Abstract
The tumor suppressor gene, p53, is rarely mutated in neuroblastomas (NB) at the time of diagnosis, but its dysfunction could result from a nonfunctional conformation or cytoplasmic sequestration of the wild-type p53 protein. However, p53 mutation, when it occurs, is found in NB tumors with drug resistance acquired over the course of chemotherapy. As yet, no study has been devoted to the function of the specific p53 mutants identified in NB cells. This study includes characterization and functional analysis of p53 expressed in eight cell lines: three wild-type cell lines and five cell lines harboring mutations. We identified two transcription-inactive p53 variants truncated in the C-terminus, one of which corresponded to the p53β isoform recently identified in normal tissue by Bourdon et al. [J. C. Bourdon, K. Fernandes, F. Murray-Zmijewski, G. Liu, A. Diot, D. P. Xirodimas, M. K. Saville and D. P. Lane (2005) Genes Dev., 19, 2122–2137]. Our results show, for the first time, that the p53β isoform is the only p53 species to be endogenously expressed in the human NB cell line SK-N-AS, suggesting that the C-terminus truncated p53 isoforms may play an important role in NB tumor development.
INTRODUCTION
The p53 tumor suppressor gene remains the most frequently altered gene in human tumors. Several p53 mutation databases have been reported previously (1–3), and to date, more than 1500 different p53 mutants have been described (4). Functional inactivation of p53 is usually due to gene mutation, deletion or protein degradation. In general, the majority of p53 mutations in human neoplasia are missense mutations affecting the DNA-binding domain (DBD). Unlike other human cancers, p53 in neuroblastoma (NB) is rarely mutated in the primary tumor at diagnosis but high levels of wild-type p53 (wt p53) protein expression have been found in the cytoplasm of undifferentiated tumors (5,6). More recently, in normal unstressed cells, wt p53 protein was found to be retained in the cytoplasm as a latent form, in huge, p53-associated protein complexes known as ‘Parc’ (7). The steady-state concentration of p53 in normal unstressed cells is usually very low because of the short half-life of the wild-type (wt) protein. Overexpression of p53 in most of the transformed cells containing a missense mutation within the p53 gene appears to be due to the increased stability of mutated p53 (8). In unstressed NB cells, high wt p53 expression may reflect the embryonic origin of NBs, in which precursor cells fail to mature (9).
p53 mutations are unusual in human NB but, when they do occur, are found in post-chemotherapy tumors. In this respect, Tweddle et al. (10) described how two NB cell lines derived from the same patient can elicit a different p53 status: wt p53 for SK-N-BE(1a) established before treatment, and mutated p53 for SK-N-BE(2c) established after relapse of the patient under treatment with cytotoxic agents such as cyclophosphamide, doxorubicin, vincristine and radiotherapy. In NB cell lines, p53 mutation has been found in multidrug-resistant cells (11). Various types of p53 mutation have been detected in NB cells and can lead to inactivation either by shut-down of protein expression or production of aberrant p53 products. Indeed, in LAN-1 cells, p53 nonsense mutation at cysteine 182 in exon 5 leads to the absence of protein (9), whereas in SK-N-BE(2) cells, missense mutation at codon 135 (C135F) leads to stable overexpressed protein (11). By analyzing IGR-N-91, a cell line established in our laboratory from the bone marrow of a patient with metastatic NB after unsuccessful Adriamycin–vincristine chemotherapy (12), we identified another type of aberrant protein that arises from the duplication of exons 7-8-9. This duplication spans from amino acids 225 to 331, which represent part of the DBD and part of the oligomerization domain (13). However, each p53 mutant has been described in the literature as a case report, and so far, no comparative study has been undertaken to link their biochemical features with functional properties.
In the present study we report two novel p53 C-terminus mutants identified in SK-N-AS and IGR-NB8 human NB cell lines. The biological properties of these two new variants were analyzed in comparison with p53 isolated from six other human NB lines: three [LAN-1, SK-N-BE(2) and IGR-N-91] expressing mutant p53 and three (SH-SY5Y, LAN-5 and IMR-32) expressing the wt protein. This characterization was done by using a range of functional assays: (i) the ability of the protein to bind with p53 consensus sequence using the functional analysis of separated allele in yeast (FASAY); (ii) the ability of the protein to transactivate the p53-responsive element (RE) identified either in the promoter of p21/WAF1 or in the first intron of BAX, using luciferase reporter assay; (iii) the induction of endogenous p21/WAF1 gene expression under stress conditions.
MATERIALS AND METHODS
Neuroblastoma cell lines, culture and drug treatments
The parental human NB SH-SY5Y, SK-N-AS, IMR-32 and SK-N-BE(2) cell lines were purchased from the European Collection of Cell Cultures (ECACC, Wiltshire, UK). The human IGR-NB8 cells (a gift of Prof. Gilles Vassal, UPRES EA 3535, Institut Gustave Roussy, Villejuif) were derived from a previously untreated localized NB (14). The LAN-1 and LAN-5 cell lines were provided by Dr Nicole Gross (Pediatric Oncology Research, Lausanne, Switzerland). The human IGR-N-91 cell line was established in our laboratory from the bone marrow of a patient with metastatic NB after unsuccessful adriamycin–vincristin chemotherapy (12). LAN-1, LAN-5 and IMR-32 were grown in RPMI medium supplemented with 2 mM l-glutamine and 10% fetal calf serum and gentamicine 10 μg/ml. Others cell lines were cultured in DMEM.
For activation of endogenous p53, cells were treated with cis-platinum (Sigma) (10 μg/ml) for 24 h then lysed for western blot analysis.
Western blot analysis
This procedure was carried out as described previously (13). Protein lysates (50 μg) were submitted to 10% SDS–PAGE, and then transferred onto nitrocellulose filters. After saturation, the membranes were incubated with primary antibody diluted in 0.1% phosphate-buffered saline, Tween-20 and 3% skim milk. The primary antibodies used were anti-p53 monoclonal antibody (clone DO-7, 1/1000, DAKO), anti-p21/WAF1 monoclonal antibody (Ab-1, 1/200, Oncogene Research) and anti-β-actin monoclonal antibody (1/1000; Chemicon) as internal control. Protein bands were detected by ECL system (Amersham).
PCR, plasmids cloning
Genomic DNA was extracted using lysis buffer containing 20 mM Tris–HCl, pH 7.5; 0.4 M NaCl; 0.5% SDS; 10 mM EDTA, treated with proteinase K (200 μg/μl), purified with phenol/chloroform, precipitated with ethanol and dissolved in DNase free water. Total RNA were purified using RNAble reagent (Eurobio), precipitated with isopropanol and dissolved in RNase free water. cDNA was obtained by reverse transcription of 1 μg of total RNA using Superscript II™ RNase H-Reverse transcriptase (Invitrogen) and Oligo-d(T)16 in conditions specified by the manufacturer. Amplification of full-length p53 coding region from SH-SY5Y, IGR-N-91, IGR-NB8 and SK-N-BE(2) cell lines was performed using forward primer at position 152 and reverse primer at position 1583 (F1 and R7, respectively, Table 1; GenBank accession no. K03199). p53 cDNA from SK-N-AS cells for cloning was obtained from RT–PCR using F1 and reverse primer i9+: 5′-GCAAAGTCATAGAACCATTTTCAT-3′ (nucleotide position 14989, GenBank accession no. X54156) primers which encompass from exon 1 to exon i9+ first identified by Flaman et al. (15) included. The PCR was done in the presence of pfu Hotstart DNA polymerase (Stratagene) for 30 cycles of 1 min at 90°C, 1 min at 65°C and 2 min 30 s at 72°C using PTC-100 thermocycler (MJ-Research).
Table 1.
Primers pairs for RT–PCR of the p53 (mRNA and gene)
| Primer | Primer location | Sequence 5′→3′ |
|---|---|---|
| F1 | Exon 1 (nt 152)* | gctttccacgacggtgaca |
| F2 | Exon 8 (nt 1001)* | aatctactgggacggaacagcttt |
| R3 | Exon 8/9 (nt 1124) | gttgggcagtgctcgcttagt |
| R4 | Exon 9 (nt 1154)* | tctttggctggggagagga |
| R5 | Exon 9 (nt 1184)* | tgaagggtgaaatattctccatc |
| R6 | Exon 10 (nt 1230)* | cctcattcagctctcggaacatct |
| R7 | Exon 11 (nt 1583)* | cccacaacaaaacaccagtgc |
| DBD-F | Exon 5 (nt 694)* | ggccatctacaagcagtcac |
| DBD-R | Exon 5 (nt 779)* | ccagaccatcgctatctgag |
| β C-ter-F | Exon 8 (n t1060)* | gcgcacagaggaagagaatc |
| β C-ter-R | Exon9/i9+ Exon 9 | aagctggtctggtcctgaag |
| wt C-ter-F | Exon 10 (nt 1141)* | caacaacaccagctcctctc |
| wt C-ter-R | Exon 10 (nt 1258) | caaggcctcattcagct tc |
| a-F | Intron 7 (nt 14367)** | aatctactgggacggaacagcttt |
| a-R | Intron 9 (nt 14981)** | gcaaagtcatagaaccattttcat |
| b-F | Intron 9 (nt 1880)** | gacaatggctcctggttgta |
| b-R | Intron 9 (nt 15624)** | ggtgtatgcctgtggtccta |
| c-F | Intron 9 (nt 16836)** | gtgatggcaggtgcctgtaa |
| c-R | Exon 10 (nt 17621)** | caaggcctcattcagctctc |
| d-F | Intron 9 (nt 17211)** | ctggctaacatggtgaag |
| d-R | Exon 10 (nt 17610) | tcagctctcggaacatct |
According to GenBank sequence accession no. *KO3199 (mRNA) and **X54156 (HSP53G); F, forward sequence; R, reverse sequence.
The p53 cDNA from SH-SY5Y, SK-N-AS, IGR-N-91, IGR-NB8 and SK-N-AS cells were then cloned into pcDNA3.1/V5-His-Topo vector (Invitrogen) according to the manufacturer's instruction. The p53 sequence of each cell line was investigated by sequencing of plasmids after cloning. Sequencing was performed by Genome Express (Meylan, France).
The pDDm-TO harboring p53 dominant negative form (p53DD) pGL3-E1bTATA and the pE1B-hWAF1 firefly luciferase reporter containing the p53-responsive element of the p21/WAF1 promoter were described previously (16,17) pE1B-BAXi contains the p53RE identified in the intron 1 of the BAX gene [(18) and D. Munsch, personal communication]. Oligonucleotides TCGAGGGCAGGCCCGGGCTTGTCG and CTAGCGACAAGCCCGGGCCTGCCC were annealed and cloned into pGL3-E1bTATA digested with NheI and XhoI to obtain pE1B-BAXi. The pcDNA3-ΔNp73α expression plasmid was a gift of Dr Daniel Caput (SANOFI, Labèges, France).
Fluorescent in situ hybridization (FISH)
Cytogenetic preparations
Metaphase spreads from healthy human male lymphocytes and tumor cell lines were prepared as described previously (19). BAC probe RP11-199F11, containing a 167 kb region spanning TP53 gene, was labeled by random priming in the presence of Alexa 594-dUTP (Molecular Probes). A commercial probe specific for chromosome 17 centromere, and labeled with spectrum green, was obtained from Vysis. After over-night cohybridization of the probes in the presence of Cot-1 DNA, the slides were washed and DNA counterstained with DAPI. The preparations were observed with an epifluorescence microscope and images captured with a Vysis imaging station. Between 3 and 14 metaphases spreads and 30–200 nuclei were examined for each cell line.
Luciferase reporter assays
LAN-1 or SH-SY5Y cells were seeded in duplicates onto 6-well plates at a density of 2 × 104 cells per cm2 and cotransfected 24 h later with 0.5 μg (2.5 μg/ml) of pGL3 firefly luciferase reporter gene plasmid under the control of either pE1B-hWAF1 or pE1B-BAX using lipofectamine 2000 and 1 μg of either a p53 expressing plasmid or an empty vector. At 24 h after transfection, cells were lysed with 200 μl/well of passive lysis buffer provided with the ‘Luciferase assay kit’ (Promega). Luciferase activity was measured using Microlumat LB96P luminometer (EG & G Berthold Instrument).
Functional assay in yeast
cDNA was obtained by RT of 1 μg of total RNA using Superscript II™ RNase H-Reverse transcriptase (Invitrogen) and random hexamers to prime the synthesis in conditions specified by the manufacturer. p53 cDNA was amplified by PCR and cotransformed into yeast, IG397 Ade2 strain, together with either pRDI-22 vector for p53-standard assay or pFW35 and pFW34 plasmid for 5′ or 3′ split assay, respectively, carrying the ADE2 open reading frame under the control of a p53-responsive promoter (20). In a selective medium lacking leucine, wt-p53 activates transcription of ADE2 gene that encodes enzyme—phosphoribosylimidazole carboxylase—implicated in adenine biosynthesis. Therefore, a colony of cells that expresses ADE2 gene is white whereas the one composed of cells where ADE2 gene is not expressed owing p53 mutation is red.
RESULTS
P53 status in SK-N-AS and IGR-NB8 cells
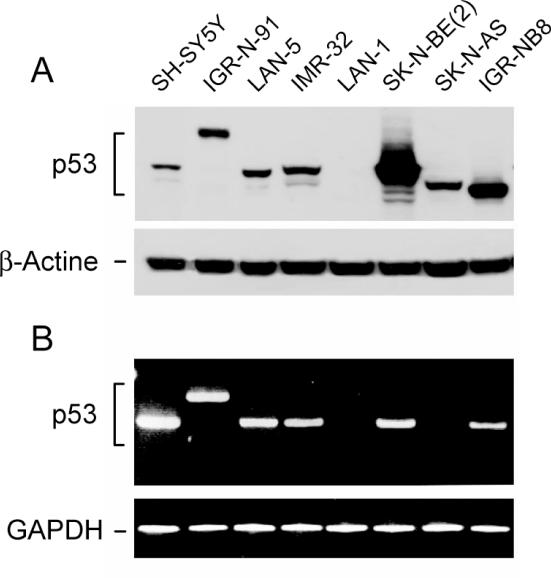
We first compared the migration profiles of p53 expressed in SK-N-AS and IGR-NB8 with those expressed in the other six NB cells, SH-SY5Y, LAN-5, LAN-1, IMR-32, SK-N-BE(2) and IGR-N-91. Western blots from 50 μg of total protein extracts were revealed with p53 monoclonal antibody (DO-7). A range of profiles was identified as shown in Figure 1A. As expected, p53 extracted from the three cell lines, SH-SY5Y, LAN-5 and IMR-32, expressing wt protein (13,21) migrated at the wt position. Of particular note in these three wt p53 cell lines was an additional faint band that migrated faster than the full-length protein. The LAN-1 cells were found to be p53 deficient (9). The SK-N-BE(2) cell line showed an intense band reflecting p53 stability due to a missense mutation at codon 135 (11). As expected, due to the previously identified duplication of exons 7-8-9 (13), p53 protein migration was delayed in the IGR-N-91 cells. In contrast, the p53 protein in the SK-N-AS cell line migrated noticeably faster than the wt protein, indicating that it was smaller in size. The p53 protein in the IGR-NB8 cell line was even smaller than that in SK-N-AS.
Figure 1.
p53 expression of different neuroblastoma cell lines. (A) Western blot from protein total extracts (upper panel); β-actin was used as loading control (lower panel). (B) RT–PCR using F1-R7 primers (Table 1); amplified fragment was normalized by GAPDH.
To analyze the coding region of each of the p53 variants, RT–PCR was performed using p53-specific F1-R7 primers (Table 1 and Figure 1B). The expected 1430 bp for full-length p53 was amplified from wt p53-expressing SH-SY5Y, LAN-5 and IMR-32 cell lines. As SK-N-BE(2) harbors a single point mutation at codon 135 (C135F), the amplified fragment analyzed by electrophoresis migrated as wt p53 (Figure 1B). The longer RT–PCR fragment from the mutated IGR-N-91 cell line resulted from the duplication of exons 7-8-9, as shown in our previous data (13), which corresponds to extra nucleic material of 321 nt. For the LAN-1 cells, no amplified fragment was observed in accordance with published data, which demonstrates the extremely low levels of p53 mRNA and the undetectable level of protein (9). An amplified fragment of the same length as the wt protein was observed in the IGR-NB8 cell line (Figure 1B). Indeed, complete gene sequencing revealed a point mutation E326STOP leading to a truncated protein at C-terminus. No fragment, however, was amplified from SK-N-AS with F1-R7 primers.
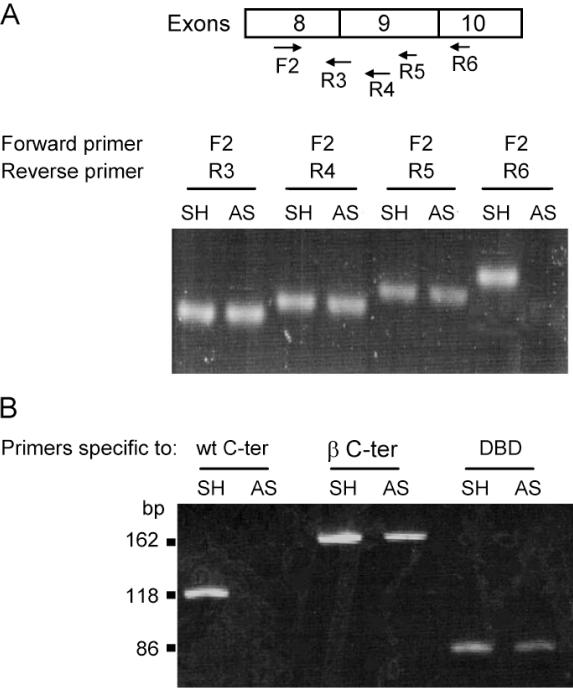
To further map the p53 mRNA transcribed in these cells, series of RT–PCR tests were performed using the forward primer, F2 (exon 8 position 1008th according to GenBank accession no. K03199), matched with different reverse primers, R3 (at the junction of exon 8/9, nt position 1124), R4, R5 (in exon 9 at positions 1154 and 1184, respectively), and R6 (in exon 10, at position 1230). The sequences of these primers are given in Table 1 and the results are presented in Figure 2A. SK-N-AS cDNA gave an amplified fragment of the same size as SH-SY5Y cDNA with the three primer pairs, F2/R3, F2/R4 and F3/R5. However, in contrast to SH-SY5Y, no fragment was obtained with SK-N-AS cDNA using the F2/R6 primer pair, which suggests the absence of exon 10 in SK-N-AS mRNA.
Figure 2.
Detection of p53 mRNA abnormalities in SK-N-AS (AS) in comparison with SH-SY5Y (SH) cells. (A) Amplification of p53 cDNA using primers from exons 8 to 10 as indicated below each arrow; for precise position see Table 1 and GenBank K03199: F2 (forward primer in exon 8); R3 (reverse primer at the junction of exon 8/9); R4 (the first moiety of exon 9); R5 (exon 9, 30 nt downstream R4); R6 (beginning of exon 10). Note that no amplification was observed in SK-N-AS from exon 10 (last lane), in contrast to SH-SY5Y. (B) RT–PCR from SK-N-AS compared to SH-SY5Y cells. Specific primers (Table 1) were used to amplify the DBD, the p53β isoform (β) and the C-terminal domain (C-ter).
The p53 protein expressed in SK-N-AS is the p53β isoform
An alternatively spliced form of human p53 mRNA with an additional 133 bp exon derived from intron 9 has been detected in normal human lymphocytes (15). This spliced variant named ‘i9+’ encodes a truncated protein of 341 amino acids including 10 new amino acids derived from the novel exon, the p53β isoform according to Bourdon et al. nomenclature (22). This led us to hypothesize that the shorter protein expressed by the SK-N-AS cell line could be the p53β isoform. To test this hypothesis, RT–PCRs were performed using primer sets designed to amplify the 3′ region of p53 mRNA encoding either the specific C-terminal part of the wt protein (wt C-ter) or the specific C-terminal part of the β isoform (β C-ter). In parallel amplification with a primer pair amplifying the DBD was used as control. The sequences of these oligonucleotides are given in Table 1. The results presented in Figure 2B are consistent with the only expression of the p53β isoform in SK-N-AS as no band was observed in lane using specific C-ter primer located in exon 10. Interestingly, RT–PCR using SH-SY5Y (SH) gave an amplified fragment not only with the primer pair specific to the C-ter domain of wt p53 but also with the primer pair specific to the p53β isoform. This result, combined with the presence of an additional faint band migrating faster than wt p53 in denaturing polyacrylamide gel (Figure 1A), strongly suggests that both the p53 full-length protein and the β isoform were expressed in the SH-SY5Y cells.
The full-length SK-N-AS p53 cDNA was then amplified with the forward primer F1 and a reverse primer located within the novel exon i9+ (Table 1). This amplified fragment was cloned in pcDNA3/V5-His-Topo, as described in Materials and Methods. Its sequence analysis confirmed that the truncated p53 expressed in SK-N-AS was encoded by the i9+ splice variant described previously by Flaman et al. (15) that encodes the p53β isoform characterized by Bourdon et al. (22).
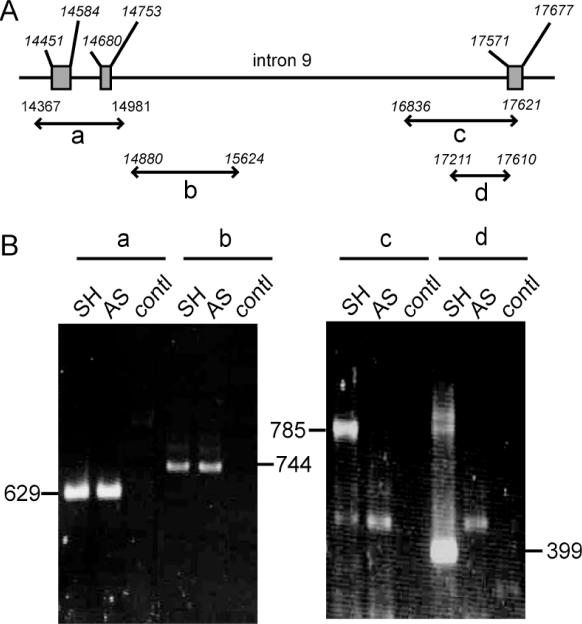
A series of genomic amplifications were performed to identify a possible deletion within the intron 9 that could account for the absence of normal size p53 in SK-N-AS cells. The primer sequences are given in Table 1. Amplifications were performed in parallel with total DNA extracted from SH-SY5Y and SK-N-AS cells. Results are presented in Figure 3. Normal size fragments that encompass the acceptor site of intron 9 were amplified with SH-SY5Y as well as with SK-N-AS DNA. On the contrary amplification fragments that encompass the intron 9 donor site were obtained only with SH-SY5Y but not with SK-N-AS DNA. These results identify a deletion of the intron 9/exon 10 junction within the SK-N-AS p53 gene.
Figure 3.
Identification of a deletion spanning the intron9/exon 10 junction of SK-N-AS p53 gene. (A) Schematic representation of p53 gene from intron 7 to intron 10 with the position of amplified fragments; (B) PCR fragments amplified from SH-SY5Y (SH) and SK-N-AS (AS) DNA with the primer pairs a, b, c and d. The primer sequences are given in Table 1; contl: PCR performed in parallel without DNA.
A yeast functional assay confirmed the absence of p53 full-length expression in SK-N-AS and IGR-NB8
It is possible to detect p53 mutation using a simple yeast colony color assay as described by Flaman et al. (23). When the strain is transformed with a plasmid-encoding wt p53, the cells express the ADE2 gene and produce white colonies (Figure 4A, a, b2 and c1, and Figure 4B, dish a). Cells containing mutant p53 fail to express ADE2 and form small red colonies (Figure 4A, b and b1, and Figure 4B, dish c). When the p53 cDNA fragment is deleted, cells are unable to form a colony (Figure 4A, c and c2). As shown in Table 2, FASAY was performed as a p53-standard test with full-length cDNA or with the split version at the 5′ and 3′ end (15). The background of FASAY experiments is around 10%. p53 wt expressing SH-SY5Y and LAN-5, 2 wt cell lines, yielded ∼92–97% of white colonies (Table 2).
Figure 4.
p53 transactivation ability using yeast-based assay (FASAY). (A) Schematic representation of the analysis of p53 mutants using the yeast homologous recombination expression vector pRDI-22 carrying the 5′ and 3′ ends of the p53 open reading frame and the split forms, pFW35 and pFW34 (lacking p53 fragment from amino acids 66 to 210 for split 5′ and 211–348 for split 3′, respectively) transfected into YPH500 Ade2 yeast strain. This strain repairs double-strand breaks in transfected plasmids by homologous recombination as ‘gap repair’ (see text). (B) Photographs of yeast colonies showing 100% wild-type p53 where all colonies are white (a), or special mutated p53 by duplication of exons 7–9 found in IGR-N-91 cells (b), where white and red colonies were mixed (see also Table 2), and mutated p53 such as those in SK-N-BE(2), where all colonies are red (c).
Table 2.
Functional activity of p53 from NB cells using FASAY assays
| Cell lines | p53-standard assay | SPLIT 5′ | SPLIT 3′ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| White (%) | Red (%) | Phenotypes*** | White (%) | Red (%) | Phenotypes*** | White (%) | Red (%) | Phenotypes*** | |
| SHSY5Y | 87* | 13** | wt | 97 | 3** | wt | 93* | 7** | wt |
| LAN-5 | 92* | 8** | wt | 93 | 7** | wt | 95* | 5** | wt |
| IGR-N-91 | 81* | 19** | mut ? | 96 | 4** | wt | 87* | 13** | wt? |
| SK-N-AS | 0 | 0 | 96 | 4** | wt | 0 | 0 | ||
| LAN-1 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| SK-N-BE(2) | 0 | 100* | mut | 0 | 100* | mut | 94* | 6** | wt |
| IGR-NB8 | 0 | 100* | mut | 95 | 5** | wt | 2** | 98* | mut |
Mean value from three independent experiments (*±6%, **±3%); ***wt and mut phenotypes: red and white colonies ≤10%, respectively; wt, wild-type; mut, mutant.
One hundred percent of the colonies carrying SK-N-BE(2) p53, which is homozygous for the C135F mutation, turned red with the standard or 5′ split assay, whereas 94% of the colonies turned white with the 3′ split assay since the missense mutation does not extend to the C′-terminus of the gene (Table 2 and Figure 4B, dish c). No colonies were observed with p53-deficient LAN-1 cells, (see also Figure 4A, c and c2). With SK-N-AS cells, the split 5′ assay gave 96% white colonies, while the p53-standard and split 3′ assay did not produce any colonies (Table 2). This means that the 5′ terminus was intact whereas the 3′ terminus had been deleted, as was confirmed by nucleotide sequence analysis. The IGR-NB8 colonies, however, were red both with the p53-standard assay and the split 3′ assay. In the IGR-N-91 cell line, where p53 harbors two contiguous sets of exons 7–9, spanning the DBD and oligomerization domain, it is interesting to note that the yeast colonies were predominantly white (Figure 4B, dish b) with the split 5′ and the split 3′ assay (96 and 87% of white colonies, respectively) This suggests that the cells express a binding ability that is specific to wild-type p53 rather than mutated p53 (Table 2).
Transcription activity of SH-SY5Y, IGR-N-91, SK-N-BE(2), SK-N-AS and IGR-NB8 p53 variants in mammalian cells
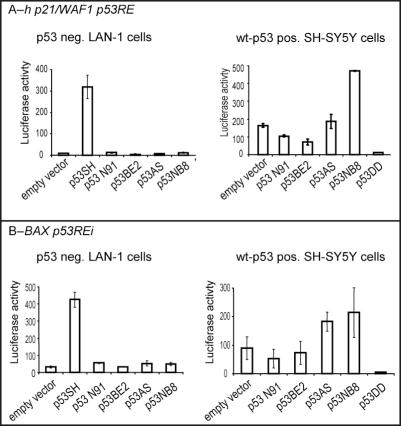
To determine the transactivation ability of p53 variants in mammalian cells, we used a reporter gene strategy. The p53RE located within either the human p21/WAF1 promoter or the intron 1 of the mouse and human BAX gene [(18) and D. Munsch, personnal communication) were cloned in a luciferase reporter gene plasmid upstream of the E1B minimal promoter as described in Materials and Methods. The p53-negative LAN-1 cells were cotransfected with the p53 vectors expressing the p53 cloned from either SH-SY5Y, IGR-N-91, SK-N-BE(2), SK-N-AS or IGR-NB8 and the luciferase reporter plasmids. Both p53RE were strongly stimulated in cells cotransfected with wt p53 cloned from SH-SY5Y, as compared to cells cotransfected with an empty plasmid. In contrast, none of the p53 variants was able to transactivate the expression of luciferase driven by either p21/WAF1 or BAX p53RE (Figure 5).
Figure 5.
p53 transactivation ability by luciferase test using plasmid pE1B-hWAF1(A) and Bax (B). p53-deficient LAN-1 cells (p53-) (left panel) or SH-SY5Y (p53+) (right panel) were cotransfected with 0.5 μg of the luciferase reporter gene containing the human p21/WAF1-p53-responsive element and with 1 μg of the expressing vector as indicated. Cells were collected and subject to luciferase assay, 24 h following cotransfection. The values represent mean relative luciferase activity from three independent experiments. SH-SY5Y, IGR-N-91, SK-N-BE(2), SK-N-AS and IGR-NB8 were termed as SH, N91, BE(2), AS and NB8, respectively.
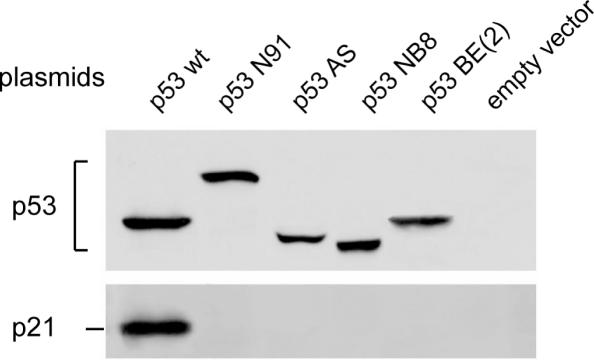
To test transactivation capability at the protein level, each variant was transfected into p53-negative LAN-1 cells and the stimulation of endogenous p21/Waf1 gene expression was analyzed by western blotting. As shown in Figure 6, in contrast to wt p53, none of the p53 variants was able to induce p21 protein accumulation.
Figure 6.
Western blot showing induction of p21/WAF1 protein by plasmid-recombinant expression vector of p53 variant transfected into p53-negative LAN-1 cells. LAN-1 cells were seeded onto 6-well plates. At a density of ∼60%, confluence cells were transfected with recombinant vector using Lipofectamine 2000 reagent according to the supplier's instructions (Invitrogen). To ascertain the transfection efficiency, cells were transfected in parallel experiments with pEGFP-C1 vector (Promega). The empty vector was used as a control. As shown in Figure 1, note that the p53 protein from the IGR-N-91 cells analyzed by SDS–PAGE migrated more slowly than the wild-type p53 due to duplication of exons 7-8-9 as described previously (13).
We then tested for a possible dominant negative effect of these various mutants on wt p53-dependent transcriptional activity. To this end, SH-SY5Y cells were cotransfected with constructs encoding the luciferase gene driven by either the p21/Waf1 or BAX p53RE and the constructs expressing the various p53s cloned from IGR-NB8, SK-N-BE(2), IGR-N-91, SK-N-AS and IGR-NB8 NB cells or p53DD, a dominant negative mutant of wt p53 (24). The stress induced by transfection activated the transcriptional activity of the wt p53 expressed in SH-SY5Y, leading to a p53-dependent expression of luciferase as illustrated by the fact that coexpression of p53DD led to a substantial decrease in luciferase activity when compared to the luciferase activity of cells cotransfected with an empty plasmid (Figure 5). Compared to p53DD, mutants within the DBD isolated from IGR-N-91 or SK-N-BE(2) had only a moderate dominant negative effect on endogenous wt p53 transcriptional activity. More surprisingly, the transfection of the C-terminal truncated variant IGR-NB8 enhanced both BAX and p21/WAF1 p53RE activity. The coexpression of p53β cloned from SK-N-AS also enhanced BAX p53RE activity. A similar effect has been reported already for p53β by Bourdon et al. (22).
When combined, these results show that all the p53 variants isolated from the NB cells had lost the ability to specifically transactivate the p53 target genes. Their effect on the transcriptional activity of endogenous wt p53 expressed in SH-SY5Y cells, however, largely depended on the p53 domain affected by the modification.
All the identified p53 variants inhibited the induction of endogenous p21/WAF1 gene expression under stress conditions
We further examined whether the four p53 variants, SK-N-BE(2), IGR-N-91, SK-N-AS and IGR-NB8, had loss their ability to stimulate the endogenous expression of the p21/WAF1 gene, the archetypical cell cycle inhibitor and the true target of p53. To this end, cellular response to genotoxic stress was analyzed by western blot following treatment of the various cell lines with cisplatin, one of the most potent antitumor agents used in neuroblastoma. Results are presented in Figure 7. None of the mutant cells, regardless of the type of mutation, was able to induce p21/WAF1 protein accumulation, unlike the 3 p53 wild-type cells (SH-SY5Y, IMR-32 and LAN-5).
Figure 7.
Western blot showing induction of endogenous p21/WAF1 in different neuroblastoma cell lines in response to 10 μM cisplatin treatment. Protein p21/WAF1 induction was observed only in the three wild-type p53 NB cell lines and the protein was differentially accumulated.
Genomic status of TP53 region in the various cell lines
According to Knudson's ‘two hit’ model of tumor suppressor gene functional inactivation, the mutation of one allele is supposed to be associated with a deletion of the second allele. To assess this genetic mechanism, we performed FISH experiments to search for deletions of one copy of the TP53 region, especially in cell lines with a mutated TP53 gene. For this purpose, metaphase preparations of the studied cell lines were cohybridized with a p53 DNA probe labeled in red (BAC clone RP11-199F11) as described previously (25) and a chromosome 17-specific centromeric probe labeled in green. The three cell lines shown previously to express a wt p53 protein (LAN5, IMR32 and SH-SY5Y), displayed as expected two signals with each probe, confirming the presence of both TP53 alleles in these cells (Figure 8 and Table 3). Conversely, IGR-N-91 and SK-N-AS cell lines displayed only one fluorescent signal for each probe, suggesting a whole chromosome 17 lost, or at least losses of the 17p arm and the centromeric region. The SK-N-BE(2) cell line has been described as containing only one chromosome 17 and one TP53 signal (10). In our analysis, only 10% of the cells displayed this characteristic, whilst most of the cells had two copies of both (Figure 8 and Table 3). p53 sequencing, however, confirmed the previously described mutation, and the absence of a normal allele, suggesting that the cells used in our study had acquired, during culture, an uniparental disomy for the TP53-mutated chromosome 17. Finally, the other two cell lines (LAN-1 and IGR-NB8) displayed highly variable genetic heterogeneity from one cell to the next (Table 3). Surprisingly, although p53 transcripts are extremely faintly expressed in LAN-1 cell line, all cells showed several FISH signals with the 167 kb BAC probe used here. To understand this apparent contradiction, an array-CGH experiment was performed on an oligo-array Agilent, which indicated a 133 kb interstitial deletion corresponding to the p53 coding region and the 97 kb upstream region. Accordingly, the fluorescent spots observed in FISH experiments on LAN-1 cells should be related to the hybridization of the 57 kb region downstream of p53 gene present in the BAC probe. IGRN-B8 cell line displayed a number of signals of both colors ranging from 0 to 4, with 87% of cells displaying a loss for one TP53 allele. Despite this genomic variability, analysis of the p53 protein showed a single shortened form in IGR-N-B8 cell line (Figure 1). Consequently, and as suggested for SK-N-BE(2) cell line, IGR-N-B8 cell line should contain a variable number of copies of chromosome 17 with mutated p53.
Figure 8.
Visualization of chromosome 17 copy number and p53 genes by FISH experiments. Chromosome 17 centromere probe is green labeled and TP53 gene probe red labeled. Only the predominant clone of each cell line is presented and its frequency indicated.
Table 3.
Genomic status of chromosome 17 centromere and TP53 gene explored by FISH
| NB cell line | Ploidy | No. of chrb 17 centromere(s) | No. of TP53 spots | % of cells |
|---|---|---|---|---|
| IMR32 | Pseudodiploid | 2 | 2 | 100 |
| LAN-5 | Pseudodiploid | 2 | 2 | 100 |
| SH-SY5Y | Pseudodiploid | 2 | 2 | 100 |
| IGR-N-91 | Pseudodiploid | 1 | 1 | 100 |
| SK-N-AS | Pseudodiploid | 1 | 1 | 95 |
| Pseudotetraploid | 2 | 2 | 5 | |
| SK-N-BE(2) | Pseudodiploid | 2 | 2 | 90 |
| Pseudodiploid | 1 | 1 | 10 | |
| LAN-1 | Pseudotriploid | 3 or 4 | 3 or 4 | 77 |
| Pseudotriploid | 3 or 4 | 1 or 2 | 20 | |
| Pseudodiploid | 2 | 2 | 3 | |
| IGR-NB8 | Pseudodiploid | 1 or 2 | 0 or 1 | 76 |
| Pseudodiploid | 2 | 2 | 3 | |
| Pseudotetraploid | 3 | 1–3 | 11 | |
| Pseudotetraploid | 4 | 4 | 10 |
DISCUSSION
The p53 gene, the ‘genome guardian’, is mutated in over 50% of human cancers, with the most common mutations being missense mutations (>2/3 of mutations) (26). In human neuroblastoma tumors, p53 mutations are rarely present at the time of diagnosis (5,27); however, oncogenic p53 mutations can be found in advanced neuroblastomas that often relapse following high-dose chemotherapy (10). In contrast, in breast cancers, it has been reported that p53 mutations might improve response to high-dose chemotherapy including therapy with epirubicin and cyclophosphamide (28).
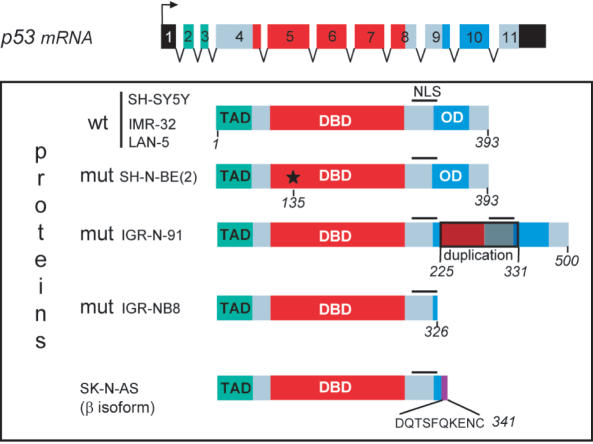
An investigation into the p53 genomic status and functions of eight human NB lines revealed that all five of the mutated cell lines had distinct genetic characteristics as is schematically represented in Figure 9: SK-N-BE(2) with a single missense mutation in the p53 gene, encoding a highly stable full-length protein. SK-N-AS and IGR-NB8 proteins, although they have intact transactivation and DBDs, were truncated at the C-terminus generating 341 and 326 amino acids respectively; they therefore lack the tetramerization domain that is essential for an active conformation. Very recently, Bourdon et al. (22) reported the putative occurrence of β and γ isoforms from different tissues due to alternate splicing that indicates the similarity to those of p73 and p63 as identified previously by Daniel Caput and co-workers (29). In the p53 isoforms scheme proposed by Bourdon et al. (22), the SK-N-AS cell line that elicits p53i9 protein expression is consistent with the p53β isoform. Genomic analysis reveals that the only occurrence of the p53β isoform in SK-N-AS results from a deletion spanning the intron 9/exon 10 junction. Similar to the p53β isoform in SK-N-AS, the p53 in IGR-NB8 that lacks 67 amino acids at C-terminus was, alone, unable to induce p21/WAF1 promoter activation except with endogenous wt-p53 on SH-SY5Y cells where transfection with IGR-NB8 significantly augmented the transcriptional activation of the p21/Waf-1 promoter (Figure 5A). Studies by other authors have reported the interaction between the C-terminal domain and another region that impedes the active conformation of p53, suggesting an allosteric model for p53 activity regulation (30). Such events have been demonstrated for the 342-stop mutant, generated by mutagenesis, which can modulate transactivation, growth and apoptosis (31). Moreover, Harms and Chen (32) reported that the C-terminal basic domain inhibits induction of the proapoptotic target gene insulin-like growth factor binding protein 3, suggesting that IGR-NB8 might induce this gene. IGR-N-91 had an abnormally high molecular weight protein due to the duplication of wild-type exons 7-8-9, thus affecting the DBD and OD; and LAN-1, with a mutation at codon 182 (Cys→stop) concurred with an earlier report showing extremely low levels of mRNA and undetectable protein expression (9).
Figure 9.
Structure of p53 proteins in different neuroblastoma cell lines. The three functional domains are represented: TAD, transactivation domain; DBD, DNA-binding domain; OD, oligomerization domain. The wild-type p53 gene in SH-SY5Y, IMR-32 and LAN-5 cells contains 11 exons that encode 393 amino acids. In SK-N-BE(2) cells, p53 is mutated at codon 135 (*), which converts cysteine to phenylalanine. In IGR-N-91 cells, a duplication of exons 7-8-9 adds an additional 107 amino acids leading to a total of 500. In SK-N-AS cells, a mutation due to alternate splicing downstream of exon 9 leads to a protein of 341 amino acids whereas in IGR-NB8 cells, the p53 protein ends at 326 amino acids owing to the mutation E326STOP.
Notably, all the p53 variants, including SK-N-AS (β isoform) and IGR-NB8 (C-terminal truncated p53), elicited a total lack of p21 promoter activation. In particular, the p53β isoform was unable to induce endogenous p21 expression in SK-N-AS (Figure 6), concurring with data obtained from in vitro transfection experiments in H1299 cells by Bourdon et al. (22). For the IGR-N-91 cells, although p53 was mutated and unable to transactivate the p21/WAF1 promoter, the FASAY global test was not conclusive since ∼80% of colonies were white and nearly 20% (see also Table 2), though not enough, were red. Moreover, in this particular line, standard sequencing on cDNA using primers located within each exon as used for routine tumor analysis was unable to detect any anomalies in p53 genetic status (data not shown). These results enlighten the limit of the conventional tests to detect a transcription inactivation of p53 brought by duplication within the DBD.
Analysis of p53 genomic status was explored by FISH experiments, in search for a potential biallelic inactivation of p53, with a mutation of one allele and a deletion of the second one. This situation was indeed clearly observed in IGR-N-91 and SK-N-AS cell lines, with an unambiguous loss of one chromosome 17p arm in all cells of both. SK-N-BE(2), LAN-1 and IGR-NB-8 cell lines showed a more complex genomic situation which should be relevant of variable copy numbers of chromosome 17 bearing in most cases (LAN-1) or in all cases [SKN-BE(2), IGR-NB8] the mutated characteristic p53 allele. Our data therefore clearly demonstrate that each technique has a role and a combination of techniques is required in order to correctly define the p53 phenotype and genotype in tumor and particularly in NB cells.
Our data enlighten a high frequency of the C-terminal abnormalities (3/5 mutated) in NB cell lines. For SK-N-AS and IGR-NB8, a part of the oligomerization domain was lost and IGR-N-91 gained an extra oligomerization domain. According to FASAY assay the p53 expressed in IGR-N-91 still specifically bind DNA but not the p53 expressed in SK-N-AS and IGR-NB8 in agreement with previous published data obtained by electrophoretic mobility shift assay (33).
With regards to biological relevance, different mutants within the DBD vary in their oncogenicity. They are classified into two types depending of the location of the mutation, mutations of class I occur in the DNA contact areas, while class II mutations occur in areas important for the conformational stability of p53 protein (30). Although both class I and class II mutants have loss its ability to specifically bind DNA, class II mutations have been shown to be more oncogenic than class I. However, to our knowledge the oncogenicity of mutant affecting the C-terminal domain have not been studied. The biological role of the C-terminal mutants needs now to be thoroughly investigated in NB tumors.
Acknowledgments
We are very grateful to Prof. Gilles Vassal for supplying the IGR-NB8 cell line, Dr Michel Barrois for assistance with the design of primers for TaqMan, and Dr Marc Lipinski and Dr David Cappellen for valuable discussions. Edited by English Booster. D.G. is supported by a ‘Ligue contre le Cancer’ fellowship. This work was partially supported by grants from the ‘Ligue contre le Cancer, Comité du Cher’ and ‘Association pour la Recherche sur le Cancer’. Funding to pay the Open Access publication charges for this article was provided by the Association pour la Recherche sur le Cancer, Villjuif, France.
Conflict of interest statement. None declared.
REFERENCES
- 1.Hainaut P., Hollstein M. p53 and human cancer: the first ten thousand mutations. Adv. Cancer Res. 2000;77:81–137. doi: 10.1016/s0065-230x(08)60785-x. [DOI] [PubMed] [Google Scholar]
- 2.Soussi T., Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nature Rev. Cancer. 2001;1:233–240. doi: 10.1038/35106009. [DOI] [PubMed] [Google Scholar]
- 3.Beroud C., Soussi T. The UMD-p53 database: new mutations and analysis tools. Hum. Mutat. 2003;21:176–181. doi: 10.1002/humu.10187. [DOI] [PubMed] [Google Scholar]
- 4.Soussi T., Kato S., Levy P.P., Ishioka C. Reassessment of the TP53 mutation database in human disease by data mining with a library of TP53 missense mutations. Hum. Mutat. 2005;25:6–17. doi: 10.1002/humu.20114. [DOI] [PubMed] [Google Scholar]
- 5.Moll U.M., Laquaglia M., Benard J., Riou G. Wild-type p53 protein undergoes cytoplasmic sequestration in undifferentiated neuroblastomas but not in differentiated tumors. Proc. Natl Acad. Sci. USA. 1995;92:4407–4411. doi: 10.1073/pnas.92.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostermeyer A.G., Runko E., Winkfield B., Ahn B., Moll U.M. Cytoplasmically sequestered wild-type p53 protein in neuroblastoma is relocated to the nucleus by a C-terminal peptide. Proc. Natl Acad. Sci. USA. 1996;93:15190–15194. doi: 10.1073/pnas.93.26.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolaev A.Y., Li M.Y., Puskas N., Qin J., Gu W. Parc: a cytoplasmic anchor for p53. Cell. 2003;112:29–40. doi: 10.1016/s0092-8674(02)01255-2. [DOI] [PubMed] [Google Scholar]
- 8.Finlay C.A., Hinds P.W., Tan T.H., Eliyahu D., Oren M., Levine A.J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol. Cell. Biol. 1988;8:531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidoff A.M., Pence J.C., Shorter N.A., Iglehart J.D., Marks J.R. Expression of p53 in human neuroblastoma- and neuroepithelioma-derived cell lines. Oncogene. 1992;7:127–133. [PubMed] [Google Scholar]
- 10.Tweddle D.A., Malcolm A.J., Bown N., Pearson A.D., Lunec J. Evidence for the development of p53 mutations after cytotoxic therapy in a neuroblastoma cell line. Cancer Res. 2001;61:8–13. [PubMed] [Google Scholar]
- 11.Keshelava N., Zuo J.J., Chen P., Waidyaratne S.N., Luna M.C., Gomer C.J., Triche T.J., Reynolds C.P. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines'. Cancer Res. 2001;61:6185–6193. [PubMed] [Google Scholar]
- 12.Ferrandis E., Da Silva J., Riou G., Benard I. Coactivation of the MDR1 and MYCN genes in human neuroblastoma cells during the metastatic process in the nude mouse. Cancer Res. 1994;54:2256–2261. [PubMed] [Google Scholar]
- 13.Goldschneider D., Blanc E., Raguenez G., Barrois M., Legrand A., Le Roux G., Haddada H., Benard J., Douc-Rasy S. Differential response of p53 target genes to p73 overexpression in SH-SY5Y neuroblastoma cell line. J. Cell Sci. 2004;117:293–301. doi: 10.1242/jcs.00834. [DOI] [PubMed] [Google Scholar]
- 14.Vassal G., Terrier-Lacombe M.J., Bissery M.C., Venuat A.M., Gyergyay F., Benard J., Morizet J., Boland I., Ardouin P., Bressac-de-Paillerets B., et al. Therapeutic activity of CPT-11, a DNA-topoisomerase I inhibitor, against peripheral primitive neuroectodermal tumour and neuroblastoma xenografts. Br. J. Cancer. 1996;74:537–545. doi: 10.1038/bjc.1996.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaman J.M., Waridel F., Estreicher A., Vannier A., Limacher J.M., Gilbert D., Iggo R., Frebourg T. The human tumour suppressor gene p53 is alternatively spliced in normal cells. Oncogene. 1996;12:813–818. [PubMed] [Google Scholar]
- 16.Drané P., Leblanc V., Miro-Mur F., Saffroy R., Debuire B., May E. Accumulation of an inactive form of p53 protein in cells treated with TNF alpha. Cell Death Differ. 2002;9:527–537. doi: 10.1038/sj.cdd.4400983. [DOI] [PubMed] [Google Scholar]
- 17.Munsch D., Watanabe-Fukunaga R., Bourdon J.C., Nagata S., May E., Yonish-Rouach E., Reisdorf P. Human and mouse Fas (APO-1/CD95) death receptor genes each contain a p53-responsive element that is activated by p53 mutants unable to induce apoptosis. J. Biol. Chem. 2000;275:3867–3872. doi: 10.1074/jbc.275.6.3867. [DOI] [PubMed] [Google Scholar]
- 18.Thornborrow E.C., Patel S., Mastropietro A.E., Schwartzfarb E.M., Manfredi J.J. A conserved intronic response element mediates direct p53-dependent transcriptional activation of both the human and murine bax genes. Oncogene. 2002;21:990–999. doi: 10.1038/sj.onc.1205069. [DOI] [PubMed] [Google Scholar]
- 19.Meddeb M., Danglot G., Chudoba I., Venuat A.M., Benard J., Avet-Loiseau H., Vasseur B., Le Paslier D., Terrier-Lacombe M.J., Hartmann O., et al. Additional copies of a 25 Mb chromosomal region originating from 17q23.1-17qter are present in 90% of high-grade neuroblastomas. Genes Chromosomes Cancer. 1996;17:156–165. doi: 10.1002/(SICI)1098-2264(199611)17:3<156::AID-GCC3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Waridel F., Estreicher A., Bron L., Flaman J.M., Fontolliet C., Monnier P., Frebourg T., Iggo R. Field cancerisation and polyclonal p53 mutation in the upper aerodigestive tract. Oncogene. 1997;14:163–169. doi: 10.1038/sj.onc.1200812. [DOI] [PubMed] [Google Scholar]
- 21.Goldschneider D., Million K., Meiller A., Haddada H., Puisieux A., Benard J., May E., Douc-Rasy S. The neurogene BTG2TIS21/PC3 is transactivated by DeltaNp73alpha via p53 specifically in neuroblastoma cells. J. Cell Sci. 2005;118:1245–1253. doi: 10.1242/jcs.01704. [DOI] [PubMed] [Google Scholar]
- 22.Bourdon J.C., Fernandes K., Murray-Zmijewski F., Liu G., Diot A., Xirodimas D.P., Saville M.K., Lane D.P. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaman J.M., Frebourg T., Moreau V., Charbonnier F., Martin C., Chappuis P., Sappino A.P., Limacher I.M., Bron L., Benhattar J. A simple p53 functional assay for screening cell lines, blood, and tumors. Proc. Natl Acad. Sci. USA. 1995;92:3963–3967. doi: 10.1073/pnas.92.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaulian E., Haviv I., Shaul Y., Oren M. Transcriptional repression by the C-terminal domain of p53. Oncogene. 1995;10:671–680. [PubMed] [Google Scholar]
- 25.Guillaud-Bataille M., Valent A., Soularue P., Perot C., Inda M.M., Receveur A., Smaili S., Crollius H.R., Benard J., Bernheim A., et al. Detecting single DNA copy number variations in complex genomes using one nanogram of starting DNA and BAC-array CGH. Nucleic Acids Res. 2004;32:e112. doi: 10.1093/nar/gnh108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigal A., Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- 27.Vogan K., Bernstein M., Leclerc J.M., Brisson L., Brossard J., Brodeur G.M., Pelletier J., Gros P. Absence of p53 gene mutations in primary neuroblastomas. Cancer Res. 1993;53:5269–5273. [PubMed] [Google Scholar]
- 28.Bertheau P., Plassa F., Espie M., Turpin E., de Roquancourt A., Marty M., Lerebours F., Beuzard Y., Janin A., De The H. Effect of mutated TP53 on response of advanced breast cancers to high-dose chemotherapy. Lancet. 2002;360:852–854. doi: 10.1016/S0140-6736(02)09969-5. [DOI] [PubMed] [Google Scholar]
- 29.Kaghad M., Bonnet H., Yang A., Creancier L., Biscan J.C., Valent A., Minty A., Chalon P., Lelias J.M., Dumont X., et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 30.May P., May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 1999;18:7621–7636. doi: 10.1038/sj.onc.1203285. [DOI] [PubMed] [Google Scholar]
- 31.Zhou X., Wang X.W., Xu L., Hagiwara K., Nagashima M., Wolkowicz R., Zurer I., Rotter V., Harris C.C. COOH-terminal domain of p53 modulates p53-mediated transcriptional transactivation, cell growth, and apoptosis. Cancer Res. 1999;59:843–848. [PubMed] [Google Scholar]
- 32.Harms K.L., Chen X. The C terminus of p53 family proteins is a cell fate determinant. Mol. Cell. Biol. 2005;25:2014–2030. doi: 10.1128/MCB.25.5.2014-2030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yonish-Rouach E., Deguin V., Zaitchouk T., Breugnot C., Mishal Z., Jenkins J.R., May E. Transcriptional activation plays a role in the induction of apoptosis by transiently transfected wild-type p53. Oncogene. 1995;11:2197–2205. [PubMed] [Google Scholar]