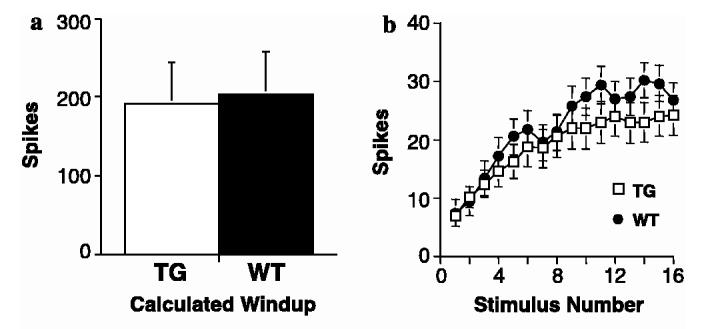
Similar windup responses in TG dorsal horn neurons. Evoked neuronal responses to windup electrical stimulation applied to the receptive fields were recorded with extracellular recordings in spinal wide dynamic neurons of WT and TG mice as described in Section 2. (a) Calculated windup responses (see Section 2) from the TG and WT mice. Numbers of spikes from each neuron were pooled and the data are presented as means ± SEM from N = 11 in each group. (b) Windup curves displayed as mean number of spikes per stimulus ±SEM from N = 11 in each group.
4. Discussion
To determine whether increased expression of the calcium channel Cavα2δ1 subunit is sufficient to mediate abnormal sensations associated with nerve injury, we generated TG mice with enhanced Cavα2δ1 expression in neuronal tissues. In general, the TG mice were indistinguishable from their WT littermates and had normal motor functions, appearance, growth rate, and fertility. However, the TG mice exhibited hypersensitivity to light touch (tactile allodynia) and thermal stimulation (thermal hyperalgesia) that was associated with a pharmacological profile consistent with the involvement of specific pathways related to elevated Cavα2δ1 expression. Data from in vitro and in vivo recordings have indicated that transgenic DRG neurons have increased densities, altered kinetics and voltage-dependence of VGCC that correlate with exaggerated dorsal horn neuronal responses to stimuli, with no detectable changes to windup stimulation and in receptive fields. Together, these data indicate that elevated Cavα2δ1 contributes to abnormal sensations through modulation of sensory neuron VGCC activity and dorsal horn neuronal responses to peripheral stimulation.
Several lines of evidence support that increased Cavα2δ1 subunit mediates abnormal sensations through a mechanism similar to that of neuropathic, but not tissue injury and inflammatory, pain. First, parallel changes in the Cavα2δ1 subunit levels and behavioral sensitivity are similar to those post-nerve injury. Second, Cavα2δ1 overexpression has additive effects on carrageenan-induced hyperalgesia and no influence on formalin test, suggesting that the inflammatory and formalininjury pain states are likely mediated by mechanisms independent of Cavα2δ1 upregulation. This is consistent with the notion that formalin-induced nociception occurs too rapidly to involve Cavα2δ1 upregulation, and carrageenan injection is not associated with DRG and spinal cord Cavα2δ1 upregulation when hyperalgesia was evident (data not shown). Third, behavioral changes associated with Cavα2δ1 overexpression in the TG mice have a pharmacological profile similar to that of neuropathic pain with Cavα2δ1 induction (Luo et al., 2002), rather than that of acute inflammatory pain. Gabapentin, which is efficacious in neuropathic pain, but not ketorolac, an anti-inflammatory agent, attenuates the abnormal sensations in the TG mice. While the efficacy of gabapentin in attenuating acute nociception after for-malin injection and inflammation varies substantially (Field et al., 1997; Shimoyama et al., 1997; Werner et al., 2001; Urban et al., 2005), emerging data indicate that gabapentin’s anti-hyperalgesic actions depend on the activation states of target tissue under different pathological conditions (Patel et al., 2000; Maneuf et al., 2001; Fehrenbacher et al., 2003). Thus, gabapentin’s actions in these pain states are likely mediated by target proteins independent of elevated Cavα2δ1 (Suzuki et al., 2005). Finally, morphine is less efficacious in reversing allodynia than gabapentin in the TG mice. It is likely that presynaptic opioid receptors that are mainly expressed on unmyelinated C-, but not myelinated A-, fiber terminals, are not regulated in the TG mice, so that presynaptically morphine only inhibits C-fiber mediated neurotransmission (Pirec et al., 2001), a less predominant pathway than that mediated by the Cavα2δ1 in sensation processing in the TG mice. This is supported by emerging data indicating that combination of morphine and gabapentin provides a better pharmacological efficacy in neuropathic pain animal models and patients (Matthews and Dickenson, 2002; Gilron et al., 2005) than each drug alone.
Our findings in the TG mice support that elevated Cavα2δ1 at the spinal level can contribute to hypersensitivity states without the influence from other nerve injury factors. Since other compensatory changes that may occur during the development of the TG mice are unlikely the same as that associated with nerve injury, the elevated Cavα2δ1 is highly likely the common factor associated with similar behavioral hypersensitivity in both models. Although the abnormal sensations in the TG mice could be mediated by elevated Cavα2δ1 subunit in different areas of the central nervous system as shown in our Western blots, the hyperexcitability was observed at the spinal level in that spinal neurons had normal windup and receptive field sizes but markedly enhanced natural responses. The exaggerated and prolonged dorsal horn neuronal responses to stimuli, and the dose-dependent allodynia reversal by spinal gabapentin in the TG mice all suggest that a spinal action mediated by elevated Cavα2δ1 is contributing, at least partially, to the behavioral and neuronal changes.
It is important to point out that non-noxious mechanical stimulation in the TG mice elicits exaggerated and prolonged dorsal horn neuronal responses in all measurements at a level at least similar to or exceeding that seen during noxious stimulation in the WT littermates. These modality-coded responses and prolonged firing are characteristics of a neuron in pain processing and indicate that elevated Cavα2δ1 is contributing to the enhanced spinal excitability. A marked feature of the neuronal responses in the TG mice was the pronounced level and abnormal duration of firing to a given stimulus. Interestingly, higher total brush-evoked pain intensity with increased duration of aftersensation to low levels of mechanical stimuli has been reported in neuropathic pain patients. The relationship between evoked pain and altered temporal-spatial stimulus parameters during allodynia induction in patients appears to relate to the neuronal changes observed here (Samuelsson et al., 2005).
One of the limitations in using the transgenic mice was that we were unable to determine the exact locus of elevated Cavα2δ1 subunit that contributed to the abnormal sensations. However, our data indicated that responses to windup stimulation and receptive field sizes were similar in both groups of mice, suggesting that the spinal wide dynamic neurons were not inherently more excitable. The enhanced and prolonged responses to stimuli in dorsal horn neurons mirrored the extent of the reduced behavioral thresholds, and thus likely due to altered excitability from afferents onto the spinal neurons. This fits well with increased Cavα2δ1 expression in transgenic DRG neurons and axons entering the dorsal horn, as well as increased peak inward current density, maximal conductance, altered activation and deactivation of VGCC seen in small DRG neurons in vitro. Since Cavα2δ1 subunit can modulate different VGCC subtypes (Bangalore et al., 1996; Gurnett et al.,1996; Qin et al., 1998; Shirokov et al., 1998; Dolphin et al., 1999; Mould et al., 2004), which are differentially distributed at different tissues and even different locations within a neuron (Wu et al., 1999; Heinke et al., 2004), elevated Cavα2δ1 subunit may mediate abnormal sensations by modulating different VGCC functions pre-synaptically. For example, an increase in low threshold T-type currents may contribute to bursting activity in DRG neurons following nerve injury (White et al., 1989), and facilitate activity and calcium-dependent long-term potentiation at spinal synapses of nociceptive fibers (Ikeda et al., 2003). Similarly, an increase in high threshold VGCC currents and excitability at the pre-synaptic terminals may cause an increase in excitatory transmitter release (Malcangio et al., 2000; Wallin and Schott, 2002), which is sensitivity to tonic inhibition by GABAB receptor activation (Marvizon et al., 1999), a mechanism different from NMDA-receptor mediated substance P release post-formalin injection (Chaplan et al., 1997; Liu et al., 1997; Honor et al., 1999). This difference may explain at least partially why responses to the formalin test are not altered in the TG mice.
However, our current data do not allow us to separate the relative influence of elevated Cavα2δ1 subunit on different types of VGCC. We hypothesize that increased whole cell calcium currents in the TG mice may derive from increased expression of subpopulations of VGCC disproportional to that of endogenous VGCC, leading to altered biophysical properties of calcium currents in TG neurons. This may explain why TG, but not WT, DRG neurons exhibit gabapentin sensitivity. This is consistent with other findings indicating that pathologically activated neurons and afferent fibers are more sensitive to gabapentin inhibition than the normal once (Stanfa et al., 1997; Pan et al., 1999; Patel et al., 2000; Kanai et al., 2004). Even though we did not observe a compensatory increase in the expression of the channel forming Cava1B sub-unit, it is possible that increased Cavα2δ1 subunit may increase the membrane assembly of subpopulations of VGCC by enhancing the processing of excess amounts of intracellular Cava1 subunits, which would otherwise be subjected to degradation. This is supported by findings in heterologous expression systems that Cavα2δ1 subunit can increase membrane expression of different types of VGCC (Gurnett et al., 1996; Walker and De Waard, 1998; Dolphin et al., 1999), and that different types of VGCC are expressed in DRG neurons (Fox et al., 1987; Mintz et al., 1992). Dissecting the functional influence of Cavα2δ1 overexpression on subtypes of VGCC will be critical in understanding the detail mechanisms of the Cavα2δ1-mediated abnormal sensations.
Acknowledgements
This work was supported in part by NIH Grants NS40135, DE14545 (ZD Luo), NS41384 (MS Gold), and Wellcome Trust London Pain Consortium (EM and AHD).
References
- Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS. Influence of L-type Ca channel alpha 2/delta-subunit on ionic and gating current in transiently transfected HEK 293 cells. Am J Physiol. 1996;270:H1521–8. doi: 10.1152/ajpheart.1996.270.5.H1521. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–38. [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, et al. Replicate high-density rat genome oligonu-cleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [Print 2002 Oct 2025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Dolphin AC, Wyatt CN, Richards J, Beattie RE, Craig P, Lee JH, et al. The effect of alpha2-delta and other accessory subunits on expression and properties of the calcium channel alpha1G. J Physiol. 1999;519(Pt 1):35–45. doi: 10.1111/j.1469-7793.1999.0035o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer F. Characterization of the analgesic activity of ketorolac in mice. Eur J Pharmacol. 1990;177:127–35. doi: 10.1016/0014-2999(90)90262-5. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–74. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–41. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513–22. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–76. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–34. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Chesa PG, Nishimura H, Rettig WJ, Maccari JE, Endo T, et al. Regulation of Thy-1 gene expression in transgenic mice. Cell. 1987;50:445–52. doi: 10.1016/0092-8674(87)90498-3. [DOI] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP. Dual function of the voltage-dependent Ca2+ channel alpha 2 delta subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–40. doi: 10.1016/s0896-6273(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Heinke B, Balzer E, Sandkuhler J. Pre- and postsynaptic contributions of voltage-dependent Ca2+ channels to nociceptive transmission in rat spinal lamina I neurons. Eur J Neurosci. 2004;19:103–11. doi: 10.1046/j.1460-9568.2003.03083.x. [DOI] [PubMed] [Google Scholar]
- Honor P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, et al. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–8. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Yaksh TL. Effect of subarachnoid gabapentin on tactileevoked allodynia in a surgically induced neuropathic pain model in the rat. Region Anesth. 1997;22:249–56. doi: 10.1016/s1098-7339(06)80010-6. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–40. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–8. doi: 10.1038/nm1060. [Epub 2004 Jun 2013] [DOI] [PubMed] [Google Scholar]
- Jiang M, Gold MS, Boulay G, Spicher K, Peyton M, Brabet P, et al. Multiple neurological abnormalities in mice deficient in the G protein Go. Proc Natl Acad Sci USA. 1998;95:3269–74. doi: 10.1073/pnas.95.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai A, Sarantopoulos C, McCallum JB, Hogan Q. Painful neuropathy alters the effect of gabapentin on sensory neuron excitability in rats. Acta Anaesthesiol Scand. 2004;48:507–12. doi: 10.1111/j.1399-6576.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Kang MG, Felix R, Campbell KP. Long-term regulation of voltagegated Ca(2+) channels by gabapentin. FEBS Lett. 2002;528:177–82. doi: 10.1016/s0014-5793(02)03295-7. [DOI] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–9. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–4. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Luo ZD. Rat dorsal root ganglia express distinctive forms of the alpha2 calcium channel subunit. Neuroreport. 2000;11:3449–52. doi: 10.1097/00001756-200011090-00010. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, et al. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, et al. Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–75. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Jones MG, McMahon SB. Abnormal substance P release from the spinal cord following injury to primary sensory neurons. Eur J Neurosci. 2000;12:397–9. doi: 10.1046/j.1460-9568.2000.00946.x. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Hughes J, McKnight AT. Gabapentin inhibits the substance P-facilitated K+-evoked release of [3H] glutamate from rat caudal trigeminal nucleus slices. Pain. 2001;93:191–6. doi: 10.1016/S0304-3959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F. Calcium channel alpha2delta subunits-structure and gabapentin binding. Mol Pharmacol. 2001;59:1243–8. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K, et al. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel subunit expression. Neuropharmacology. 2002;42:353–66. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Grady EF, Stefani E, Bunnett NW, Mayer EA. Substance P release in the dorsal horn assessed by receptor internalization: NMDA receptors counteract a tonic inhibition by GABA(B) receptors. Eur J Neurosci. 1999;11:417–26. doi: 10.1046/j.1460-9568.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Dickenson AH. A combination of gabapentin and morphine mediates enhanced inhibitory effects on dorsal horn neuronal responses in a rat model of neuropathy. Anesthesiology. 2002;96:633–40. doi: 10.1097/00000542-200203000-00020. [DOI] [PubMed] [Google Scholar]
- Mintz IM, Adams ME, Bean BP. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Mould J, Yasuda T, Schroeder CI, Beedle AM, Doering CJ, Zamponi GW, et al. The alpha2delta auxiliary subunit reduces affinity of omega-conotoxins for recombinant N-type (Cav2.2) calcium channels. J Biol Chem. 2004;279:34705–14. doi: 10.1074/jbc.M310848200. [Epub 32004 May 34727] [DOI] [PubMed] [Google Scholar]
- Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res Mol Brain Res. 2001;95:1–8. doi: 10.1016/s0169-328x(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Pan HL, Eisenach JC, Chen SR. Gabapentin suppresses ectopic nerve discharges and reverses allodynia in neuropathic rats. J Pharmacol Exp Ther. 1999;288:1026–30. [PubMed] [Google Scholar]
- Patel MK, Gonzalez MI, Bramwell S, Pinnock RD, Lee K. Gabapentin inhibits excitatory synaptic transmission in the hyperalgesic spinal cord. Br J Pharmacol. 2000;130:1731–4. doi: 10.1038/sj.bjp.0703530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirec V, Laurito CE, Lu Y, Yeomans DC. The combined effects of N-type calcium channel blockers and morphine on A delta versus C fiber mediated nociception. Anesth Analg. 2001;92:239–43. doi: 10.1097/00000539-200101000-00046. [DOI] [PubMed] [Google Scholar]
- Qin N, Olcese R, Stefani E, Birnbaumer L. Modulation of human neuronal alpha 1E-type calcium channel by alpha 2 delta-subunit. Am J Physiol. 1998;274:C1324–31. doi: 10.1152/ajpcell.1998.274.5.C1324. [DOI] [PubMed] [Google Scholar]
- Samuelsson M, Leffler AS, Hansson P. Dynamic mechanical allodynia: on the relationship between temporo-spatial stimulus parameters and evoked pain in patients with peripheral neuropathy. Pain. 2005;115:264–72. doi: 10.1016/j.pain.2005.03.001. [Epub 2005 Apr 2019] [DOI] [PubMed] [Google Scholar]
- Scott RH, Pearson HA, Dolphin AC. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol. 1991;36:485–520. doi: 10.1016/0301-0082(91)90014-r. [DOI] [PubMed] [Google Scholar]
- Shimoyama N, Shimoyama M, Davis AM, Inturrisi CE, Elliott KJ. Spinal gabapentin is antinociceptive in the rat formalin test. Neurosci Lett. 1997;222:65–7. doi: 10.1016/s0304-3940(97)13331-6. [DOI] [PubMed] [Google Scholar]
- Shirokov R, Ferreira G, Yi J, Rios E. Inactivation of gating currents of L-type calcium channels. Specific role of the alpha 2 delta subunit. J Gen Physiol. 1998;111:807–23. doi: 10.1085/jgp.111.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by alpha 2/delta and beta subunits in Xenopus oocytes: contribution of changes in channel gating and alpha 1 protein level. J Physiol. 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfa LC, Singh L, Williams RG, Dickenson AH. Gabapentin, ineffective in normal rats, markedly reduces C-fibre evoked responses after inflammation. Neuroreport. 1997;8:587–90. doi: 10.1097/00001756-199702100-00002. [DOI] [PubMed] [Google Scholar]
- Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol. 2002;135:257–65. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Hunt SP, Dickenson AH. The coding of noxious mechanical and thermal stimuli of deep dorsal horn neurones is attenuated in NK1 knockout mice. Neuropharmacology. 2003;45:1093–100. doi: 10.1016/s0028-3908(03)00281-8. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rahman W, Rygh LJ, Webber M, Hunt SP, Dickenson AH. Spinal-supraspinal serotonergic circuits regulating neuropathic pain and its treatment with gabapentin. Pain. 2005;117:292–303. doi: 10.1016/j.pain.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Tao F, Tao YX, Zhao C, Dore S, Liaw WJ, Raja SN, Johns RA. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience. 2004;128:421–30. doi: 10.1016/j.neuroscience.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Urban MO, Ren K, Park KT, Campbell B, Anker N, Stearns B, et al. Comparison of the antinociceptive profiles of gabapentin and 3-methylgabapentin in rat models of acute and persistent pain: implications for mechanism of action. J Pharmacol Exp Ther. 2005;313:1209–16. doi: 10.1124/jpet.104.081778. [Epub 2005 Feb 1225] [DOI] [PubMed] [Google Scholar]
- Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J. Neurochem. 2003;87:560–73. doi: 10.1046/j.1471-4159.2003.02016.x. [DOI] [PubMed] [Google Scholar]
- Vidal M, Morris R, Grosveld F, Spanopoulou E. Tissue-specific control elements of the Thy-1 gene. EMBO J. 1990;9:833–40. doi: 10.1002/j.1460-2075.1990.tb08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, De Waard M. Subunit interaction sites in voltage-dependent Ca2+ channels: role in channel function. Trends Neurosci. 1998;21:148–54. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- Wallin J, Schott E. Substance P release in the spinal dorsal horn following peripheral nerve injury. Neuropeptides. 2002;36:252–6. doi: 10.1016/s0143-4179(02)00024-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, et al. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114:529–46. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Werner MU, Perkins FM, Holte K, Pedersen JL, Kehlet H. Effects of gabapentin in acute inflammatory pain in humans. Reg Anesth Pain Med. 2001;26:322–8. doi: 10.1053/rapm.2001.25070. [DOI] [PubMed] [Google Scholar]
- White G, Lovinger DM, Weight FF. Transient low-threshold Ca2+ current triggers burst firing through an afterdepolarizing potential in an adult mammalian neuron. Proc Natl Acad Sci USA. 1989;86:6802–6. doi: 10.1073/pnas.86.17.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, et al. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science. 1992;257:389–95. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- Wu LG, Westenbroek RE, Borst JG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci. 1999;19:726–36. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]