Abstract
Senile cataracts are associated with progressive oxidation, fragmentation, cross-linking, insolubilization, and yellow pigmentation of lens crystallins. We hypothesized that the Maillard reaction, which leads browning and aroma development during the baking of foods, would occur between the lens proteins and the highly reactive oxidation products of vitamin C. To test this hypothesis, we engineered a mouse that selectively overexpresses the human vitamin C transporter SVCT2 in the lens. Consequently, lenticular levels of vitamin C and its oxidation products were 5- to 15-fold elevated, resulting in a highly compressed aging process and accelerated formation of several protein-bound advanced Maillard reaction products identical with those of aging human lens proteins. These data strongly implicate vitamin C in lens crystallin aging and may serve as a model for protein aging in other tissues particularly rich in vitamin C, such as the hippocampal neurons and the adrenal gland. The hSVCT2 mouse is expected to facilitate the search for drugs that inhibit damage by vitamin C oxidation products.
Keywords: advanced glycation end products, ascorbic acid, cataract, diabetes, oxidation
The ocular lens in most animal species contains a unique set of proteins, the crystallins, which have virtually no turnover. Thus, these proteins are highly susceptible to chemical and physical damage, and the lifespan of the lens would be extremely short unless evolution had equipped the lens with powerful defense mechanisms. These include a special protein structure with chaperone function that guarantees extraordinary stability and transparency throughout life (1) and a very low oxygen concentration that minimizes oxidative damage (2). The latter is further kept in check thanks to millimolar concentrations of glutathione, which, in concert with vitamin C, can detoxify a host of oxidative and photoxidative reaction products (3).
In recent years a major form of protein damage has been recognized and shown to originate from almost any reactive carbonyl compound that is generated during the Maillard reaction. This reaction is commonly observed during cooking or baking of foods and may involve sugars, oxoaldehydes such as methyglyoxal, oxidized lipids, or ascorbic acid (ASA) itself (4) (Fig. 1). Indeed, there is unequivocal evidence that the Maillard reaction proceeds in the aging lens (5–7). Many of the products formed are yellow and cross-linked. Because of the high reactivity of ASA oxidation products with proteins and its presence in high concentrations in the human lens, Bensch (8) first proposed that vitamin C oxidation could play a role in the browning process of lens crystallins.
Fig. 1.
Selected chemical pathways linking vitamin C oxidation with the formation of advanced Maillard reaction products assayed in this study.
Although in the past we were able to detect Maillard reaction products derived from vitamin C in the lens (5, 9), it has not been possible to unequivocally implicate ASA in lenticular aging, because none of the advanced glycation products have been shown to be unique for this vitamin.
Mice, being nocturnal animals, have very low lenticular levels of ASA and SVCT2 transporter activity (10). We hypothesized that overexpressing SVCT2 in the lens would result in accelerated modification of crystallins by the Maillard reaction with vitamin C oxidation products.
Results
An SmaI-XbaI fragment containing the entire human SVCT2 (hSVCT2) coding sequence was amplified by PCR from a constructed clone plasmid (pcDNA3.1–hSVCT2) (11). The SmaI-XbaI fragment was inserted into the SmaI-XbaI polycloning site of the δenαA lens-specific promoter vector (12). This expression vector contains a chick δ1-crystallin lens enhancer placed upstream of the mouse αA crystallin promoter linked to a rabbit β-globin intron and human growth hormone polyA signal. It has the unique ability to allow the specific and sustained expression of genes not only in the epithelium of the lens but also in the fiber-like cells (12). Pronuclear injection of the construct resulted in the generation of nine founder lines, which were backcrossed into C57BL background for eight generations.
Expression of hSVCT2 in Lens Epithelium and Fiber Cells Leads to Increased Lenticular Levels of ASA and Dehydroascorbic Acid (DHA).
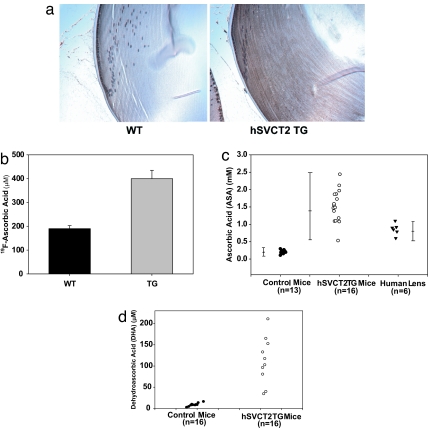
Evidence of lens-specific expression of the transporter was obtained in several ways. First, immunohistochemical staining with a hSVCT2 antibody (Alpha Diagnostic International, San Antonio, TX) revealed staining throughout the epithelium and the fiber-like cells of the transgenic mouse lens, whereas only the lens epithelium of the wild type was positive (Fig. 2a). Furthermore, when the lenses were incubated as before (13) with 100 μM 6-fluoro-6-deoxy ASA, a specific probe for SVCT1/2 function (13, 14), uptake at 2 h was 2-fold increased in the transgenic vs. the wild-type mice (Fig. 2b). Finally, lenticular levels of ASA and DHA measured by HPLC in metaphosphoric acid extract were 5- to 15-fold elevated. ASA increased from ≈0.2 mM to 1–2.5 mM, and DHA increased from 10 μM to 40–250 μM in the transgenic lenses (Fig. 2 c and d). ASA levels are shown for comparison in human lenses obtained at autopsy and stored frozen (Fig. 2c). Because of autooxidation during storage, these ascorbate levels are likely to be at the lower end. DHA levels in human lenses have been reported to be ≈20 μM (15). Most importantly, in contrast to previous lens-specific transgenes (16), sustained age-related expression of the hSVCT2 transgene persisted throughout the 12-month testing period (data not shown).
Fig. 2.
Effects of hSVCT2 overexpression in mouse lens epithelial and fiber cells on uptake and lenticular levels of vitamin C and DHA. Immunohistochemical staining of a typical control (a Left) and transgenic (a Right) lens with an antibody against the C-terminal portion of hSVCT2 reveals a diffuse presence of hSVCT2 along the cell membrane in cortical layers of the lens. (b) 6-fluoro-6-deoxy ASA uptake into cultured lenses after incubation with 100 μM for 120 min (P < 0.001). (c) ASA concentration in 16 lenses from hSVCT2 mice, 13 controls from the same litter, and six human lenses. (d) DHA concentration in 10 lenses from hSVCT2 and control mice.
Expression of hSVCT2 in Mouse Lens Leads to Increased Levels of ASA-Derived Advanced Ascorbylation End Products.
The incubation of ASA and other reducing sugars with proteins under oxidative conditions is known to result in the formation of various protein-bound fluorophores and cross-links such as pentosidine and vesperlysine A, as well as the colorless glycoxidation/lipoxidation product carboxymethyl-lysine (CML), all of which have been documented in the aging human lens (5, 17, 18) (Fig. 1). In addition, a lysine–lysine cross-link named K2P (Fig. 1) was recently identified in the human lens as a major fluorophore and UVA active protein modification (19). It could be synthesized from the reaction of ASA with human lens proteins (20). All these compounds, including protein-bound fluorescence, were assayed at 6, 9, and 12 months of age either in the enzymatic digest (K2P and protein-bound fluorescence) or acid hydrolyzate of the mouse lens crystallins (pentosidine, vesperlysine A, and CML) following established procedures (5, 17, 18). The glucose-derived Amadori product fructose-lysine (assayed as furosine) and the methylglyoxal-derived carboxyethyl-lysine (CEL) (21) were also measured.
Protein-bound fluorescence at 335/385 nm and 370/440 nm was significantly increased in the transgenic vs. the wild-type lenses and increased with age (P < 0.0001) (Fig. 3 a and b). The fluorescent cross-links K2P, pentosidine, and vesperlysine A were all significantly increased in the transgenic lenses at all time points (P < 0.001 to P < 0.0001) (Fig. 3 c–e). CML was also increased, although to a lesser degree (P < 0.001 or less) (Fig. 3f). CEL was barely increased (Fig. 3g), suggesting that methylglyoxal is likely only mildly elevated, although other markers of methylglyoxal levels will be needed to confirm this conclusion. Similarly, furosine was not increased (Fig. 3h), excluding the presence of high lenticular glucose concentrations as a cause for the elevated advanced glycation end-products in the transgenic lenses. For comparison, it should be noted that, when all data are expressed as a picomole/micromole leucine equivalent, assuming 45 lysine residues per 1,000 aa, absolute levels of CML are similar to those of K2P and vesperlysine A, whereas those of CEL are similar to pentosidine (Table 1).
Fig. 3.
Effects of hSVCT2 overexpression on lens protein-bound fluorescence and advanced ascorbylation product. Student's t test was used for all comparisons. (a) Fluorescence at 335/385 nm excitation/emission in enzymatic protein digest was increased at all ages in hSVCT2 compared with wild type (P < 0.05 to P < 0.0001). (b) Fluorescence at 370/440 nm was similarly increased in transgenic mice (P value from nonsignificant at 6 months to P < 0.0001 for 12 vs. 9 months. (c) Cross-link K2P content in transgenic vs. wild type was higher at all ages (P < 0.0001). (d) Pentosidine levels were significantly higher in transgenics at all ages (P < 0.001 to P < 0.0001). (e) Vesperlysine A was detectable only in transgenic mice. (f) The CML level was significantly higher in transgenic mice (P < 0.0001 for 6 months; P < 0.003 for 9 months; P < 0.001 for 12 months). (g) CEL was elevated in transgenic mice (P < 0.05) but did not increase with age. (h) Fructosyl-lysine measured as furosine was unchanged in transgenic mice (P > 0.05).
Table 1.
Comparative analysis of the highest level of protein modification detected in the SVCT2 lenses
| Glycation product | Lysine, μmol/mol | Leucine, pmol/μmol |
|---|---|---|
| CML | 600* | 13.3 |
| CEL | 80* | 1.8 |
| Furosine | 1,400* | 31.1 |
| Pentosidine | – | 2* |
| Vesperlysine A | – | 15* |
| K2P | – | 20* |
Assuming ≈45 lysine residues per 1,000 amino acids (i.e., 1,000 leucine equivalents) for mouse αA-crystallin, 100 μmol of advanced glycation end-products per mole of lysine would be the equivalent to 4.5 pmol/μmol leucine.
*Data taken from Fig. 3.
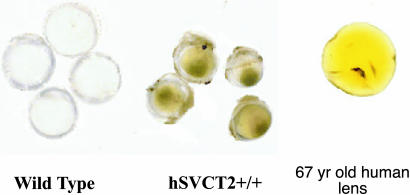
Finally, we noticed in the course of this work that the 12-month-old transgenic lenses had acquired a yellow color similar to that observed in older human lenses (Fig. 4).
Fig. 4.
Macroscopic appearance of the hSVCT2+/+ lenses at 12 months. The transgenic lenses are colored yellow. All mouse lenses were photographed in the same dish to allow accurate comparison between wild-type and transgenic lenses. A fresh 67-year-old normal human lens obtained at autopsy is shown for comparison.
Discussion
The above data strongly implicate vitamin C in at least one form of the chemical processes that affect the aging human lens crystallins. Furthermore, the hSVCT2–δenαA mouse is an animal model capable of reproducing, within a very short time, the yellow discoloration that increases over several decades in the human lens. These findings suggest that a substantial part of the yellowing process to aging human lens crystallins likely arises from vitamin C.
These two statements and their significance for the aging human lens and cataractogenesis need to be examined. At the outset it should be noted that cataracts, i.e., the formation of light-scattering protein aggregates, can occur at any age as a result of mutations in any of the lens proteins that are critically involved in lens transparency. Thus, chemical modification of lens crystallins is not needed for opacification to occur, and, in the absence of overt cataractogenic conditions and risk factors, the healthy lens can remain transparent for decades while being progressively discolored. Concerning this latter process, however, several epidemiological and clinical studies have revealed a strong association among lens color, lens fluorescence, and the nuclear sclerosis that accompany age-onset cataract formation (22, 23). It is therefore reasonable to postulate that the accumulation of protein modifications by vitamin C oxidation and other Maillard reaction products may predispose lens crystallins toward destabilization and aggregation, as supported by several in vitro studies (24–26).
Our study currently does not address the extent to which the yellow color of the transgenic lenses is protein-linked or exists free in solution. In the human lens, soluble and protein-bound kynurenine glucosides have been proposed to account for some of the lens color in young primates (27). These disappear with age and bind to the lens proteins. Clearly, the aging human lens is under attack from various carbonyl sources that include not only vitamin C and kynurenine oxidation products but also nonoxidative modifications such as glucosepane and methylglyoxal-derived hydroimidazolones (7, 28). Of particular interest, in the latter case, is that these reversible protein adducts may actually protect arginine residues from the formation of irreversible ascorbylation adducts and enhance crystallin chaperone function (24).
What are the implications of our findings concerning vitamin C supplementation, lenticular aging, and cataractogenesis? On one hand, the vanishing low vitamin C levels in the mouse lens imply that millimolar concentrations of the vitamin are not necessary for maintaining lens clarity. On the other hand, low serum levels of the vitamin have been associated with increased risk of cataractogenesis in the human (29), and various studies suggest that supplementation of the vitamin is beneficial for decreasing the risk of cataractogenesis (30), in particular under conditions that favor oxidant stress (31). Proposed mechanisms include the scavenging of free radicals by the vitamin itself or in conjunction with glutathione that is present in millimolar concentrations in rodent lenses. Thus, vitamin C joins the ranks of those metabolites that are essential for life, such as glucose, fatty acids, and oxygen, but can inflict damage when the cell's defenses are weakened by diabetes, end-stage renal disease, poor nutrition, exposure to UV light, or old age itself.
The discovery that high cellular concentrations of vitamin C can inflict damage in such a short time, as in the hSVCT2–δenαA mouse, could be relevant to other tissues with high SVCT2 activity, such as the hippocampus and the adrenal glands, in which ascorbate concentrations reach up to 10 mM (32, 33). Both tissues can be severely affected in their function during aging, and thus the possibility emerges that excess vitamin C oxidation in hippocampal neurons (34) may, for example, contribute to age-related neurodegenerative diseases. Further work is required to understand how vitamin C damages lens crystallins and other molecular structures during aging. In that regard, the hSVCT2–δenαA mouse provides an extraordinary tool for the development of drugs that can delay this aspect of the lenticular aging process.
Materials and Methods
Transgene Construct.
An SmaI-XbaI fragment containing the entire hSVCT2 (human sodium-dependent vitamin C transporter 2) coding sequence was amplified by PCR by using primers 5′-GCGCCCCGGGATGATGGGTATTGGTAAGAA-3′ (sense) and 5′-GCGCTCTAGACTATCCCGTGGCCTGGGAGT-3′ (antisense) from a constructed clone plasmid (pcDNA3.1–hSVCT2) (35). The SmaI-XbaI fragment was linked to the SmaI-XbaI polycloning site of a minigene construct containing chick δ1-crystallin lens enhancer, which was placed upstream of αA crystallin promoter, and a rabbit β-globin intron and human growth hormone polyA signal, which were placed downstream from the δenαA promoter (published in ref. 12). The hSVCT2 minigene was released by BSSHII and NotI digestion. The minigene was further confirmed by DNA sequence analysis.
Generation of Transgenic Mice.
Mice were housed under diurnal lighting conditions and allowed free access to food and water. All animals were used in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmology and Vision Research. The hSVCT2 minigene construct was microinjected into fertilized eggs of B6SJLF1 mice, and the eggs were then transferred into the oviducts of pseudopregnant females (Transgenic Animal Model Core, University of Michigan, Ann Arbor, MI). Genomic DNAs from mouse tails were isolated and subjected to PCR screening by PCR using specifically designed primers, 5′-TCTTCCGGTGGTGATAAATGGA-3′ (sense) and 5′-GCTCTGCTGTTCCATTGGCA-3′ (antisense). A 500-bp fragment was expected in transgenic but not in wild-type mice. As a positive control, a 350-bp fragment of γ-glutamate-cysteine ligase was amplified by using primers 5′-CCA TTC AGT TCG ATG TGC TCA GAT-3′ (sense) and 5′-AGC AGT TGC CCA TCC GGA AT-3′ (antisense). The offspring were crossed with C57BL/6 mice. F1 offspring were genotyped by the same method, and founder lines were chosen.
Aging Study Design.
Transgenic and age-matched control mice, 10 mice per group, were maintained on a standard mouse diet (Prolab 5P75 Isopro 3000; LabDiet, Richmond, IN). Mice were killed at 6, 9, and 12 months. Eyes were removed from mice and decapsulated to release lenses. For immunohistology, the eyes were fixed in formalin immediately after death.
Immunohistochemistry.
Transgenic and age-matched normal mouse lenses were fixed in 10% neutral buffered formalin and gradient adapted to 70% ethanol, embedded in paraffin, and cut into 5-μm sections. After dehydration, heat-induced epitope retrieval was done in 10 mM citrate buffer (pH 6.0), and the sections were treated with 3.0% hydrogen peroxide to block endogenous peroxidases. The sections were blocked with 1.5% normal horse serum and incubated for 2 h either with the anti-hSVCT2 polyclonal antibody (Alpha Diagnostic International) diluted in PBS or with nonimmune rabbit IgG diluted to the same protein concentration as the primary antibody. After washing, the slides were incubated with biotinylated anti-rabbit IgG (Vector Laboratories, Burlingame, CA) followed by ABC reagent (Vector Laboratories). They were stained initially with 3,3′-diaminobenzidine, counterstained with hematoxylin, and then permanently mounted.
Measurement of ASA and DHA.
ASA and DHA concentrations were determined based on dimethyl-o-phenylene-diamine derivatization as previously described, with few modifications (36). In brief, after mice were killed, eyes were removed and decapsulated immediately. Mouse lenses were homogenized in 200 μl of 10% cold trichloroacetic acid and kept on ice for 10 min. For transgenic mice 50 μl of supernatant was used for derivatization after 20-fold dilution because of high ASA content. After oxidation with 10 μl of 0.01 M iodine in 2.7% potassium iodide, 20 μl of 0.01 M thiosulfate was added to reduce excess of iodine. Samples were then derivatized by adding 50 μl of Na-phosphate buffer (pH 5.4) and 20 μl of dimethyl-o-phenylene-diamine (1 mg/ml in 0.1 M HCl). The derivatized samples were injected into HPLC and separated by C18 reverse-phase (5-μm diameter) column, 25 cm × 4.6 mm (Grace Vydac, Atlanta, GA). The mobile phase was 50% methanol/50% phosphate buffer (80 mmol/liter, pH 7.4), with a final pH of 8.4, and the column was eluted at 1.0 ml/min. The derivative was detected by using a fluorescent detector with excitation at 360 nm and emission at 440 nm. For quantitation of DHA the lens extract was directly derivatized under same conditions, but without the oxidation step (iodine and thiosulfate).
Enzymatic Digestion of Lens Proteins.
The trichloroacetic acid precipitation of lens protein was washed twice with 500 μl of ethyl ether and allowed to dry at room temperature for 10 min. The pellet was then reconstituted in an Eppendorf tube with 500 μl of 5.0 mM argon-exchanged Chelex-treated phosphate buffer (pH 7.0), 5 μl of proteinase K (10 mg/ml in argon-exchanged water; Roche, Indianapolis, IN), 1.5 μl of chloroform, and 1.5 μl of toluene. The digestion mixture was mixed by rotation at 37°C for 24 h under argon in the dark with sequential addition every 24 h of 10 μl of peptidase (0.36 mg/ml; P7500; Sigma, St. Louis, MO), 10 μl of pronase solution (0.36 mg/ml; P5147; Sigma), and 12 milliunits of aminopeptidase M (Roche). Digestion efficiency varied from 75% to 85%. Corresponding enzyme blanks incubated without added protein were used as background control. After digestion, the samples were reconstituted with water for fluorescence measurement, dried, reconstituted with 0.01 M heptafluorobutyric acid (HFBA) in water, and subjected to HPLC assay for determination of K2P. The protein concentrations of samples were analyzed by means of a ninhydrin assay expressed as leucine equivalent after enzymatic digestion and also after 6 M HCl hydrolyzed to evaluate the digestion efficiency and also used for the calculation of fluorescence and other assays.
Fluorescence Spectroscopy.
The fluorescence at λex/em 370/440 and 335/385 nm of the lens protein digest was measured with a spectrofluorometer (821-F; Jasco, Easton, MD). The data are expressed as fluorescence units per micromolar leucine equivalent.
Measurement of K2P.
The lens protein digest was dried and reconstituted into 1 ml of 0.01 M HFBA water, and 75 μl was injected into HPLC equipped with a C18 reversed-phase analytical column (15 cm × 2.1 mm, 3 μm; Discovery HS C18; Supelco, St. Louis, MO) and a UV detector (343 nm). The mobile phase A was 2% acetonitrile/0.01 M HFBA water, and mobile phase B was 60% acetonitrile/0.01 M HFBA water. The mobile phase was maintained at 100% A for 5 min and followed by a linear gradient to 55% A/45% B within 100 min. The K2P was eluted for ≈53 min. The data are expressed as picomole/micromole leucine equivalent.
Measurement of Pentosidine.
The trichloroacetic acid precipitation of lens protein was washed twice with 500 μl of ethyl ether and dried at room temperature. The protein pellet was hydrolyzed with 1 ml of 6 M HCl at 110°C for 16 h. The HCl used for hydrolysis was extensively exchanged with argon, and samples were hydrolyzed for 16 h at 110°C under argon. The hydrolyzed solution was dried and reconstituted in 1 ml of 0.01 M HFBA water, and amino acid concentration was determined by means of a ninhydrin assay. A total of 75 μl of sample was injected into HPLC and analyzed under the same conditions as for the K2P separation, except for detection at 335/385-nm excitation/emission fluorescence. The data were expressed as picomoles of pentosidine per micromole leucine equivalent. Chemically synthesized pentosidine was used as a standard for identification and calculation.
Measurement of Vesperlysine A.
The two-step HPLC procedure from Tessier et al. (18) was used to determine vesperlysine A in lens protein acid hydrolyzate. An ≈4 nmol leucine equivalent of hydrolyzed lens protein was injected into a HPLC equipped with C18 reverse-phase column (25 cm × 4.6 mm, 5 μm; Grace Vydac, Atlanta, GA). The column was eluted with 1% acetic acid (vol/vol) for 20 min with a flow rate of 0.7 ml/min followed by linear gradient to 60% acetonitrile using 95% acetonitrile mobile phase. The 8- to 15-min fractional region was collected and dried. The dried fraction was then reconstituted in 150 μl of 0.01 M HFBA water, and 75 μl was injected into HPLC equipped with the Discovery HS C18 reverse-phase column (Supelco) as for pentosidine analysis.
Measurement of CML, CEL, and Furosine by GC-MS.
CML, furosine, and CEL were determined as trifluoroacetic acid methyl ester derivatives in acid hydrolyzed delipidated protein samples by GC-MS using an isotope dilution method as described by Miyata et al. (37) using a 5890 Series II gas chromatograph (Hewlett–Packard, Santa Clara, CA) with a 5971 Series Mass Selective Detector and a 6890 Series automatic injector, a 25-m × 0.2-mm × 0.33-μmol/liter Ultra2 column, and the temperature program of Dunn et al. (38). Internal standards were 121.2 nmol of D8-lysine and 195 pmol of D4-CML. Lysine, D8-lysine, CML, D4-CML, CEL, and furosine were monitored at ions m/z = 320, 328, 392, 396, 379, and 110, which eluted at 25 (lysine and D8-lysine), 31 (CML and D4-CML), 32 (CEL), and 38 (furosine) min.
Statistical Analysis.
All values were expressed as means ± SE. Statistical significance of difference in mean values was assessed by repeated-measures ANOVA or Student's t test. Only P values of <0.05 were considered statistically significant.
Acknowledgments
We thank Drs. Joram Piatigorsky and Paul Overbeek for helpful discussions; Dr. S. Thorpe for providing samples of D4-CML; the National Disease Research Interchange (Philadelphia, PA) for providing human lenses; Dr. Venkat Reddy (University of Akron, Akron, OH) for assistance with 19F-NMR spectroscopy; and Denise Hatala and the Case Visual Sciences Core Laboratory for assistance with immunohistochemistry. This work was supported by National Eye Institute Grants EY07099 (to V.M.M.), NIA18629 (to V.M.M.), and EY07070 (to B.J.O.); National Institutes of Health Grants EY13146 (to L.W.R.) and P30EY-11373; a departmental grant from Research to Prevent Blindness, Inc; and Visual Sciences Training Program T32 EY 07157 (to M.E.O.).
Abbreviations
- CEL
carboxyethyl-lysine
- CML
carboxymethyl-lysine
- ASA
ascorbic acid
- DHA
dehydroascorbic acid
- HFBA
heptafluorobutyric acid.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Wang K, Spector A. J Biol Chem. 1994;269:13601–13608. [PubMed] [Google Scholar]
- 2.Eaton JW. Free Radical Biol Med. 1991;11:207–213. doi: 10.1016/0891-5849(91)90173-z. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VN. Exp Eye Res. 1990;50:771–778. doi: 10.1016/0014-4835(90)90127-g. [DOI] [PubMed] [Google Scholar]
- 4.Baynes JW, Monnier VM, Ames JM, Thorpe SR, editors. Ann NY Acad Sci. 2005;1043:1–432. doi: 10.1196/annals.1333.035. [DOI] [PubMed] [Google Scholar]
- 5.Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. Proc Natl Acad Sci USA. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyons TJ, Silvestri G, Dunn JA, Dyer DG, Baynes JW. Diabetes. 1991;40:1010–1015. doi: 10.2337/diab.40.8.1010. [DOI] [PubMed] [Google Scholar]
- 7.Biemel KM, Friedl DA, Lederer MO. J Biol Chem. 2002;277:24907–24915. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- 8.Bensch KG, Fleming JE, Lohmann W. Proc Natl Acad Sci USA. 1985;82:7193–7196. doi: 10.1073/pnas.82.21.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng R, Lin B, Lee KW, Ortwerth BJ. Biochim Biophys Acta. 2001;1537:14–26. doi: 10.1016/s0925-4439(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 10.Obrenovich ME, Fan X, Satake M, Reneker L, Reddan JR, Jarvis SM, Monnier VM. Mol Cell Biochem. 2006 doi: 10.1007/s11010-006-2678-7. [DOI] [PubMed] [Google Scholar]
- 11.Liang WJ, Johnson D, Ma LS, Jarvis SM, Wei-Jun L. Am J Physiol. 2002;283:C1696–C1704. doi: 10.1152/ajpcell.00461.2001. [DOI] [PubMed] [Google Scholar]
- 12.Reneker LW, Chen Q, Bloch A, Xie L, Schuster G, Overbeek PA. Invest Ophthalmol Vis Sci. 2004;45:4083–4090. doi: 10.1167/iovs.03-1270. [DOI] [PubMed] [Google Scholar]
- 13.Satake M, Dmochowska B, Nishikawa Y, Madaj J, Xue J, Guo Z, Reddy DV, Rinaldi PL, Monnier VM. Invest Ophthalmol Vis Sci. 2003;44:2047–2058. doi: 10.1167/iovs.02-0575. [DOI] [PubMed] [Google Scholar]
- 14.Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KL, Levine M. J Biol Chem. 2005;280:5211–5220. doi: 10.1074/jbc.M412925200. [DOI] [PubMed] [Google Scholar]
- 15.Tessier F, Moreaux V, Birlouez-Aragon I, Junes P, Mondon H. Int J Vitam Nutr Res. 1998;68:309–315. [PubMed] [Google Scholar]
- 16.Cammarata PR, Zhou C, Chen G, Singh I, Kuszak JR, Robinson ML. Invest Ophthalmol Vis Sci. 1999;40:1727–1737. [PubMed] [Google Scholar]
- 17.Dunn JA, Ahmed MU, Murtiashaw MH, Richardson JM, Walla M, Thorpe SR, Baynes JW. Biochemistry. 1990;29:10964–10970. doi: 10.1021/bi00501a014. [DOI] [PubMed] [Google Scholar]
- 18.Tessier F, Obrenovich M, Monnier VM. J Biol Chem. 1999;274:20796–20804. doi: 10.1074/jbc.274.30.20796. [DOI] [PubMed] [Google Scholar]
- 19.Cheng R, Feng Q, Argirov OK, Ortwerth BJ. J Biol Chem. 2004;279:45441–45449. doi: 10.1074/jbc.M405664200. [DOI] [PubMed] [Google Scholar]
- 20.Cheng R, Feng Q, Ortwerth BJ. Biochim Biophys Acta. 2006;1762:533–543. doi: 10.1016/j.bbadis.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. Biochem J. 1997;324:565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chylack LT, Jr, Wolfe JK, Friend J, Khu PM, Singer DM, McCarthy D, del Carmen J, Rosner B. Optom Vis Sci. 1993;70:886–895. doi: 10.1097/00006324-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Siik S, Chylack LT, Jr, Friend J, Wolfe J, Teikari J, Nieminen H, Airaksinen PJ. Acta Ophthalmol Scand. 1999;77:509–514. doi: 10.1034/j.1600-0420.1999.770504.x. [DOI] [PubMed] [Google Scholar]
- 24.Nagaraj RH, Oya-Ito T, Padayatti PS, Kumar R, Mehta S, West K, Levison B, Sun J, Crabb JW, Padival AK. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 25.Dickerson JE, Jr, Lou MF, Gracy RW. Curr Eye Res. 1995;14:163–166. doi: 10.3109/02713689508999929. [DOI] [PubMed] [Google Scholar]
- 26.Prabhakaram M, Ortwerth BJ. Exp Eye Res. 1992;55:451–459. doi: 10.1016/0014-4835(92)90118-c. [DOI] [PubMed] [Google Scholar]
- 27.Hood BD, Garner B, Truscott RJ. J Biol Chem. 1999;274:32547–32550. doi: 10.1074/jbc.274.46.32547. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM. Invest Ophthalmol Vis Sci. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- 29.Robertson JM, Donner AP, Trevithick JR. Am J Clin Nutr. 1991;53:346S–351S. doi: 10.1093/ajcn/53.1.346S. [DOI] [PubMed] [Google Scholar]
- 30.Jacques PF, Taylor A, Hankinson SE, Willett WC, Mahnken B, Lee Y, Vaid K, Lahav M. Am J Clin Nutr. 1997;66:911–916. doi: 10.1093/ajcn/66.4.911. [DOI] [PubMed] [Google Scholar]
- 31.Hegde KR, Varma SD. Biochim Biophys Acta. 2004;1670:12–18. doi: 10.1016/j.bbagen.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Hediger MA. Nat Med. 2002;8:445–446. doi: 10.1038/nm0502-445. [DOI] [PubMed] [Google Scholar]
- 33.Grunewald RA. Brain Res Brain Res Rev. 1993;18:123–133. doi: 10.1016/0165-0173(93)90010-w. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues Siqueira I, Fochesatto C, da Silva Torresqq IL, Dalmaz C, Alexandre Netto C. Life Sci. 2005;78:271–278. doi: 10.1016/j.lfs.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 35.Liang WJ, Johnson D, Jarvis SM. Mol Membr Biol. 2001;18:87–95. doi: 10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- 36.Tessier F, Birlouez-Aragon I, Tjani C, Guilland JC. Int J Vitam Nutr Res. 1996;66:166–170. [PubMed] [Google Scholar]
- 37.Miyata T, Fu MX, Kurokawa K, van Ypersele de Strihou C, Thorpe SR, Baynes JW. Kidney Int. 1998;54:1290–1295. doi: 10.1046/j.1523-1755.1998.00093.x. [DOI] [PubMed] [Google Scholar]
- 38.Dunn JA, McCance DR, Thorpe SR, Lyons TR, Baynes JW. Biochemistry. 1991;30:1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]