Abstract
Vibrio cholerae, the causative agent of Asiatic cholera, has been reported to make large quantities of polyphosphate. Inorganic polyphosphate is a ubiquitous molecule with a variety of functions in prokaryotic and eukaryotic cells. We constructed a V. cholerae mutant with a deletion in the polyphosphate kinase (ppk) gene. The mutant was defective in polyphosphate biosynthesis. Deletion of ppk had no significant effect on production of cholera toxin, hemagglutinin/protease, motility, biofilm formation, and colonization of the suckling mouse intestine. The wild type and mutant had similar growth rates in rich and minimal medium and exhibited similar phosphate uptake and alkaline phosphatase induction. In contrast to ppk mutants from other gram-negative bacteria, the V. cholerae mutant survived prolonged starvation in LB medium and artificial seawater basal salts. The ppk mutant was significantly more sensitive to low pH, high salinity, and oxidative stress when it was cultured in low-phosphate minimal medium. The ppk mutant failed to induce catalase when it was downshifted to phosphorus-limiting conditions. Furthermore, the increased sensitivity of the ppk mutant to environmental stressors in phosphate-limited medium correlated with a diminished capacity to synthesize ATP from intracellular reservoirs. We concluded that polyphosphate protects V. cholerae from environmental stresses under phosphate limitation conditions. It has been proposed that toxigenic V. cholerae can survive in estuaries and brackish waters in which phosphorus and/or nitrogen can be a limiting nutrient. Thus, synthesis of large polyphosphate stores could enhance the ability of V. cholerae to survive in the aquatic environment.
Cholera is an acute diarrheal disease characterized by the passing of voluminous rice water stools. Vibrio cholerae strains belonging to serogroups O1 and O139 continue to cause seasonal cholera outbreaks that affect highly populated regions in Asia, Africa, and Latin America. The bacterium adheres to and colonizes the small intestine and secretes cholera toxin, which causes the major clinical symptoms of the disease. The quorum-sensing regulator HapR coordinates cell density-dependent expression of virulence factors (23). At a high cell density, expression of HapR switches the metabolism of V. cholerae from a “virulence mode” characterized by the expression of cholera toxin and toxin-coregulated pilus to an “exit mode” typified by expression of hemagglutinin (HA)/protease (13, 39). HA/protease in turn promotes detachment of V. cholerae to initiate new infection foci or exit from the host (5, 12).
The fate of V. cholerae outside the human host is still unresolved. V. cholerae strains belonging to non-O1 and non-O139 serogroups are known to be part of the microbial flora of estuarine and coastal waters, from which they are commonly isolated (9, 11). Interestingly, virulence factors typical of O1 and O139 pathogenic strains have also been found dispersed among non-O1 non-O139 strains isolated in areas where O1 and O139 pathogenic strains are not endemic (9). The existence of an aquatic reservoir of O1 and O139 toxigenic strains has not been established. However, the capacity of these strains to survive and persist in estuarine and brackish waters is widely accepted (9, 11). A better understanding of the mechanisms that V. cholerae employs to survive in the environment between epidemics could be important to the control of this disease.
The ability of V. cholerae to associate with phytoplankton and zooplankton and its ability to form biofilms on biotic and abiotic surfaces have been proposed to play important roles in environmental survival (16, 29). Survival in a dormant viable but not culturable stage has been proposed (37). However, it is not clear to what extent these mechanisms are found specifically in toxigenic O1 and O139 strains. Cyclical cholera outbreaks have been correlated with numerous environmental factors (15), but the genetic and biochemical determinants of the correlations remain unknown.
It has recently been reported that V. cholerae biosynthesizes very large amounts of inorganic polyphosphate (poly-P) (25). The enzyme polyphosphate kinase (PPK) catalyzes the reversible transfer of a terminal phosphate from ATP to poly-P (1, 2, 18). This enzyme can act as a nucleotide diphosphate kinase, or it can transfer a pyrophosphoryl group to GDP to generate the stringent response mediator guanosine tetraphosphate (ppGpp) (19). Escherichia coli has been shown to accumulate poly-P in response to nutritional and osmotic stresses (4, 30). An E. coli polyphosphate kinase (ppk) mutant expressed lower levels of RpoS and was more sensitive to H2O2 (4, 30). Poly-P was reported previously to be essential for long-term survival of Shigella and Salmonella spp. (17). Although rpoS mutants of V. cholerae (and other members of the genus Vibrio) are more sensitive to starvation, osmotic, and oxidative stresses (14, 20, 26, 38), inactivation of the V. cholerae ppk gene did not reveal stress-related phenotypes (25). Finally, inorganic poly-P has been shown to affect motility in several bacterial pathogens, including E. coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, V. cholerae, and Salmonella spp (28).
Recently, V. cholerae was reported to make 100 times more poly-P than E. coli makes in response to extracellular phosphate (25). The presence of large poly-P stores in V. cholerae was explained by reduced expression of poly-P exophosphatase (3, 25). Surprisingly, despite the accumulation of large amounts of poly-P, the V. cholerae ppk mutant exhibited very few altered phenotypes compared to ppk mutants of E. coli, Salmonella, and Pseudomonas (25). This raises an intriguing question. Why does V. cholerae makes such large amounts of poly-P?
In the present study we addressed this question by constructing a V. cholerae mutant with a deletion in the ppk gene. The ppk mutant was significantly more sensitive to low pH, salinity, or H2O2 in minimal medium with a low phosphate content. In this study we investigated the mechanism of this phenomenon, and below we discuss the potential significance of this finding to the ecology of cholera.
MATERIALS AND METHODS
Strains and media.
V. cholerae C6709-1 (El Tor, Peru, 1991 isolate) was used as the wild-type strain to construct a ppk mutant. V. cholerae AC-W66 (32, 34) and AJB41 (33) are lacZ- and relA-defective derivatives of C6709-1, respectively. Strain AJB2 contains a hapA-lacZ fusion integrated into the chromosome of AC-W66 (32). E. coli strains TOP10 (Invitrogen), SEY327λpir, and SM10λpir (24) were grown in LB medium. V. cholerae was grown in LB medium or in MOPS (morpholinepropanesulfonic acid) minimal medium (Teknova Laboratories) containing 0.4% d-glucose (MOPS-G medium) supplemented with 2 mM or 0.1 mM inorganic phosphate (K2HPO4). When required, ampicillin was added to LB medium at a concentration of 0.1 mg/ml.
Construction and analysis of a V. cholerae Δppk deletion mutant.
Chromosomal DNA was purified from V. cholerae C6709-1 using a QIAGEN DNeasy kit. A DNA fragment containing sequences 5′ of the ppk open reading frame (ORF) was amplified with primers 5′-AAAGCATGCACGCAAATACAGGGTC and 5′-GTTGGATCCCGCTTCTTGTAATACG using an Advantage 2 PCR kit (BD Biosciences Clontech). A second fragment containing sequences immediately 3′ of the ppk ORF was amplified using primers 5′-GAAAGGATCCGCAGAAAAGTACGCT and 5′-GCAGAGCTCAAACGGATATCGGAAT. The amplicons were sequentially cloned in pUC19 as SacI-BamHI and BamHI-SphI fragments and were confirmed by DNA sequencing. A SacI-SphI fragment containing chromosomal sequences flanking the ppk gene was subcloned in pCVD442 (8) digested with the enzymes mentioned above. The resulting suicide vector was constructed in SEY327λpir, transferred to strain SM10λpir, and mobilized to V. cholerae C6709-1. Exconjugants were selected in LB medium plates containing ampicillin and polymyxin B (100 U/ml). The ampicillin-sensitive segregant AJB37 was isolated by sucrose selection. DNA from AJB37 was purified as described above, digested with EcoRI, and analyzed by Southern hybridization. A SacI-SphI DNA fragment flanking the ppk ORF was labeled with digoxigenin (DIG High Prime; Roche Applied Science) and used as a hybridization probe. In addition, chromosomal DNA flanking the ppk locus was amplified from strain AJB37 using primers 5′-ATTCGGTGATCTATATTGCGCTCCA and 5′-GATTCGCGCGCAAGATATTTTACTG. The amplicon was cloned in pUC19 and sequenced using M13 forward and reverse primers.
Virulence studies.
Cholera toxin contents were measured by a GM1 enzyme-linked immunosorbent assay as described previously (31). Production of HA/protease was detected using an azocasein assay (5, 13) and was expressed in azocasein units per unit of optical density at 600 nm (OD600) of the culture. For motility, strains were stabbed into LB medium containing 0.3% agar (swarm agar). Biofilm formation was measured by the crystal violet staining method (39). To compare the abilities of our wild type and the ppk mutant to colonize the suckling mouse intestine, mixtures (1:1) of C6709-1 and AC-W66 and of AJB37 and AC-W66 were orally inoculated into 4- to 5-day-old CD-1 mice. After 16 h of incubation mice were sacrificed by cervical dislocation, and the small intestine was homogenized in phosphate-buffered saline (pH 7.4) and plated on LB agar containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (20 μg/ml). The competitive index was calculated from the ratio of the mutant to the wild type after intraintestinal growth divided by the input ratio.
Measurement of inorganic phosphate uptake.
Phosphate uptake was measured as described by Ogawa et al. (25). Briefly, V. cholerae was grown in LB medium for 24 h at 37°C, and the cells were collected by centrifugation, washed with phosphate-free MOPS-G minimal medium, and resuspended in 5 ml of the same medium at an OD600 of 0.1. The cells were then incubated for 2 h at 37°C in a rotary shaker (250 rpm). Carrier-free 32P-labeled K2HPO4 (Amersham Biosciences) was added to a specific activity of 20 μCi/ml, and the preparation was incubated as described above. Samples (0.1 ml) were taken at different times, and the cells were collected on 0.45-μm Costar Spin-X centrifuge tube filters (Corning Inc.). The filters were washed three times with phosphate-free MOPS-G medium, and the radioactivity was measured by Cherenkov counting with a Beckman Coulter LS 6500 scintillation counter.
Measurement of poly-P biosynthesis.
Poly-P biosynthesis was assessed by measuring the incorporation of 32P into high-molecular-weight poly-P, binding to glassmilk (Qbiogene, California), and thin-layer chromatography (TLC) (4). V. cholerae was grown in LB medium for 24 h at 37°C, and cells were collected by centrifugation, resuspended in MOPS-G medium containing 0.1 mM phosphate and 20 μCi/ml of carrier-free K2H32PO4, and incubated for 4 h at 37°C as described above. After incubation, aliquots (1 ml) of the cell suspension were centrifuged and extracted with 0.5 ml of guanidine isothiocyanate in 50 mM Tris (pH 7.0) at 95°C for 5 min. The extract was then treated with 15 μl of 20% sodium dodecyl sulfate (SDS), 0.5 ml of 95% ethanol, and 5 μl of glassmilk. The tubes were incubated for 1 min with vortexing and centrifuged to pellet the glassmilk. Next, the glassmilk was washed twice with 5 mM Tris (pH 7.4)—50 mM NaCl—5 mM EDTA-50% ethanol (wash buffer), resuspended in 50 μl of 50 mM Tris (pH 7.4)—10 mM MgCl2 containing 20 μg per ml of DNase and RNase, and incubated for 15 min at 37°C. The glassmilk was subsequently washed once with 150 μl of guanidine isothiocyanate, then with 95% ethanol, and finally twice with wash buffer. Poly-P was eluted from the glassmilk with 50 μl of 50 mM Tris (pH 8.0) at 95°C for 2 min. Five microliters of the poly-P solution was applied to polyethyleneimine-cellulose F TLC plates (EMD Chemicals, Inc.) and developed with 0.75 M KH2PO4 (pH 3.5). The plates were air dried, and high-molecular-weight poly-P was visualized by autoradiography. In this assay, high-molecular-weight poly-P stayed at the origin.
Alkaline phosphatase and catalase assays.
Alkaline phosphatase was detected as described by Manoil and Beckwith (21) using p-nitrophenyl phosphate as the substrate. Briefly, cells were diluted in 50 mM Tris (pH 8.0) and permeabilized with SDS-chloroform. The reaction was started by addition of substrate and was stopped by addition of 0.5 ml of 0.1 M K2HPO4. Optical densities at 420 nm were recorded, and Miller units were calculated as described elsewhere (22). To measure catalase activity, V. cholerae was grown in MOPS-G medium containing 2 mM or 0.1 mM inorganic phosphate, and samples (5 ml) were collected at different times. Cells were collected and lysed in 0.2 ml of CelLytic B cell lysis reagent (Sigma Chemical Co.). Catalase activity was determined with a catalase assay kit (Sigma Chemical Co.) and was expressed in micromoles of H2O2 decomposed per minute per milligram of protein.
Western blot analysis.
Cells grown in MOPS-G medium containing 0.1 mM phosphate were collected by centrifugation and lysed with CelLytic B cell lysis reagent (Sigma), and the insoluble debris was removed by high-speed centrifugation. Samples were boiled in SDS-polyacrylamide gel electrophoresis loading buffer, and 15 μg of protein was loaded into each well in a 12% polyacrylamide gel. The gel was electroblotted onto a polyvinylidene difluoride membrane, and RpoS protein was detected with a rabbit anti-RpoS serum (obtained from F. Norel, Pasteur Institute, France) and peroxidase-conjugated anti-rabbit immunoglobulin G (whole molecule; Sigma).
Measurement of ATP biosynthesis.
ATP biosynthesis was measured using the firefly luciferase-based BacTiter-Glo microbial cell viability assay (Promega Corporation). Light production was standardized on the basis of the number of CFU in the reaction mixture.
Growth curves and survival assays.
Growth curves were constructed by measuring OD600 and dilution plating in LB agar. Long-term survival experiments were performed by dilution plating V. cholerae cells after prolonged incubation at 37°C in LB medium or artificial seawater (ASW) basal salts. For pH stress, salinity stress, and oxidative stress, wild-type and mutant V. cholerae strains were grown for 24 h in LB or MOPS-G medium containing 2 mM phosphate. The cells were centrifuged, washed, and resuspended in 1 volume of the appropriate medium in the presence of specific environmental stressors. At different times, viability was assessed by dilution plating on LB agar.
RESULTS
Construction of a V. cholerae Δppk deletion mutant.
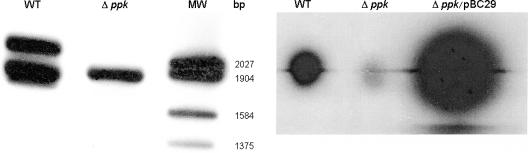
A standard approach was used to generate ppk mutant AJB37 from strain C6709-1. Southern hybridization showed that AJB37 harbored a deletion that removed a unique EcoRI site located within the ppk ORF (Fig. 1, left panel). To confirm deletion of the ppk gene, we cloned a DNA fragment from AJB37 using primers flanking the ppk locus. Sequencing of the cloned DNA confirmed that 1,839 bp was deleted, which removed 87% of the ppk ORF. Figure 1 (right panel) shows that the ppk mutant was defective in poly-P biosynthesis. In order to confirm that the material which remained at the origin of the TLC plate was poly-P, a plasmid overexpressing PPK (pBC29) (3) (obtained from C. Fraley, Stanford University School of Medicine) was electroporated into strain AJB37. The complemented strain produced a large amount of poly-P that was visible as a strong signal at the TLC plate origin (Fig. 1).
FIG. 1.
Structural and functional analysis of a V. cholerae ppk mutant. (Left panel) Southern hybridization analysis. DNA from strains C6709-1 (WT) and AJB37 (ppk) was digested with EcoRI, and the fragments were separated in a 0.8% agarose gel and transferred to a positively charged nylon membrane. The membrane was hybridized with DNA flanking the ppk gene labeled with digoxigenin. MW, molecular weight markers. (Right panel) Poly-P biosynthesis. Strains C6709-1 (wild type) and AJB37 (ppk) were grown in LB medium, and incorporation of 32P into high-molecular-weight poly-P was determined by TLC as described in Materials and Methods.
V. cholerae Δppk mutant is fully virulent.
In an initial study of our ppk mutant we examined production of cholera toxin, motility, biofilm formation, HA/protease production, and colonization of the suckling mouse intestine. Strain AJB37 produced levels of cholera toxin (2.1 μg/ml/unit of OD600) similar to the levels produced by its wild type precursor (1.5 μg/ml/unit of OD600). No difference in the abilities of the wild type and the mutant to swarm away from the inoculation site was demonstrated by the motility assay (data not shown). Consistent with the observation that both strains were motile, there were not significant differences in biofilm formation (expressed as OD570/OD660) between the ppk mutant (3.0 ± 0.1) and its wild-type precursor (3.9 ± 0.6) at 24 h. Furthermore, there was no difference in production of RpoS-dependent HA/protease between the ppk mutant (17.4 ± 0.6 azocasein units/unit of OD600) and its wild-type precursor (16.5 ± 0.5 azocasein units/unit of OD600). Finally, the growth of strain C6709-1 (lacZ positive) in the suckling mouse intestine and the growth of strain AJB37 (lacZ positive) in the suckling mouse intestine were compared to the growth of strain AC-W66 (lacZ negative) in the suckling mouse intestine. Strains C6709-1 and AJB37 showed competitive indices of 1 and 1.9, respectively, compared to AC-W66. These results indicate that poly-P stores are not required for the expression of virulence and for colonization of the suckling mouse intestine.
V. cholerae ppk mutant AJB37 is not impaired in starvation survival.
There were no differences in the specific growth rate between the wild-type strain and its ppk mutant in LB medium or MOPS-G minimal medium containing 2 mM phosphate (data not shown). However, overexpression of poly-P from plasmid pBC29 significantly reduced the growth rates of C6709-1 and AJB37 (data not shown), suggesting that increasing the level of poly-P above its already high levels in V. cholerae could be deleterious. Additionally, we studied the survival of the ppk mutant in LB medium and ASW basal salts (see Fig. S1 in the supplemental material). The ppk mutant did not exhibit increased sensitivity to prolonged incubation in LB medium or ASW basal salts.
In E. coli, starvation conditions can also induce the stringent response characterized by increased levels of ppGpp. Considering the capacity of PPK to synthesize ppGpp from GDP and poly-P (19), we examined our ppk mutant to determine whether it had a relA (relaxed) phenotype. V. cholerae relA mutants are sensitive to the histidine analog 1,2,4-triazole due to their inability to derepress histidine biosynthetic genes (33). Strain C6709-1 and ppk mutant AJB37 were resistant to 1,2,4-triazole compared to the isogenic relA mutant AJB41 (see Fig. S2 in the supplemental material).This result indicates that a lack of PPK activity does not significantly impact the ability of V. cholerae to synthesize ppGpp.
Sensitivity of ppk mutant to environmental stressors in different culture media.
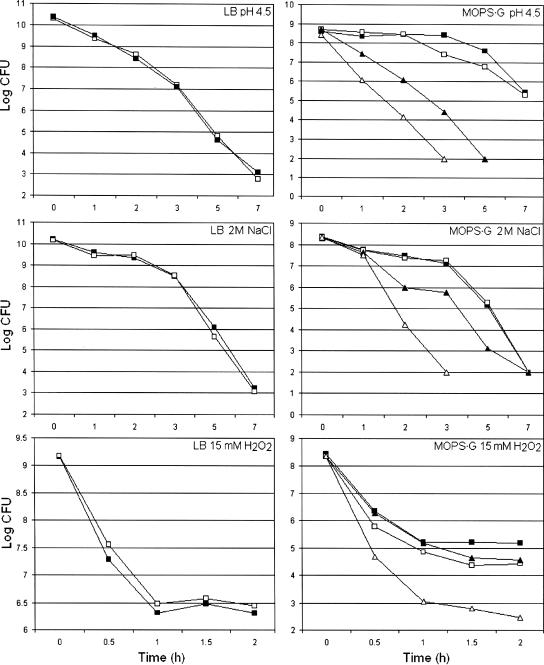
Next, we studied the sensitivities of the wild-type strain and its isogenic ppk mutant to low pH, high salinity, and H2O2 in different culture media. As shown in Fig. 2, no differences between the wild type and the mutant were found in LB medium or MOPS-G medium containing 2 mM phosphate. In striking contrast, the ppk mutant was significantly more sensitive to all these stresses in MOPS-G medium at a low phosphate concentration (0.1 mM) (Fig. 2). This result suggests that poly-P plays an important role in stress resistance under phosphate limitation conditions. Strain AJB37 containing the ppk gene on a plasmid grew poorly in minimal medium (data not shown), suggesting that overexpression of poly-P is deleterious. This prevented us from testing if the ppk gene provided in trans restored stress resistance in low-phosphate medium.
FIG. 2.
Resistance of V. cholerae ppk mutant to environmental stresses. Strains C6709-1 (wild type) and AJB37 (ppk) were grown to stationary phase in LB medium or MOPS-G medium containing 2 mM phosphate. The cells were centrifuged, washed, and resuspended in 1 volume of fresh LB medium with different stresses (symbols: ▪, wild type; □, ppk). The cultures grown in MOPS-G medium containing 2 mM phosphate were centrifuged, washed, and resuspended in 1 volume of MOPS-G medium containing 2 mM phosphate (symbols: ▪, wild type; □, ppk) or 1 volume of MOPS-G medium containing 0.1 mM phosphate (symbols: ▴, wild type; ▵, ppk) in the presence of different stresses. Each value is the mean of three independent cultures.
Mechanism of poly-P-mediated stress resistance.
To explore the mechanism by which poly-P could enhance survival to stress in low-phosphate medium, we first examined whether the ppk mutant had a growth defect in MOPS-G medium at a low phosphate concentration. We found that there was no difference between the growth rates of strains C6709-1 and AJB37 (see Fig. S3 in the supplemental material). The V. cholerae PhoBR regulatory system has recently been shown to work like the system in E. coli by controlling numerous genes involved in phosphate acquisition (35, 36). Since a previously constructed ppk mutant showed a slightly lower rate of phosphate uptake (25), we examined whether the phosphate incorporation of the wild type and the phosphate incorporation of our mutant differed. Again, no significant differences were observed for inorganic phosphate uptake under our experimental conditions (see Fig. S3 in the supplemental material). In addition, the wild type and the mutant expressed similar levels of PhoA (alkaline phosphatase) activity, indicating that both strains are capable of inducing the pho genes under phosphate limitation conditions (see Fig. S3 in the supplemental material).
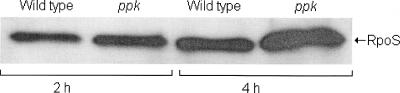
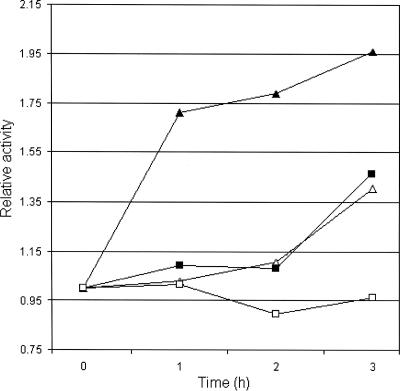
In E. coli, phosphate starvation induces expression of rpoS by a mechanism different from the mechanism observed with carbon and nitrogen starvation (27). Therefore, it was conceivable that our ppk mutant could induce sufficient RpoS expression to survive starvation in LB medium or ASW salts but could not produce enough RpoS in low-phosphate medium. To test this possibility, we first confirmed expression of RpoS in MOPS-G medium by using the reporter strain AJB2 (32). Expression of the hapA-lacZ fusion in AJB2 is strictly dependent on RpoS (32). When cultures of AJB2 were diluted in MOPS-G medium containing 2 and 0.1 mM phosphate, the β-galactosidase activity steadily increased from 5 to 20 Miller units in both media over 4 h of incubation, indicating that there was RpoS expression. Next, we performed a Western blot experiment to compare production of RpoS in low-phosphate medium by strain C6709-1 and production of RpoS in low-phosphate medium by strain AJB37. As shown in Fig. 3, the production of RpoS protein by ppk mutant AJB37 in MOPS-G medium containing 0.1 mM phosphate was not affected. In fact, strain AJB37 appeared to make slightly more RpoS protein than the wild type made. In order to examine the possibility that the ppk mutant exhibited lower RpoS activity in phosphate-limited medium, we measured the relative increase in catalase activity after a phosphate downshift. It has been shown that V. cholerae RpoS induces catalase production (38). As shown in Fig. 4, transfer of wild-type strain C6709-1 to low-phosphate minimal medium resulted in a sharp increase in catalase specific activity. In contrast, significantly lower induction was observed for the ppk mutant (Fig. 4). These results suggest that the increased sensitivity of the ppk mutant to environmental stressors in phosphorus-limited medium could be a consequence of lower RpoS activity.
FIG. 3.
Production of RpoS by V. cholerae in low-phosphate medium. Strains C6709 (wild type) and AJB37 (ppk) were grown to stationary phase in MOPS-G medium containing 2 mM phosphate. Cells were centrifuged and resuspended in 1 volume of MOPS-G medium containing 0.1 mM phosphate. Cultures were incubated at 37°C, and samples were taken at 2 and 4 h and used for Western blot analysis.
FIG. 4.
Derepression of catalase activity in low-phosphate minimal medium. V. cholerae C6709-1 (wild type) and AJB37 (ppk) were grown to stationary phase in MOPS-G medium containing 2 mM phosphate and diluted in fresh MOPS-G medium containing 2 or 0.1 mM phosphate. Samples were taken at 0, 1, 2, and 3 h for determination of catalase activity, and the data were normalized based on the time-zero activity. Symbols: ▴, wild type, 0.1 mM phosphate; ▵, wild type, 2 mM phosphate; ▪, ppk, 0.1 mM phosphate; □, ppk, 2 mM phosphate.
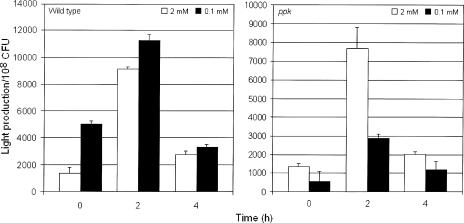
Since poly-P can be used to make ATP (1, 2), we tested the possibility that the ppk mutant had a defect in maintaining an adequate energy charge to cope with environmental stresses under conditions in which ATP could be derived mostly from intracellular reservoirs rather than from externally supplied phosphate. To this end, C6709-1 and AJB37 were grown to stationary phase in MOPS-G medium containing 2 mM phosphate, and the cells were resuspended in fresh MOPS-G medium containing either 2 mM or 0.1 mM phosphate. When C6709-1 was refreshed in minimal media containing the two phosphate concentrations, a peak level of ATP per cell was observed at 2 h, followed by a decrease to the initial basal level (Fig. 5). As in the stress response experiments (Fig. 2), the ppk mutant behaved like the wild type with 2 mM phosphate but the ATP levels did not increase with 0.1 mM phosphate (Fig. 5), conditions in which it exhibited increased sensitivity to low pH, salinity, and H2O2 (Fig. 2). This result suggests that under phosphate limitation conditions, a significant fraction of ATP is derived from poly-P stores. The ppk mutant appears to be incapable of maintaining an adequate energy charge to overcome environmental stresses in a low-phosphate environment.
FIG. 5.
ATP biosynthesis in V. cholerae ppk mutants. Strains C6709 (wild type) and AJB37 (ppk) were grown to stationary phase in MOPS-G medium containing 2 mM phosphate. Cells were centrifuged and resuspended in 1 volume of MOPS-G medium containing 2 mM phosphate (open bars) or 0.1 mM phosphate (solid bars). Light production and the number of CFU were determined as described in Materials and Methods. The error bars indicate the standard deviations of the means for three independent cultures.
DISCUSSION
The ability to make poly-P does not appear to affect the expression of RpoS in V. cholerae grown in rich or high-phosphate minimal medium. We reached this conclusion based on the findings that our ppk mutant made similar amounts of RpoS-dependent HA/protease and did not exhibit rpoS phenotypes unless it was grown in phosphate-limited medium. These results are in close agreement with a previous report showing that a V. cholerae ppk mutant did not exhibit phenotypes predicted from E. coli studies (25). In a previous study the motility of and biofilm formation by a V. cholerae ppk mutant were found to be slightly affected (25). We did not observe these effects in our ppk mutant, which was isolated from a V. cholerae El Tor biotype strain belonging to a different lineage. The finding that our ppk mutant expressed RpoS and motility is fully consistent with its ability to effectively colonize the suckling mouse intestine.
A previous report showed that V. cholerae makes about 100 times more poly-P than E. coli makes, yet a mutant had fewer phenotypic changes (25). Our results show that a V. cholerae ppk mutant is indeed more sensitive to environmental stresses, but only when the extracellular concentration of phosphate is low (Fig. 2). We investigated possible mechanisms for the sensitivity of our ppk mutant to multiple environmental stresses. The results indicated that inactivation of ppk does not affect growth in low-phosphate medium, the ability to derepress the pho regulon, and the ability to express the RpoS protein in low-phosphate medium (see Fig, S1, S2, and S3 in the supplemental material). However, these experiments did not rule out the possibility that RpoS activity is affected in the ppk mutant grown in low-phosphate medium. To examine this possibility, we measured the induction of catalase activity in the wild type and mutant after a downshift to low-phosphate conditions. The inability of the ppk mutant to induce catalase in response to phosphate limitation (Fig. 4) explains its increased sensitivity to H2O2 in this medium and could have been due to lower RpoS activity.
Studies with E. coli have shown that different starvation diets induce the RpoS-mediated stress responses by different mechanisms involving rpoS transcription, translation protein stability, and activity (27). ppk mutant AJB37 survived prolonged carbon, nitrogen, and phosphorus starvation in ASW salts like the wild type survived but expressed stress-related phenotypes in low-phosphate MOPS medium containing a carbon and nitrogen source. Therefore, we suggest that poly-P specifically protects V. cholerae from environmental stresses under phosphorus imbalance conditions. Furthermore, we observed that the ATP levels of the ppk mutant did not increase when the mutant was transferred to low-phosphate medium (Fig. 5). Consequently, we propose that the increased stress sensitivity of strain AJB37 (ppk) in low-phosphate medium is a consequence of a more drastic metabolic defect, an inability to generate ATP by mobilizing intracellular poly-P reservoirs. The paucity of ppk-related phenotypes in V. cholerae suggests that large amounts of poly-P are made in this bacterium only to be used under very specific conditions. Why has V. cholerae evolved to make such large amounts of poly-P? Clearly, poly-P is not required for the expression of virulence and intestinal colonization. However, it has been proposed that V. cholerae can survive outside the human host in estuaries and brackish waters (9-11, 29). It is well established that phosphorus and nitrogen play crucial roles in the ecology of aquatic ecosystems (6, 7). Phosphorus has been proposed to be the most common cause of eutrophication in freshwater lakes, reservoirs, streams, and the headwaters of estuaries, while nitrogen is believed to be the key mineral nutrient controlling primary production in the ocean (6, 7). Depending on the specific aquatic environment, both nitrogen and phosphorus could become limiting nutrients simultaneously or in a cyclic manner (6, 7). It is likely that in an environment in which phosphorus is limiting, bacteria capable of synthesizing large poly-P stores could have a competitive advantage. Salinity gradients have been recognized to be an important environmental stressor in aquatic ecosystems. Poly-P-defective mutant AJB37 was found to be remarkably sensitive to high NaCl concentrations (Fig. 2). It will be of interest to examine if more widespread inhabitants of aquatic ecosystems (e.g., V. cholerae non-O1 and non-O139 and other members of the genus Vibrio) have similarly large poly-P stores.
The major differences in poly-P metabolism between E. coli and V. cholerae could reflect the evolutionary adaptation of these organisms to different habitats. While E. coli is a normal inhabitant of the lower gastrointestinal tract of humans and animals, long-term human carriage of V. cholerae is very unusual. Consequently, V. cholerae could have evolved to make more poly-P to resist longer exposure to phosphate-limited conditions outside the gastrointestinal tract.
In summary, many mechanisms that potentially enhance the survival of V. cholerae outside the human host have been proposed. These mechanisms include the general stress response, formation of biofilm communities, association with phytoplankton and zooplankton, and a viable but not culturable stage. In this paper we describe a novel mechanism: synthesis of large poly-P stores for ATP biosynthesis. Our results show that availability of a large poly-P high-energy phosphate depository enhances the capacity of V. cholerae to survive environmental stresses in a low-phosphate environment.
Supplementary Material
Acknowledgments
This work was supported by Public Health Service grant AI063187 from the National Institutes of Health to J.A.B.
We are grateful to Cres Fraley of the Kornberg laboratory (Stanford University School of Medicine) for advice concerning poly-P biochemistry and to Richard A. Finkelstein (University of Missouri School of Medicine) for critical reading of the manuscript and helpful suggestions.
Footnotes
Published ahead of print on 1 September 2006.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Ahn, K., and A. Kornberg. 1990. Polyphosphate kinase from Escherichia coli. J. Biol. Chem. 265:11734-11739. [PubMed] [Google Scholar]
- 2.Akiyama, M., E. Crooke, and A. Kornberg. 1992. The polyphosphate kinase of Escherichia coli. J. Biol. Chem. 267:22556-22561. [PubMed] [Google Scholar]
- 3.Akiyama, M., E. Crooke, and A. Kornberg. 1993. An exopolyphosphatase of Escherichia coli. J. Biol. Chem. 268:633-639. [PubMed] [Google Scholar]
- 4.Ault-Riche, D., C. D. Fraley, C. M. Tzeng, and A. Kornberg. 1998. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 180:1841-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benitez, J. A., A. J. Silva, and R. A. Finkelstein. 2001. Environmental signals controlling production of hemagglutinin/protease in Vibrio cholerae. Infect. Immun. 69:6549-6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benitez-Nelson, C. R. 2000. The biogeochemical cycling of phosphorus in marine systems. Earth-Sci. Rev. 51:109-135. [Google Scholar]
- 7.Correll, D. L. 1999. Phosphorus: a rate limiting nutrient in surface waters. Poultry Sci. 78:674-682. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faruque, S. M., and G. B. Nair. 2002. Molecular ecology of toxigenic Vibrio cholerae. Microbiol. Immunol. 46:59-66. [DOI] [PubMed] [Google Scholar]
- 10.Faruque, S. M., G. B. Nair, and J. J. Mekalanos. 2004. Genetics of stress adaptation and virulence in toxigenic Vibrio cholerae. DNA Cell Biol. 23:723-741. [DOI] [PubMed] [Google Scholar]
- 11.Faruque, S. M., M. John Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkelstein, R. A., M. Boesman-Finkelstein, Y. Chang, and C. C. Häse. 1992. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect. Immun. 60:472-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Häse, C. C., and R. A. Finkelstein. 1991. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J. Bacteriol. 173:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulsmann, A., T. M. Rosche, I. S. Kong, H. M. Hassan, D. E. M. Beam, and J. D. Oliver. 2003. RpoS-dependent stress response and exoenzyme production in Vibrio vulnificus. Appl. Environ. Microbiol. 69:6114-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huq, A., R. B. Sack, A. Nizam, I. M. Longini, G. B. Nair, A. Ali, J. Glen Morris, Jr., M. N. Huda Khan, A. K. Siddique, M. Yanus, J. Albert, D. A. Sack, and R. R. Colwell. 2005. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl. Environ. Microbiol. 71:4645-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joelsson, A., Z. Liu, and J. Zhu. 2006. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect. Immun. 74:1141-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, K.-S., N. N. Rao, C. D. Fraley, and A. Kornberg. 2002. Inorganic polyphosphate is essential for long-term survival and virulence factors in Shigella and Salmonella spp. Proc. Natl. Acad. Sci. USA 99:7675-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornberg, A., N. N. Rao, and Dana Ault-Riche. 1999. Inorganic polyphosphate: a molecule of many functions. Annu. Rev. Biochem. 68:89-125. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda, A., and A. Kornberg. 1997. Polyphosphate kinase as a nucleoside diphosphate kinase in Escherichia coli and Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 94:439-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, Y. H., C. Miyamoto, and E. A. Meighen. 2002. Cloning, sequencing, and functional studies of the rpoS gene from Vibrio harveyi. Biochem. Biophys. Res. Commun. 293:456-462. [DOI] [PubMed] [Google Scholar]
- 21.Manoil, C. D. F., and J. Beckwith. 1985. TnphoA: a transposon probe for protein export signals. Proc. Natl. Acad. Sci. USA 82:8129-8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1971. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 24.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane protein and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa, N., C.-M. Tzseng, C. D. E. Fraley, and A. Kornberg. 2000. Inorganic polyphosphate in Vibrio cholerae: genetics, biochemical, and physiological features. J. Bacteriol. 182:6687-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, K. J., M. J. Kang, S. H. Kim, H. J. Lee, J. K. Lim, S. H. Choi, S. J. Park, and K. H. Lee. 2004. Isolation and characterization of rpoS from a pathogenic bacterium, Vibrio vulnificus: role of σS in survival of exponential phase cells under oxidative stress. J. Bacteriol. 186:3304-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peterson, C. N., M. J. Mandel, and T. J. Silhavy. 2005. Escherichia coli starvation diets: essential nutrients weigh in distinctly. J. Bacteriol. 187:7549-7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rashid, M. H., N. N. Rao, and A. Kornberg. 2000. Inorganic polyphosphate is required for motility of bacterial pathogens. J. Bacteriol. 182:225-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoolnik, G., and F. H. Yildiz. 2000. The complete genome sequence of Vibrio cholerae: a tale of two chromosomes and two lifestyles. Genome Biol. 1:REVIEWS1016.1-1016.3. [Online.] http://genomebiology.com/2000/1/3/reviews/1016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiba, T., K. Tsutsumi, H. Yano, Y. Ihara, A. Kameda, K. Tanaka, H. Takahashi, M. Munekata, N. N. Rao, and A. Kornberg. 1997. Inorganic polyphosphate and the induction of rpoS expression. Proc. Natl. Acad. Sci. USA 94:11210-11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silva, A. J., R. Fando, and J. A. Benitez. 1998. Overexpression of a mutant B subunit in toxigenic Vibrio cholerae diminishes production of active cholera toxin in vivo. Curr. Microbiol. 37:231-235. [DOI] [PubMed] [Google Scholar]
- 32.Silva, A. J., and J. A. Benitez. 2004. Transcriptional regulation of Vibrio cholerae hemagglutinin/protease expression by the cyclic AMP receptor protein and RpoS. J. Bacteriol. 189:6374-6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva, A. J., and J. A. Benitez. 2006. A Vibrio cholerae relaxed (relA) mutant expresses major virulence factors, biofilm, exhibits motility, and colonizes the suckling mouse intestine. J. Bacteriol. 188:794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva, A. J., K. Pham, and J. A. Benitez. 2003. Hemaglutinnin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149:1883-1891. [DOI] [PubMed] [Google Scholar]
- 35.von Kruger, W. M. A., L. M. Santos Lery, M. Regina Soares, F. S. de Neves-Manta, C. M. Batista e Silva, A. G. Da Costa Neves-Ferreira, J. Perales, and P. Mascarello Bisch. 2006. The phosphate-starvation response in Vibrio cholerae O1 and phoB mutant under proteomic analysis: disclosing functions involved in adaptation, survival and virulence. Proteomics 6:1495-1511. [DOI] [PubMed] [Google Scholar]
- 36.von Kruger, W. M. A., S. Humphreys, and J. M. Ketley. 1999. A role for the PhoBR regulatory system homologue in the Vibrio cholerae phosphate limitation response and intestinal colonization. Microbiology 145:2463-2475. [DOI] [PubMed] [Google Scholar]
- 37.Xu, H.-S., N. Roberts, F. L. Singleton, R. W. Attwell, D. J. Grimes, and R. R. Colwell. 1982. Survival and viability of non-culturable Escherichia coli and Vibrio cholerae in the estuaries and marine environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
- 38.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.