Abstract
Objective
We have previously shown that short-term energy restriction followed by modest lifestyle changes improves glucose tolerance for up to 1 year in obese individuals. The purpose of the present study was to determine the mechanism by which improvements in glucose tolerance occur in obese African Americans with insulin resistance and abnormal glucose tolerance.
Research Design and Methods
Nine subjects (53 ± 2 years; body mass index, 37 ± 3 kg/m2 [mean ± SEM]) received a low-energy diet (3883 ± 222 kJ/d) for 1 week, and then followed a modest lifestyle intervention program for up to 1 year. Body composition was estimated by hydrostatic weighing, and insulin secretion and action were assessed during a hyperglycemic clamp with superimposed arginine infusion and fat meal. Baseline and final tests were performed during weight stability.
Results
Significant improvements (P < .05) were observed for body weight (−6.1 ± 1.1 kg), body composition (−5.5 ± 1.3 kg fat mass), fasting plasma glucose (−1.1 ± 0.3 mmol/L), fasting insulin (−52 ± 21 pmol/L), oral glucose tolerance, and insulin action (+24%), defined as an increase in glucose disposal rate relative to plasma insulin concentration during the hyperglycemic clamp. These improvements were independent of an acute effect of energy restriction or weight loss, because body weight was stable.
Conclusions
These results suggest that the improvements in glucose tolerance with a modest lifestyle intervention were attributable to an improvement in insulin action, and provide evidence that despite persistent obesity (body mass index, 34.7 ± 2.4 kg/m2), long-term benefits can be achieved with relatively small weight loss in obese African Americans.
1. Introduction
The metabolic syndrome [1], characterized by abdominal obesity, insulin resistance, fasting hyperglycemia, hypertension, and dyslipidemia, contributes to the development of impaired glucose tolerance (IGT) and type 2 diabetes [2–4]. Because obesity and type 2 diabetes are more prevalent among African Americans than Caucasians [5,6], interventions aimed at reducing body weight and improving glucose regulation are particularly important in this population. Negative energy balance, achieved by energy restriction [7–9] or exercise [7,8,10], can reduce insulin resistance and ameliorate hyperglycemia and hyperinsulinemia. Exercise has the additional advantage of providing a weight loss–independent enhancement of insulin action [8,11–13], and prospective studies have shown a preventive effect of physical activity on the incidence of type 2 diabetes [14–19]. Therefore, we hypothesized that a treatment program consisting of short-term energy restriction followed by modest changes in diet and physical activity habits would have beneficial effects on glucose tolerance that are mediated by improvements in insulin action in obese individuals with abnormal glucose tolerance.
Although earlier studies have evaluated the effects of long-term lifestyle interventions on glycemic control in subjects with IGT [20], there is little information regarding the mechanism by which such improvements occur. Previous studies of obese individuals with impaired or diabetic glucose tolerance conducted in our laboratory demonstrated that short-term diet and exercise interventions improve glucose concentrations and insulin action [8], and a modest decrease in dietary fat combined with a small increase in physical activity can maintain improvements in glucose tolerance for up to 1 year [21]. The mechanisms responsible for improvements in glucose tolerance may include an increase in pancreatic beta-cell insulin secretion, enhanced insulin action on skeletal muscle and liver, and an increase in GLUT4 glucose transporter content of skeletal muscle [22]. We previously found that acute improvements in glucose concentrations after 10 days of energy restriction or exercise training were attributable to improvements in insulin action [8]. In the present study, we sought to determine whether similar results are observed when improvements in oral glucose tolerance are sustained for up to 1 year. Therefore, the aim of the current study was to determine the mechanism by which long-term modest lifestyle changes maintain or enhance the improvements in glucose tolerance in response to a 1-week diet in obese African Americans with insulin resistance and impaired or diabetic glucose tolerance. We used a modified hyperglycemic clamp technique, in which an arginine infusion and fat ingestion were superimposed on hyperglycemia, to assess insulin secretory response and insulin action.
2. Research design and methods
2.1. Subjects
Obese African American men and women with IGT or type 2 diabetes according to the 1997 guidelines of the Expert Committee on the Diagnosis and Categorization of Diabetes Mellitus [23] were invited to participate. Insulin therapy was exclusionary. Subjects who were being treated with oral hypoglycemic agents withheld their medications for 24 hours before testing. Screening tests included a medical history, physical examination, standard blood chemistries, and oral glucose tolerance test. Eligible subjects were weight stable and not engaged in a regular exercise program before enrollment. The study was approved by the Human Studies Committee of Washington University School of Medicine, St Louis, Mo, and the Scientific Advisory Committee of the Washington University General Clinical Research Center (GCRC). Written informed consent was obtained from each participant before enrollment.
These subjects were part of a larger study evaluating the effects of short-term energy restriction and modest lifestyle changes on body weight and glucose tolerance over a 1-year period in 69 obese African Americans with IGT or type 2 diabetes [21]. A subgroup of subjects who underwent an initial hyperglycemic clamp at baseline, and whose oral glucose tolerance improved significantly by the end of the 1-year intervention, was asked to undergo a second hyperglycemic clamp. A significant improvement in oral glucose tolerance was defined as a change in glucose tolerance status from IGT to normal, from diabetic glucose tolerance to IGT, or from diabetic to normal glucose tolerance. Of the 30 subjects who underwent a hyperglycemic clamp at baseline, 19 completed the 1-year intervention, 10 of whom showed a significant improvement in oral glucose tolerance. Body weight changes were −6.8 ± 1.1 kg (P = .0004) in these 10 subjects and −2.3 ± 1.2 kg (P = .047) among the 9 others who did not show a significant improvement in oral glucose tolerance after 1 year. Of the 10 eligible subjects, one declined the final clamp, leaving a sample of 9.
2.2. Intervention
The intervention began with a 1-week low-energy diet (63 kJ/kg fat-free mass [FFM] per day, 3883 ± 222 kJ/d) designed to initiate weight loss and improve plasma glucose and insulin concentrations [8,21]. The diet contained 60% of energy as carbohydrate, 25% protein, and 15% fat, and consisted of common foods prepared in the metabolic kitchen of the GCRC. Throughout the ensuing 12 months, subjects were instructed to follow a modest lifestyle program of reduced dietary fat (−14 g/d, −523 kJ/d) and increased physical activity (+523 kJ/d). Rather than formal exercise training, the physical activity goal was to increase daily energy expenditure either with daily physical activities or aerobic exercise (unsupervised).
2.3. Anthropometrics
Height was measured without shoes to the nearest 0.1 cm. Body weight was obtained on a balance scale in the morning after a 12-hour fast. Body mass index was calculated by dividing body weight (in kilogram) by the square of height (in meter).
2.4. Body composition
Total body fat and FFM were assessed by hydrodensitometry. Body fat percentage was calculated from body density using the equation of Brozek et al [24].
2.5. Oral glucose tolerance tests
Oral glucose tolerance tests were performed in the morning after a 12-hour fast. Blood samples were drawn for the determination of plasma glucose, insulin, and C-peptide concentrations before and every 30 minutes for 2 hours after the administration of a 75-g glucose beverage. Plasma concentrations of glucose were measured by the glucose oxidase method (Beckman Instruments, Fullerton, Calif) and insulin and C peptide by radioimmunoassay. Total areas under the curves for glucose, insulin, and C peptide were calculated using the trapezoidal rule.
2.6. Hyperglycemic clamps
Insulin secretion and action were evaluated during a 2-hour hyperglycemic clamp procedure in which plasma glucose concentrations were raised to and maintained at 13.9 mmol/L, and during which an arginine infusion and fat meal were superimposed [25]. After an overnight fast, subjects were admitted to the GCRC between 0700 and 0800 hours, voided, weighed, and had 2 polyethylene intravenous catheters placed: one in an antecubital vein for the infusion of dextrose and arginine hydrochloride, and the other in a hand or wrist vein for blood sampling. The hand was placed in a heated (65°C) warming box to arterialize the blood.
After 30 minutes, 3 baseline blood samples were obtained at 5-minute intervals to determine basal plasma glucose, insulin, and C-peptide concentrations. The hyper-glycemic clamp was initiated with a 10-minute priming infusion of 20% dextrose in water with a Harvard pump (Harvard, Natick, Mass) to raise the plasma glucose concentration to 13.9 mmol/L. The priming infusion rate decreased each minute throughout the first 10 minutes based upon a computer-generated algorithm. Thereafter, a variable rate dextrose infusion was adjusted at 5-minute intervals or as necessary to minimize fluctuations in plasma glucose concentrations. Adjustments were based upon the plasma glucose concentrations determined every 5 minutes throughout the hyperglycemic clamp.
Forty-five minutes after the start of the dextrose infusion, a 5-g dose of arginine monohydrochloride diluted with 0.9% NaCl to a total volume of 50 mL was administered over a 2-minute period (infusion rate, 25 mL/min). This is a maximally stimulating dose of the amino acid arginine on pancreatic beta-cell insulin secretion. A continuous infusion of arginine hydrochloride, diluted to the same concentration as the bolus dose, was administered at a rate of 15.0 g/m2 per hour throughout the remaining 75 minutes of hyperglycemia. Seventy-five minutes after the start of the dextrose infusion (30 minutes after the start of the arginine infusion), subjects consumed a liquid fat drink (37.5 mL) containing 25 g of fat in the form of corn oil (Lipomul; Upjohn, Kalamazoo, Mich). The hyperglycemic clamp was terminated at 120 minutes, the arginine infusion was discontinued, and the dextrose infusion rate was reduced and continued as long as needed to maintain the plasma glucose concentration at or above the fasting value.
Glucose disposal rates (GDRs) were calculated during each period of hyperglycemia: hyperglycemia alone (15–45 minutes), hyperglycemia with superimposed arginine infusion (45–75 minutes), and hyperglycemia with arginine after the fat meal (75–120 minutes), as well as for the entire period of hyperglycemia (15–120 minutes). Glucose disposal rates are expressed relative to FFM.
2.7. Statistical analyses
Analyses were performed using SPSS statistical software, version 11.5 (SPSS, Inc, Chicago, Ill). Paired t tests and repeated measures analysis of variance, when appropriate, were used to determine if the changes in body weight, body composition, oral glucose tolerance, and GDRs during the hyperglycemic clamp were statistically significant at an α level of .05. All data represent mean ± SEM.
3. Results
3.1. Subject characteristics
Nine African American subjects (8 women, 1 man) were studied. The baseline characteristics of the subjects are shown in Table 1. Five participants had IGT, and 4 had type 2 diabetes at baseline. All but one subject had impaired fasting glucose using the most recent criteria of 5.6 mmol/L as the cut point [26]. One subject was on medication for diabetes management (an α glucosidase inhibitor) but withheld this for 24 hours before testing.
Table 1.
Subject characteristics before the intervention and changes in response to the intervention
| Before | Change | P | |
|---|---|---|---|
| Height (cm) | 163 ± 2 | ||
| Weight (kg) | 99.7 ± 8.6 | −6.1 ± 1.1 | .0003 |
| Body mass index (kg/m2) | 37.0 ± 2.5 | −2.3 ± 0.4 | .0003 |
| Fat mass (%)a | 40.2 ± 2.1 | −3.1 ± 1.2 | .018 |
| Fat mass (kg)a | 41.0 ± 5.2 | −5.5 ± 1.2 | .002 |
| FFM (kg)a | 58.7 ± 4.2 | −1.5 ± 0.6 | .019 |
| Waist circumference (cm) | 108.0 ± 4.5 | −5.6 ± 1.8 | .009 |
| Hip circumference (cm) | 124.2 ± 5.3 | −3.9 ± 1.8 | .039 |
| Waist-to-hip ratio | 0.87 ± 0.01 | −0.03 ± 0.02 | .055 |
| Fasting plasma glucose (mmol/L) | 6.5 ± 0.5 | −1.1 ± 0.3 | .006 |
| Fasting plasma insulin (pmol/L) | 152 ± 33 | −52 ± 21 | .018 |
Values are mean ± SEM.
Change data for fat mass and FFM are missing for one subject.
3.2. Body weight and composition
Rapid weight loss was observed in response to the 1-week low-energy diet provided at the beginning of the program (−3.2 ± 0.7 kg, P = .002), which was attributable, in part, to reductions in water and glycogen stores. By the end of the long-term modest intervention, significant reductions were observed for body weight (−6.1 ± 1.1 kg, −6.4% ± 1.2% relative to baseline), fat mass (−5.5 ± 1.2 kg), and waist circumference (Table 1). Body weight was stable during the baseline and final testing periods (change in weight = 0.0 ± 0.1 kg during the week preceding the final hyperglycemic clamp, −0.2 ± 1.5 during the month preceding the final clamp).
3.3. Oral glucose tolerance
The responses of plasma glucose, insulin, and C peptide during the oral glucose tolerance tests before and after the intervention are shown in Fig. 1. All 5 subjects with IGT at baseline had normal glucose tolerance after the intervention. Of the 4 subjects with diabetic glucose tolerance at baseline, 1 had normal and 3 had IGT after the intervention. The area under the glucose curve was 21% ± 4% lower (P < .001) after the intervention. Impaired fasting glucose persisted in 4 subjects using the revised criteria [26] and in 2 subjects using the older criteria [23].
Fig. 1.
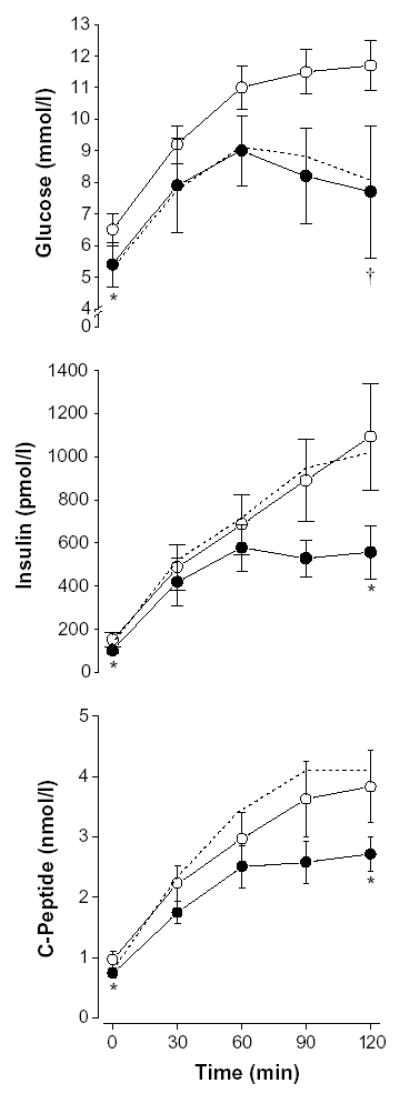
Oral glucose tolerance test results for glucose, insulin, and C-peptide at baseline (white circles), after 1 week (dotted line), and after the 1-year intervention (black circles). Asterisk indicates P < .05 for reductions in fasting glucose, insulin, and C-peptide values; 2-hour insulin and C-peptide values; and area under the curve values for insulin and C-peptide (1 year vs baseline). Dagger indicates P < .001 for reductions in 2-hour glucose and glucose area under the curve (1 year vs baseline). Values are mean ± SEM.
3.4. Glucose disposal rates and plasma insulin concentrations
During the hyperglycemic clamps, plasma glucose concentrations averaged 14.1 ± 0.3 and 14.0 ± 0.3 mmol/L (15–120 minutes) before and after the intervention, respectively. Glucose disposal rates, expressed per kilogram of FFM during hyperglycemia (15–120 minutes), tended to be higher (30% ± 19%, P = .054) during the second clamp study. When expressed relative to plasma insulin concentrations, however, GDR improved 24% overall (P = .012, Fig. 2). Improvements were observed during the last 2 stages of the clamp, when plasma insulin concentrations were highest (P = .025 for 45–75 minutes, P = .013 for 75–120 minutes). No improvements in GDR were observed during hyperglycemia alone (15–45 minutes).
Fig. 2.
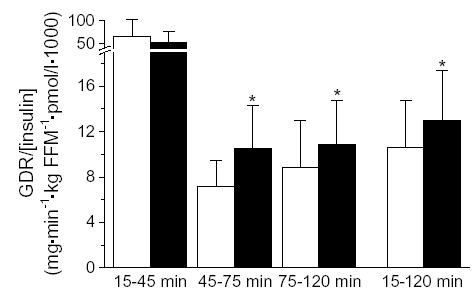
Glucose disposal rates (mg/kg FFM per minute) expressed relative to mean insulin concentration (pmol/L) during hyperglycemia alone (15–45 minutes), hyperglycemia + arginine (45–75 minutes), hyperglycemia + arginine + fat meal (75–120 minutes), and during the entire period of hyperglycemia (15–120 minutes) before (white bars) and after (black bars) the intervention. Asterisk indicates P < .05.
Plasma insulin concentrations throughout the hyperglycemic clamp are shown in Fig. 3. We were unable to detect significant changes in the first phase insulin response, whether defined as the insulin change during the first 10 minutes of the hyperglycemic clamp relative to the preclamp fasting concentration, the mean insulin concentration during the first 10 minutes, or the area under the insulin curve during the first 10 minutes (all P ≥ .143). Likewise, the final phase insulin response (75–120 minutes) did not change significantly in response to the intervention (P = .186). However, there may have been trends in the acute and the final insulin responses that did not reach significance because of the relatively small sample size.
Fig. 3.
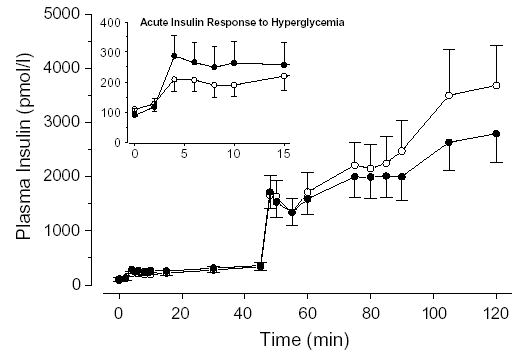
Plasma insulin responses throughout the hyperglycemic clamp before (white circles) and after (black circles) the intervention. The acute insulin response to hyperglycemia (0–15 minutes) is shown (inset).
4. Discussion
We have shown previously that short-term energy restriction followed by modest lifestyle changes results in sustained improvements in glucose tolerance for as long as 1 year in obese African American men and women [21]. In the current study, we used a hyperglycemic clamp technique to assess the mechanism by which long-term improvements occur. Our results indicate that insulin action improved, as evidenced by higher GDRs relative to plasma insulin concentrations during hyperglycemia. Importantly, these improvements were independent of an acute energy deficit, because body weight was stable during the weeks preceding testing.
Previous studies have shown improvements in insulin action in response to short-term (ie, 10-day) diet [8] and exercise [8,27] interventions, after 6- to 14-week diet and exercise regimens [28,29] and after long-term (ie, 9-month) vigorous endurance exercise training [30]. There is less evidence, however, that long-term modest lifestyle interventions produce similar physiological benefits. In the Oslo diet and exercise study [31], a 1-year, large-scale (n = 219), randomized lifestyle intervention trial that compared the independent and combined effects of diet and exercise interventions, improvements in insulin resistance were correlated with changes in body mass index. Insulin resistance, however, was calculated using fasting glucose and insulin measurements. The use of the hyperglycemic clamp technique [32] in the current study enables a mechanistic explanation for the observed improvements in glucose regulation in this and other lifestyle intervention studies.
In agreement with some previous reports [31,33], there was not a detectable improvement in pancreatic beta-cell secretory capacity, as evidenced by the lack of a significant change in either the acute or the late phase insulin response to hyperglycemia. Arciero et al [8] observed a significant reduction in the acute insulin response during a hyperglycemic clamp after 10 days of exercise training, but not after a 10-day low-energy diet in obese individuals with IGT or type 2 diabetes. In contrast, Kirwan et al [30] reported no change in the acute insulin response to hyperglycemia with exercise training in 60- to 70-year-old men and women with normal glucose tolerance, but a significant reduction in the maximal insulin response to hyperglycemia. The increases in glucose disposal relative to plasma insulin concentration in our current and previous studies in which hyperglycemic clamps were performed [8,30] imply that the improvements in glucose tolerance induced by exercise training were mediated by enhanced insulin action, not by improved insulin secretion.
Defects in insulin action resulting in IGT or type 2 diabetes generally are attributable, at least in part, to reduced translocation of the GLUT4 isoform of the glucose transporter from intracellular sites to the cell surface, and therefore reduced glucose transport in skeletal muscle [2]. Improvements in insulin action with exercise training frequently are due to increases in skeletal muscle GLUT4 content [34,35], resulting in translocation of more GLUT4 to the cell surface and increased skeletal muscle glucose transport [36]. One important distinction between these previous studies and the current study, however, is the use of short-term vigorous exercise in the former [34,35] vs modest lifestyle changes in the current study. The exercise performed by the participants in this study was low intensity, and the periods of exercise were relatively brief. Although it seems unlikely that the improvement in insulin action was mediated by an increase in muscle GLUT4 content, we cannot conclude whether GLUT4 played a role, because we did not measure it in this study.
Another more likely mediator of the improvement in insulin action was the decrease in body fat content. It is remarkable that the participants in this study had a sustained improvement in glucose tolerance and insulin action despite the fact that they were still obese after losing only 5.5 kg of fat. There is evidence that both food restriction [37] and exercise [38] have a more powerful effect on visceral fat than on subcutaneous fat, and that reductions in visceral fat have favorable metabolic effects. It is also well established that the insulin resistance associated with obesity is more closely related to abdominal obesity than to lower body obesity [37]. In the present study, there was a highly significant decrease in waist circumference, providing evidence for a decrease in abdominal fat.
There is a large body of evidence that chronic physical activity prevents the decline in insulin sensitivity and the incidence of type 2 diabetes with aging. Because a high percentage of the adult population in the United States is obese, and an even higher percentage is sedentary, the progression from normal to impaired or diabetic glucose tolerance is occurring more frequently and at earlier ages. Although short-term diet and exercise interventions have proven successful at reversing insulin resistance and improving glucose regulation acutely [8,39], there has been little evidence that modest long-term interventions allow maintenance of these beneficial changes [31]. The results of the current study provide a mechanism, that is, a decrease in abdominal fat and improvement of insulin action, by which modest lifestyle changes sustain improvements in glucose tolerance for as long as 1 year. Furthermore, although it has been evident that an acute energy deficit produces dramatic improvements in glucose regulation [7], the current study provides evidence that modest changes in lifestyle can result in persistence of improved insulin action for a prolonged period after acute weight loss. Our results provide strong evidence that public health recommendations to engage in physical activity and modify dietary intake to control body weight are beneficial for improving insulin action and glucose control in African American adults with IGT or mild type 2 diabetes.
Footnotes
This research was supported by NIH research grant DK48128, Diabetes Research and Training Center grant DK20579, General Clinical Research Center grant 5M01RR00036, and Institutional National Research Service Award AG-00078.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Zierath J, Krook A, Wallberg-Henriksson H. Insulin action in skeletal muscle from patients with NIDDM. Mol Cell Biochem. 1998;182:153–60. [PubMed] [Google Scholar]
- 3.Fujimoto WY. The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. Am J Med. 2000;108:9S–14S. doi: 10.1016/s0002-9343(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 4.Lorenzo C, Okoloise M, Williams K, et al. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care. 2003;26:3153–9. doi: 10.2337/diacare.26.11.3153. [DOI] [PubMed] [Google Scholar]
- 5.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 6.Brancati FL, Kao WHL, Folsom AR, et al. Incident type 2 diabetes mellitus in African American and white adults. JAMA. 2000;283:2253–9. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 7.Kelley DE, Wing R, Buonocore C, et al. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;77:1287–93. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 8.Arciero PJ, Vukovich MD, Holloszy JO, et al. Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol. 1999;86:1930–5. doi: 10.1152/jappl.1999.86.6.1930. [DOI] [PubMed] [Google Scholar]
- 9.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1985;61:917–25. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- 10.Dengel DR, Hagberg JM, Pratley RE, et al. Improvements in blood pressure, glucose metabolism, and lipoprotein lipids after aerobic exercise plus weight loss in obese, hypertensive middle-aged men. Metabolism. 1998;47:1075–82. doi: 10.1016/s0026-0495(98)90281-5. [DOI] [PubMed] [Google Scholar]
- 11.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–61. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 12.Kelley DE, Goodpaster BH. Effects of physical activity on insulin action and glucose tolerance in obesity. Med Sci Sports Exerc. 1999;31:S619–23. doi: 10.1097/00005768-199911001-00021. [DOI] [PubMed] [Google Scholar]
- 13.Mayer-Davis EJ, D’Agostino R, Jr, Karter AJ, et al. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279:669–74. doi: 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson KF, Lindgarde F. Prevention of type 2 (non–insulin-dependent) diabetes mellitus by diet and physical exercise: the 6-year Malmo feasibility study. Diabetologia. 1991;34:891–8. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- 15.Helmrich SP, Ragland DR, Leung RW, et al. Physical activity and reduced occurrence of non –insulin dependent diabetes mellitus. N Engl J Med. 1991;325:147–52. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 16.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non–insulin dependent diabetes mellitus in women. Lancet. 1991;338:774–8. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 17.Pan X-R, Li G-W, Hu Y-H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and diabetes study. Diabetes Care. 1997;20:537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 18.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 19.Diabetes Prevention Program Reasearch Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourn DM, Mann JI, McSkimming BJ, et al. Impaired glucose tolerance and NIDDM: does a lifestyle intervention program have an effect? Diabetes Care. 1994;17:1311–9. doi: 10.2337/diacare.17.11.1311. [DOI] [PubMed] [Google Scholar]
- 21.Racette SB, Weiss EP, Obert KA, et al. Modest lifestyle intervention and glucose tolerance in obese African Americans. Obes Res. 2001;9:348–55. doi: 10.1038/oby.2001.45. [DOI] [PubMed] [Google Scholar]
- 22.Hughes VA, Fiatarone MA, Fielding RA, et al. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol. 1993;264:E855–62. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- 23.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 24.Brozek J, Grande F, Anderson JT, et al. Densitometric analysis of body composition: revision of some quantitative assumptions. Ann NY Acad Sci. 1963;110:113–40. doi: 10.1111/j.1749-6632.1963.tb17079.x. [DOI] [PubMed] [Google Scholar]
- 25.King DS, Staten MA, Kohrt WM, et al. Insulin secretory capacity in endurance-trained and untrained young men. Am J Physiol. 1990;259:E155–81. doi: 10.1152/ajpendo.1990.259.2.E155. [DOI] [PubMed] [Google Scholar]
- 26.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 27.Brown MD, Moore GE, Korytkowski MT, et al. Improvement of insulin sensitivity by short-term exercise training in hypertensive African-American women. Hypertension. 1997;30:1549–53. doi: 10.1161/01.hyp.30.6.1549. [DOI] [PubMed] [Google Scholar]
- 28.Yamanouchi K, Shinozaki T, Chikada K, et al. Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care. 1995;18:775–8. doi: 10.2337/diacare.18.6.775. [DOI] [PubMed] [Google Scholar]
- 29.Ross R, Janssen I, Dawson J, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res. 2004;12:789–98. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 30.Kirwan JP, Kohrt WM, Wojta DW, et al. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-yr-old men and women. J Gerontol. 1993;48:M84–90. doi: 10.1093/geronj/48.3.m84. [DOI] [PubMed] [Google Scholar]
- 31.Torjesen PA, Birkeland KI, Anderssen SA, et al. Lifestyle changes may reverse development of the insulin resistance syndrome: the Oslo diet and exercise study: a randomized trial. Diabetes Care. 1997;20:26–31. doi: 10.2337/diacare.20.1.26. [DOI] [PubMed] [Google Scholar]
- 32.Elahi D. In praise of the hyperglycemic clamp. A method for assessment of beta-cell sensitivity and insulin resistance. Diabetes Care. 1996;19:278–86. doi: 10.2337/diacare.19.3.278. [DOI] [PubMed] [Google Scholar]
- 33.Hughes TA, Gwynne JT, Switzen BR, et al. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med. 1984;77:7–17. doi: 10.1016/0002-9343(84)90429-7. [DOI] [PubMed] [Google Scholar]
- 34.Gulve EA, Spina RJ. Effects of 7–10 days of cycle ergometer exercise on skeletal muscle GLUT-4 protein content. J Appl Physiol. 1995;79:1562–6. doi: 10.1152/jappl.1995.79.5.1562. [DOI] [PubMed] [Google Scholar]
- 35.Houmard JA, Hickey MS, Tyndall GL, et al. Seven days of exercise increase GLUT-4 protein content in human skeletal muscle. J Appl Physiol. 1995;79:1936–8. doi: 10.1152/jappl.1995.79.6.1936. [DOI] [PubMed] [Google Scholar]
- 36.Holloszy JO, Hansen PA. Regulation of glucose transport into skeletal muscle. In: Blaustein MP, Grunicke H, Habermann E, et al., editors. Reviews of physiology, biochemistry and pharmacology. Berlin: Springer-Verlag; 1996. pp. 99–193. [DOI] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Kelley DE, Wing RR, et al. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–47. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163:1343–50. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 39.Houmard JA, Tanner CJ, Slentz CA, et al. Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol. 2004;96:101–6. doi: 10.1152/japplphysiol.00707.2003. [DOI] [PubMed] [Google Scholar]


