Abstract
Deregulation of the Sonic hedgehog pathway has been implicated in an increasing number of human cancers. In this pathway, the seven-transmembrane (7TM) signaling protein Smoothened regulates cellular proliferation and differentiation through activation of the transcription factor Gli. The activity of mammalian Smoothened is controlled by three different hedgehog proteins, Indian, Desert, and Sonic hedgehog, through their interaction with the Smoothened inhibitor Patched. However, the mechanisms of signal transduction from Smoothened are poorly understood. We show that a kinase which regulates signaling by many “conventional” 7TM G-protein-coupled receptors, G protein-coupled receptor kinase 2 (GRK2), participates in Smoothened signaling. Expression of GRK2, but not catalytically inactive GRK2, synergizes with active Smoothened to mediate Gli-dependent transcription. Moreover, knockdown of endogenous GRK2 by short hairpin RNA (shRNA) significantly reduces signaling in response to the Smoothened agonist SAG and also inhibits signaling induced by an oncogenic Smoothened mutant, Smo M2. We find that GRK2 promotes the association between active Smoothened and β-arrestin 2. Indeed, Gli-dependent signaling, mediated by coexpression of Smoothened and GRK2, is diminished by β-arrestin 2 knockdown with shRNA. Together, these data suggest that GRK2 plays a positive role in Smoothened signaling, at least in part, through the promotion of an association between β-arrestin 2 and Smoothened.
The Sonic hedgehog (Shh) signaling pathway plays an important role in the development and homeostasis of an organism. Indeed, this cascade has been implicated in an ever-growing number of human malignancies. While the importance of this pathway has been recognized for some time, the details of signal transmission have not yet been fully elucidated in mammalian systems. Most of what is known about the Shh signaling cascade comes from studies of Drosophila, where the pathway was originally identified (35). In Drosophila, signaling from the 7TM protein Smoothened (Smo) arises when the secreted hedgehog ligand (hh) binds to a 12-transmembrane transporter-like protein, Patched (Ptc). The binding of hh to Ptc relieves Ptc-mediated inhibition of Smo. Intracellularly, activation of Smo leads to the disruption of a large protein complex composed of a kinesin-like protein, Costal-2 (Cos2), the serine/threonine kinase Fused, the Suppressor of Fused protein, and the transcription factor effector Cubitus interruptus (Ci). In the absence of the hh signal, this protein complex is tethered to microtubules in the cytoplasm, providing one mechanism for excluding Ci from the nucleus. Ci is further inhibited by protein kinase A (PKA) phosphorylation, which leads to the proteolytic processing of Ci from a full-length transcriptional activator into a 75-kDa transcriptional repressor. Pathway activation through hh binding to Ptc leads to inhibition of Ci proteolysis and allows nuclear accumulation of active Ci (for reviews, see references 8, 16, and 21).
While the functions of Smo and Ptc are conserved in mammals, the transduction of the signal through intracellular signaling pathways remains less clear. The function of the single Ci transcription factor in Drosophila is, in mammalian systems, ascribed to a family of zinc finger transcription activators and repressors, Gli1 to Gli3, of which Gli3 is also proteolytically processed in a manner similar to that of Ci (17). As in Drosophila, the Suppressor of Fused protein antagonizes Gli1 activity by sequestering it in the cytoplasm (33, 44). Recently, it has been shown that, in Drosophila, Cos2 interacts directly with Smo, providing a link between Smo activation at the membrane and the inhibition of proteolysis and nuclear accumulation of Ci (19, 31, 36). While Kif27/Kif7 seems to be the mammalian ortholog of Cos2, its functional conservation in the pathway remains to be fully elucidated (23).
The closest structural relative of Smo is the Wnt receptor Frizzled, which, like Smo, is a 7TM protein. However, while the Wnt proteins are ligands for Frizzled, Smo has no known natural ligand. Several pieces of evidence link Frizzled signaling to G proteins; however, evidence implicating G proteins in Smo signaling is very limited (1, 11, 22, 30, 45). It is tempting, however, to speculate that Smo signaling may share some similarities with other 7TM receptors (7TMRs). Classically, upon agonist binding, 7TMRs undergo a conformational change that mediates signal transduction through the activation of heterotrimeric G proteins. At the same time, phosphorylation of the receptor by G-protein-coupled receptor kinases (GRKs) leads to feedback mechanisms that desensitize the 7TMR by uncoupling it from its G protein and promote internalization of the receptor. A critical component in the desensitization/internalization pathway is the β-arrestin family of proteins. β-Arrestins are multifunctional cytoplasmic scaffold proteins that mediate desensitization and internalization by recruiting components of the endocytic machinery such as AP-2 and clathrin to a GRK-phosphorylated receptor. The recruitment of β-arrestin to an activated receptor at the membrane directs the receptor to clathrin-coated pits that are then endocytosed and trafficked elsewhere in the cell for degradation or for recycling of the receptor back to the membrane (for a review, see reference 7). More recently, it has been appreciated that the β-arrestin protein can also play an important role in signal transmission through the recruitment and scaffolding of cellular signaling factors such as the tyrosine kinase Src and members of the mitogen-activated protein kinase cascades JNK and ERK (for reviews, see references 26 and 27). Thus, β-arrestins and GRKs have the capacity to turn off some signaling pathways and promote signaling through others.
The structural similarity of Smo to the 7TMR family suggests the possibility that Smo signaling involves the actions of GRK and β-arrestin. Supporting this possibility is the finding that, in Drosophila, Smo is hyperphosphorylated upon activation (12, 39, 43, 48, 49). Furthermore, in mammalian systems, GRK2 has recently been identified as a major kinase in activation-dependent Smo phosphorylation in human embryonic kidney (HEK) cells (6). Finally, β-arrestin has also recently been shown to be a critical component in Smo-mediated zebra fish development (46). Thus, given this supportive evidence, we undertook the present studies to examine if GRK2 and β-arrestin 2 play a role in Smo-dependent signaling.
MATERIALS AND METHODS
Plasmids.
The 16.3 mutant Gli and 16.2 wild-type Gli reporter plasmids and the Myc-Smoothened, Smoothened M2, Ptc, and Shh N terminus expression plasmids were obtained from M. Scott (40). The Flag-β-arrestin, green fluorescent protein (GFP)-β-arrestin, bovine GRK2, and bovine GRK2 K220R expression plasmids were generated as described previously (3). The Myc-Smoothened, GRK2, and GRK2 K220R retroviruses used for making stable lines of C3H10T1/2 cells were constructed by introducing the coding sequences into the pLXRN (Smo) and pLPCX (GRK2 and GRK2 K220R) Clontech vectors. The short hairpin RNAs (shRNAs) were cloned into the pSilencer 3.0 hygro vector (Ambion) with the following sequences: control, 5′ GAT CCG TTC TCC GAA CGT GTC ACG TTT CAA GAG AAC GTG ACA CGT TCG GAG AAT TTT TTG GAA A 3′ and 5′ AGC TTT TCC AAA AAA TTC TCC GAA CGT GTC ACG TTC TCT TGA AAC GTG ACA CGT TCG GAG AAC G 3′; GRK2 1, 5′ GAT CCA TAT GAG AAG CTG GAG ACA TTC AAG AGA TGT CTC CAG CTT CTC ATA TTT TTT TGG AAA 3′ and 5′ AGC TTT TCC AAA AAA ATA TGA GAA GCT GGA GAC ATC TCT TGA ATG TCT CCA GCT TCT CAT ATG 3′; GRK2 2, 5′ GAT CCG AAT GTT GAG CTC AAC ATC TTC AAG AGA GAT GTT GAG CTC AAC ATT CTT TTT TGG AAA 3′ and 5′ AGC TTT TCC AAA AAA GAA TGT TGA GCT CAA CAT CTC TCT TGA AGA TGT TGA GCT CAA CAT TCG 3′; GRK2 3, 5′ GAT CCG GAA TCA AGT TAC TGG ACA TTC AAG AGA TGT CCA GTA ACT TGA TTC CTT TTT TGG AAA 3′ and 5′ AGC TTT TCC AAA AAA GGA ATC AAG TTA CTG GAC ATC TCT TGA ATG TCC AGT AAC TTG ATT CCG 3′; Barr2 1, 5′ GAT CCC AGT GAA GCT GGT GGT GTC TTT CAA GAG AAG ACA CCA CCA GCT TCA CTT TTT TTG GAA A 3′ and 5′ AGC TTT TCC AAA AAA AGT GAA GCT GGT GGT GTC TTC TCT TGA AAG ACA CCA CCA GCT TCA CTG G 3′; Barr2 2, 5′ GAT CCG CAA GAT GAC CAG GTG TCT CTT CAA GAG AGA GAC ACC TGG TCA TCT TGT TTT TTG GAA A 3′ and 5′ AGC TTT TCC AAA AAA CAA GAT GAC CAG GTG TCT CTC TCT TGA AGA GAC ACC TGG TCA TCT TGC G 3′.
Mutagenesis.
shRNA-resistant bovine GRK2 (GRK2 2*) was made with the QuikChange site-directed mutagenesis kit (Stratagene) and primers 5′ GTG GAA GAA TGT AGA ACT AAA TAT ACA CCT GAC CAT GAA C 3′ and 5′ GTT CAT GGT CAG GTG TAT ATT TAG TTC TAC ATT CTT CCA C 3′.
Tissue culture.
C3H10T1/2 cells were cultured in Eagle's basal medium containing 10% fetal bovine serum, 1% penicillin-streptomycin, 0.1% gentamicin, 1.5 g/liter sodium bicarbonate, and 2 mM l-glutamine. HEK 293 cells were cultured in Eagle's minimum essential medium (MEM) with 10% fetal bovine serum, 0.1% gentamicin, and 1% penicillin-streptomycin. Shh-LIGHT cells were cultured in Dulbecco's modified Eagle's medium with 10% newborn calf serum, 0.1% gentamicin, and 1% penicillin-streptomycin. Experiments with SAG (see Fig. 3A and C) were performed in the presence of 2% serum. Experiments with SAG (see Fig. 2) were performed in the presence of 10% serum.
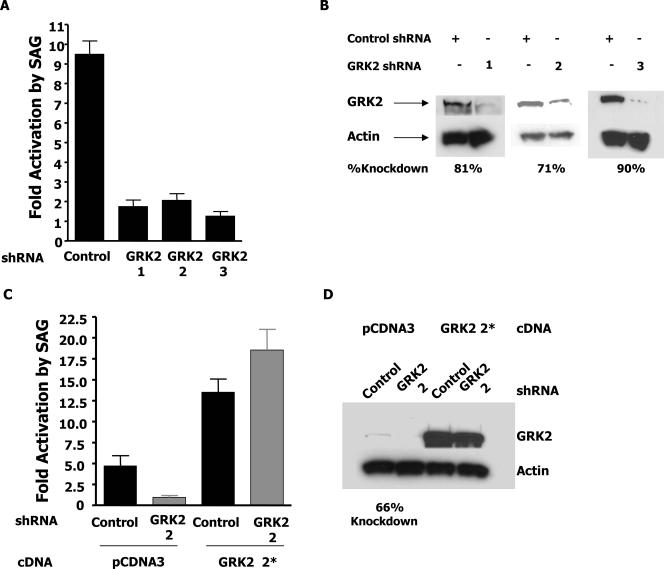
FIG. 3.
Gli activation through SAG is GRK2 dependent. (A) C3H10T1/2 cells were transfected with the indicated shRNA and the Gli reporter vector. After 24 h, the cells were split in half and treated with either SAG or the vehicle for 48 h. Cells were then assayed for luciferase activity. Fold activation by SAG refers to the induction of luciferase activity in SAG-treated cells relative to that of vehicle-treated cells. (B) Cells were treated as for panel A and sorted by flow cytometry with a cotransfected GFP vector. Extracts from equal numbers of cells were immunoblotted with GRK2 and actin antibodies. (C) Cells were treated as for panel A with the additional transfection of pCDNA3 or an shRNA-resistant GRK2 2* expression vector. (D) Immunoblotting was performed as for panel B.
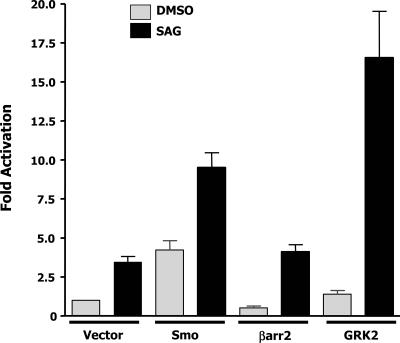
FIG. 2.
SAG and GRK2 synergy. C3H10T1/2 cells were transfected with the indicated expression plasmids. After 24 h, cells were split in half and treated with either the vehicle or SAG for 48 h. Cells were harvested and assayed for luciferase and βgal activities. Luciferase values were normalized for transfection efficiency with the βgal values of the sample containing the reporter alone. Fold activation refers to the increase in luciferase activity relative to that of the vehicle-treated sample containing the reporter alone. The data shown are the results of at least three independent experiments performed in duplicate.
Focus formation assays.
C3H10T1/2 cells stably expressing Smo alone, GRK2 alone, GRK2 K220R alone, Smo together with GRK2, or Smo together with GRK2 K220R were plated in 35-mm dishes at a density of 1.5 × 105/dish. Cells were transfected with 2 μg of a plasmid expressing Ras 61L with the TransIT-LT1 transfection reagent (Mirus). One plate of each was also transfected with a plasmid expressing β-galactosidase (βgal) to verify that the transfection efficiencies were equal. After 48 h, the cells were split and plated into 60-mm dishes. The medium was changed every 3 days for 21 days. Foci were then stained with crystal violet stain and counted. Counted foci were brightly stained areas of dense cell growth greater than approximately 1.5 mm in diameter.
Luciferase assay.
C3H10T1/2 cells were plated at a density of 1.5 × 105/well in a six-well dish. After 16 h, the cells were transiently transfected with the indicated expression plasmids, a Gli reporter plasmid (0.5 μg), and a cytomegalovirus (CMV)-βgal (0.25 μg) internal transfection control plasmid by using the TransIT-LT1 transfection reagent (Mirus). The total amount of DNA transfected was kept constant with the empty pCDNA3 plasmid. Cells were allowed to grow for 72 h posttransfection with no change in medium. After 72 h, cells were harvested and lysed in 1× reporter buffer (Promega.) Luciferase activity was measured with the Luciferase Assay system (Promega) and normalized to βgal internal controls. For assays with agonists and antagonists, cells were plated at a density of 3 × 105/60-mm plate. Cells were transiently transfected as described above, and the reagent and DNA were adjusted for cell number. After 24 h, cells were split into two six-well dishes and treated (i) with 5 mM cyclopamine in dimethyl sulfoxide (DMSO) at a final concentration of 0.001% or with the DMSO vehicle for 48 h or (ii) with 0.3 mM benzo[b]thiophene-2-carboxamide, 3-chloro-N-[4-(methylamino)cyclohexyl]-N-{[3-(4-pyridinyl)phenyl]methyl}-(9CI) (SAG) (5) in DMSO at a final concentration of 0.001% or with the DMSO vehicle for 48 h. Cells were then assayed for luciferase and βgal activities. For experiments with hairpins, cells were plated at a density of 3 × 105/60-mm plate. Cells were transiently transfected as described above with the reporter plasmid (1 μg), CMV-βgal (0.5 μg), and the indicated shRNA (2 μg). After 24 h, the cells were split into two six-well dishes and treated with SAG or the vehicle for 48 h. Cells were then assayed for luciferase and βgal activities. shRNA rescue experiments were performed as described above but with the addition of either pCDNA3 or shRNA-resistant GRK2 (2 μg) to the transfection. Experiments with Smo and GRK2 to activate Smo-mediated signaling were performed by plating cells at 1.5 × 105/well in a six-well plate. Cells were transfected with the reporter plasmid (0.5 μg), CMV-βgal (0.25 μg), pCDNA3 or Smo and GRK2 (2 μg pCDNA3 or 1 μg Smo plus 1 μg GRK2), and the indicated shRNA (1 μg). Cells were assayed for luciferase and βgal activities at 72 h posttransfection.
βgal assay.
βgal activity was measured by adding a third of the extract prepared for the luciferase assays to 570 μl of 0.1 mg/ml chlorophenol red-β-d-galactopyranoside in lacZ buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 38 mM 2-mercaptoethanol, pH 7.0). After incubation at 37°C until a visible color change takes place in all samples (5 to 40 min), the absorbance of each sample is measured at 570 nm.
Coimmunoprecipitation assays.
HEK 293 cells were transiently transfected with the indicated expression plasmids by the calcium phosphate method. After 15 h of transfection, the medium was replaced with fresh complete MEM or MEM containing 5 μM cyclopamine where indicated. Forty hours posttransfection, cells were harvested and lysed in 1 ml immunoprecipitation buffer containing 50 mM HEPES, 0.5% NP-40, 250 mM NaCl, 10% glycerol, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and a 1× protease inhibitor cocktail tablet (Complete Mini; Roche). An aliquot of the extract (2%) was used as the input, while the remaining extract was incubated with Flag-antibody beads (Sigma) that had been preblocked with bovine serum albumin for 1 h. Extracts and beads were allowed to mix at 4°C for 3 h. Beads were then washed five times in immunoprecipitation buffer. Sample buffer was then added directly to the washed beads and run on sodium dodecyl sulfate-polyacrylamide gels. The gels were transferred to membranes, which were then blotted with the appropriate antibody.
Western blotting.
For Western blotting of a transfected protein, samples were loaded with βgal values to normalize for transfection efficiency. Otherwise, samples were loaded with an equal amount of total protein as measured by a DC protein assay read at 750 nm (Bio-Rad). To detect the extent of knockdown with GRK2 or βarr2 shRNA, six 60-mm plates of cells were transfected with the indicated plasmids and an empty GFP vector (0.5 μg). Five plates of cells were collected and sorted for GFP expression by flow cytometry. One plate was assayed for luciferase activity and used as a data point for the experiment. Sorted cells were lysed, and equal total protein was run on a 10% polyacrylamide gel. Western blotting was then performed with the appropriate antibody (Myc-tagged rabbit antibody from Abcam, human Smo [A2668] antibody from MBL, Flag-M2-peroxidase antibody from Sigma, GRK2 [C-15] antibody from Santa Cruz, actin antibody from Chemicon, or A1CT β-arrestin antibody [2]). All Western blot assays were done with SuperSignal West Pico chemiluminescent substrate (Pierce), except for Western blot assays of Myc-Smo overexpression in C3H10T1/2 cells (see Fig. 1B), which were done with the SuperSignal West femto maximum-sensitivity substrate (Pierce).
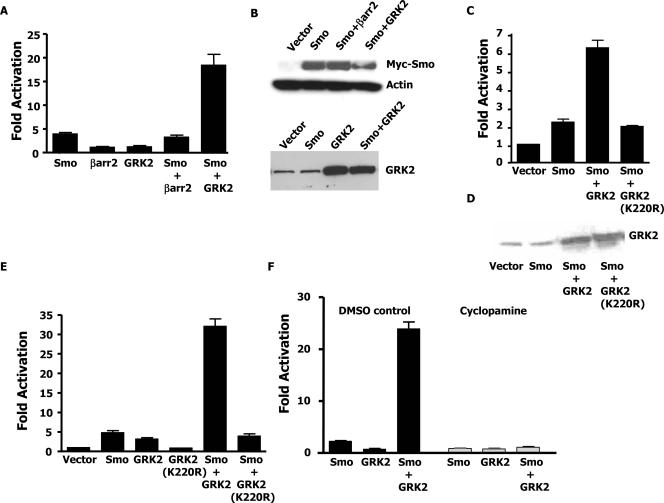
FIG. 1.
Synergistic activation of Gli by GRK2 and active Smo. (A) C3H10T1/2 cells were transiently transfected with the indicated expression plasmids, a Gli luciferase reporter, and a βgal transfection control. Luciferase values were adjusted for transfection efficiency relative to that of a sample containing the reporter alone. Fold activation refers to the increase in luciferase activity of each sample relative to that of the sample containing the reporter alone. (B, top) Cells were transfected as for panel A with the addition of a GFP plasmid. Cells were sorted by flow cytometry, and lysates from equal numbers of cells were immunoblotted with Myc and actin antibodies. (B, bottom) Cells were transfected as for panel A. Extracts used for luciferase assays were normalized for transfection efficiency and immunoblotted with a GRK2 antibody. A representative blot of three independent experiments is shown. (C) Cells were transfected and assayed as for panel A. (D) Extracts from the experiments whose results are shown in panel C were adjusted for transfection efficiency and immunoblotted with a GRK2 antibody. (E) C3H10T1/2 cells stably expressing the indicated proteins were transfected with the Gli reporter and a βgal transfection control. Luciferase assays were performed as for panel A. (F) Cells were transfected as for panel A. After 24 h, the cells were split in half and treated with either the vehicle or cyclopamine for 48 h. Cells were then assayed for luciferase activity as for panel A. The data shown are the results of three independent experiments.
Confocal microscopy.
Shh-LIGHT cells (42) were transfected with the indicated expression plasmids. After 24 h, cells were replated onto a confocal dish with a glass bottom. At 48 h posttransfection, cells were examined by confocal microscopy. Twenty-five green cells (indicating transfection with β-arrestin 2-GFP) per plate (three plates per experiment) were counted for translocation of β-arrestin 2 to the membrane.
RESULTS
Smo and GRK2 synergize to activate the Shh pathway.
To identify a potential role for β-arrestin 2 and GRK2 in signaling through a Smo-dependent pathway, we first assayed the effects of these proteins on the activation of Gli. C3H10T1/2 cells are well known to respond to Shh by inducing Gli-dependent genes (24, 34); therefore, this cell line was used to examine Smo-dependent signaling. Transfection of C3H10T1/2 cells with Smo and a luciferase reporter under the control of eight Gli binding sites results in induction of the reporter by greater than fourfold, revealing that exogenous expression of Smo can overcome endogenous Ptc inhibition to induce signaling (Fig. 1A). Expression of β-arrestin 2 or GRK2 alone is not sufficient to activate pathway signaling, nor does β-arrestin 2 further enhance the induction of the reporter by Smo in cells transfected with Smo and β-arrestin 2 together. However, interestingly, expression of Smo together with GRK2 results in a large synergistic activation (15- to 20-fold) of the reporter (Fig. 1A). The Western analysis in Fig. 1B confirms that the exogenous Smo and GRK2 proteins are expressed equally.
The finding that GRK2 can synergize with Smo to activate a Gli-dependent reporter is interesting in the light of recent data that reveal that GRK2 can also phosphorylate Smo (6). It is therefore possible that the observed synergy between GRK2 and Smo is dependent on the ability of GRK2 to phosphorylate Smo or some other target. To address the necessity of the kinase activity of GRK2 for its ability to cooperate with Smo, we examined if a kinase-inactive version of GRK2, GRK2 K220R, could also synergize with Smo (25). Figure 1C and D reveal that, at equal levels of expression, only wild-type GRK2 is capable of synergizing with Smo to activate the reporter. This suggests that the kinase domain of GRK2 is necessary for GRK2 to synergize with Smo. Similar Gli reporter assays were also performed with C3H10T1/2 cells stably expressing Smo, GRK2, GRK2 K220R, Smo and GRK2, or Smo and GRK2 K220R, with similar results (Fig. 1E).
To further examine the nature of the observed Smo-GRK2 synergy, we next assessed if activation of Smo is necessary by testing the effect of the Smo antagonist cyclopamine. Figure 1F shows that while GRK2 could synergize with Smo to activate Gli-dependent transcription in vehicle (DMSO)-treated cells, inhibition of Smo through cyclopamine abolished the ability of GRK2 to enhance Smo-mediated, Gli-dependent signaling. These data indicate that active Smo is required for the observed Smo/GRK2 synergy.
Given that exogenous Smo can synergize with GRK2 to activate Gli-dependent signaling, we next examined if endogenous Smo might also synergize with GRK2. We reasoned that activation of endogenous Smo, with the Smo agonist SAG, should mimic exogenous overexpression of Smo. Addition of SAG to C3H10T1/2 cells in the presence of 10% serum was sufficient to activate the transfected Gli-responsive promoter approximately 3.5-fold because of the activation of endogenous Smo (Fig. 2), (5). Treatment of cells transfected with exogenous Smo or β-arrestin 2 with SAG showed an additive induction over the induction caused by transfection alone. As expected, transfection of GRK2 into C3H10T1/2 cells that were subsequently treated with SAG caused a large synergistic induction (∼15-fold) of the reporter relative to cells transfected with GRK2 and treated with the vehicle (DMSO) or cells transfected with an empty expression vector and treated with SAG (Vector lane, black bar). Together, these results suggest that GRK2 can synergize with endogenous Smo to facilitate Gli-dependent signaling.
Signaling through the Smo agonist SAG is GRK2 dependent.
As we have observed that Smo-dependent signaling through Gli can be enhanced by the activity of GRK2, we next examined the physiological role of endogenous GRK2 in agonist-induced Smo signaling. C3H10T1/2 cells cultured in low-serum medium respond to the addition of Shh or SAG by activating Smo and inducing Gli transcriptional activity (5). These cells were therefore transfected with the Gli-dependent reporter and a pSilencer vector expressing either a control shRNA or one of three different shRNAs designed to reduce the expression of GRK2. Figure 3A shows that SAG-treated cells that were transfected with the control shRNA induced Gli-dependent transcription by greater than ninefold over vehicle-treated cells. However, cells transfected with GRK2 shRNA 1, 2, or 3 were all significantly impaired in the ability to induce the Gli-dependent reporter upon SAG addition, revealing the importance of endogenous GRK2 in SAG-induced signaling. Cells expressing these shRNAs were sorted by flow cytometry and used for Western blot analysis (Fig. 3B). Figure 3B shows that all of the hairpin RNAs used were capable of significantly inhibiting the expression of endogenous GRK2. Thus, the ability of SAG to induce Smo-dependent activation of Gli depends on the activity of GRK2.
To verify that the effects of the GRK2 shRNAs are in fact specific, we created an amino acid conservation mutant form of bovine GRK2 that renders it insensitive to GRK2 2 shRNA. This mutant form, GRK2 2*, was transfected with GRK2 2 shRNA to rescue SAG-induced signaling. Figure 3C shows that while transfection of multiple plasmids into these cells reduces the control induction of the reporter by SAG, inhibition of signaling by the shRNA is still observed. Addition of the amino acid conservation mutant GRK2 2* to cells expressing the GRK2 2 shRNA is sufficient to return SAG-induced signaling to control levels. The GRK2 Western blot assay in Fig. 3D reveals that in fact the GRK2 2* cDNA is expressed at high levels even in the presence of the GRK2 2 shRNA. Indeed, the level of GRK2 expression from the CMV promoter is such that even wild-type GRK2 can rescue SAG-mediated signaling (data not shown). This is likely because the overexpression of GRK2 is high enough to overwhelm the activity of the hairpin, resulting in sufficient remaining GRK2 to mediate signaling. Together, these data indicate that endogenous GRK2 is required for agonist-induced Smo-mediated signaling.
GRK2 contributes to the transforming potential of Smo.
Given that GRK2 facilitates signaling through endogenous Smo, we next wanted to determine if GRK2 plays a similar role in aberrant signaling through oncogenic Smo mutants. The Smo M2 mutant SmoW535L, originally identified in human basal cell carcinoma and subsequently found in human medulloblastomas, has been shown to superactivate the Gli reporter (42, 47). Cells were transfected with either wild-type Smo or the Smo M2 mutant together with either the control or GRK2 2 shRNA. Figure 4A reveals that the GRK2 2 shRNA inhibits the signaling from both wild-type and mutant Smo. The expression levels of the Smo and Smo M2 plasmids were compared by expressing the plasmids in HEK 293 cells, which do not express endogenous Smo. Figure 4B shows that Smo and Smo M2 are expressed relatively equally. These data thus suggest that GRK2 plays a role in both wild-type and aberrant Smo signaling.
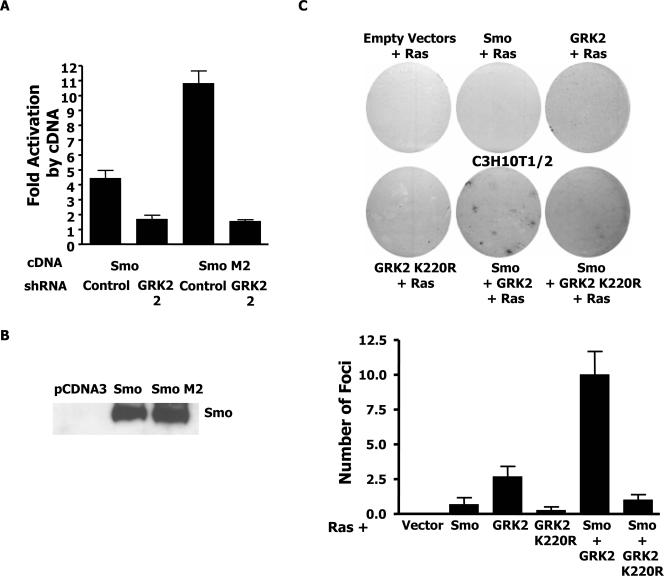
FIG. 4.
GRK2 contributes to oncogenic Smo M2 signaling and cooperates with Smo and Ras to transform C3H10T1/2 cells. (A) C3H10T1/2 cells were transfected with the Gli luciferase reporter, a βgal transfection control, and the indicated cDNA and shRNA plasmids. Fold activation refers to the induction of luciferase activity by the indicated cDNA relative to that of the control sample with the reporter alone containing the same shRNA. The data shown are the results of three independent experiments. (B) HEK 293 cells were transfected with the indicated expression plasmids. Cell extracts were normalized for transfection efficiency. Immunoblotting was performed with human Smo antibody. (C) Focus formation assays. C3H10T1/2 cells stably expressing the indicated proteins were transfected with Ras and stained for foci with crystal violet after 21 days. Representative fields are shown. At the bottom is a quantification of the number of foci per 60-mm plate (three independent experiments in duplicate).
Smo M2 is believed to have its oncogenic effects in part because of an ability to activate the expression of Shh target genes involved in cell proliferation. Indeed, Smo M2 in this cell system and others can activate Gli-dependent reporters between 2.5- and 13-fold more strongly than wild-type Smo (38, 42, 49). Given that the overexpression of Smo together with GRK2 similarly induces a Gli-dependent reporter better than wild-type Smo alone, we examined if Smo/GRK2 could cooperate with Ras 61L to allow the growth of foci in C3H10T1/2 cells, as has been shown for Smo M2 and E1A in REF52 cells (47).
Parental C3H10T1/2 cells or cells stably expressing Smo alone, GRK2 alone, GRK2 K220R alone, Smo together with GRK2, or Smo together with GKR2 K220R were all transfected with H-Ras 61L. As a control, one set of plates was also transfected with βgal to verify that all cell lines were transfected equally with Ras. Foci were counted after 21 days of growth. Under these conditions, few foci grew in plates with the parental cells, Smo alone, GRK2 K220R alone, or Smo together with GRK2 K220R. Interestingly, several foci repeatedly formed in cells expressing GRK2 alone. However, the most significant number of densely packed foci formed in the plates containing cells expressing Smo and GRK2 together (Fig. 4C), indicating that Smo and GRK2 together can cooperate with Ras to produce a transformed phenotype in C3H10T1/2 cells.
GRK2 facilitates the interaction between active Smo and β-arrestin 2.
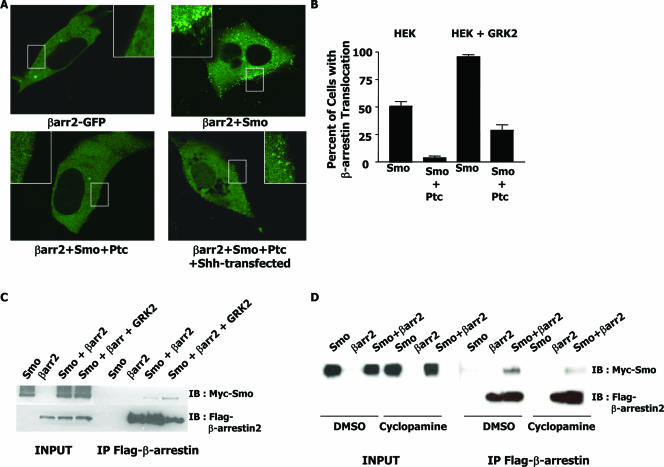
As GRK2 activity seems to be required for SAG-induced signaling, we hypothesized that perhaps GRK2 facilitates the recruitment of a signaling protein to Smo. One protein known to associate with the C terminus of GRK-phosphorylated 7TMRs is β-arrestin. Indeed, β-arrestin has recently been shown to regulate zebra fish development through the Shh pathway and to mediate activation-dependent internalization of Smo in HEK cells (6, 46). To examine if Smo can recruit β-arrestin 2 to the membrane in signaling-competent cells, we chose to examine β-arrestin 2 recruitment in Shh-LIGHT cells (42), which are more transfectable and less autofluorescent than C3H10T1/2 cells. Shh-LIGHT cells were first transfected with a GFP-tagged version of the β-arrestin 2 protein with or without Smo. Figure 5A reveals that while β-arrestin 2-GFP expression is uniformly cytoplasmic when transfected alone, in the presence of cotransfected Smo, it is localized to the plasma membrane in a punctate pattern. This pattern of β-arrestin 2 localization is reminiscent of patterns seen when β-arrestin is recruited to ligand-stimulated 7TMRs (3). As transfection of Shh-LIGHT cells with Smo induces pathway stimulation, (42), it is likely that Smo requires activation in order to recruit β-arrestin 2 to the membrane. To further examine if the recruitment of β-arrestin 2 to the membrane is dependent on Smo activity, we transfected β-arrestin 2-GFP and Smo together with the Smo inhibitor Ptc. As previously observed in HEK cells, in the presence of Ptc, β-arrestin 2 is no longer recruited to the membrane but is again diffusely cytoplasmic (Fig. 5A) (6). Finally, when the inhibition of Smo by Ptc is relieved by cotransfection of the N terminus of the Shh protein, β-arrestin 2 is again recruited to the membrane in a punctate pattern, albeit less robustly than with Smo alone. It therefore seems that this recruitment of β-arrestin 2 to the membrane correlates with Smo activation (6).
FIG. 5.
Interaction between β-arrestin 2 and Smo. (A) Confocal images of Shh-LIGHT cells expressing β-arrestin 2-GFP and different Shh pathway components. The insets are magnifications of the boxed area of the membrane. (B) Quantification of experiments as for panel A with HEK cells with or without exogenous expression of GRK2. Percentage of cells with β-arrestin 2 translocation refers to the percentage of GFP-positive cells that show punctate β-arrestin 2-GFP localization at the plasma membrane. (C) Effect of GRK2 on Smo and β-arrestin 2 association. HEK cells were transfected with the indicated expression plasmids, and extracts were immunoprecipitated (IP) with Flag affinity gel. Immunoprecipitates and total lysates were immunoblotted (IB) with Myc and Flag antibodies. (D) Effect of cyclopamine on Smo and β-arrestin 2 association. Experiments were performed as for panel C, by treating HEK cells with either the vehicle or cyclopamine for 24 h.
In canonical 7TMR biology, the activation of a 7TMR via an agonist results in the phosphorylation of that 7TMR by a GRK. This phosphorylation event promotes β-arrestin 2 recruitment. To examine if GRK2 can facilitate the observed β-arrestin recruitment to Smo at the membrane, HEK 293 cells and HEK 293 cells stably expressing GRK2 were transfected with GFP-β-arrestin and Smo. Figure 5B shows that while approximately 50% of all Smo-transfected, GFP-positive cells without exogenous GRK2 show β-arrestin translocation, almost 95% of all Smo-transfected, GFP-positive cells show β-arrestin translocation when GRK2 is stably expressed. This suggests that GRK2 can enhance the recruitment of β-arrestin to Smo at the membrane. Interestingly, while coexpression of Smo and Ptc inhibits the translocation of β-arrestin, this inhibition of translocation is less pronounced in the presence of exogenous GRK2.
Given that β-arrestin 2 is recruited to the membrane in the presence of Smo, we next examined if Smo and β-arrestin 2 could interact directly and if this interaction could be facilitated by GRK2. HEK cells were transiently transfected with Flag-β-arrestin 2, Myc-Smo, or Flag-β-arrestin 2 and Myc-Smo together with or without GRK2. Cells were lysed and immunoprecipitated with a Flag affinity gel matrix to precipitate Flag-β-arrestin 2. As shown in Fig. 5C, Myc-Smo can be specifically immunoprecipitated with Flag antibody only when it is coexpressed with Flag-β-arrestin 2. Moreover, the coexpression of GRK2 enhances the association of Myc-Smo with Flag-β-arrestin 2 by 2.6-fold ± 0.3-fold (n = 3), again suggesting that GRK2 facilitates the association between Smo and β-arrestin 2.
Figure 5A reveals a correlation between the recruitment of β-arrestin 2 to Smo at the membrane and the activity of the Smo signaling pathway. The Smo antagonist cyclopamine is known to inhibit Smo signaling. Indeed, Fig. 2A confirms that cyclopamine abolishes signaling through Smo in our experiments. We therefore tested whether inhibition of Smo activity by cyclopamine reduces the association between β-arrestin 2 and Smo. HEK cells were transfected with Myc-Smo, Flag-β-arrestin 2, or Myc-Smo and Flag-β-arrestin 2 together. The cells were then split into two plates, and one plate was treated with the vehicle (DMSO) and the other was treated with 5 μM cyclopamine. The immunoprecipitation experiments in Fig. 5D show that cyclopamine does, in fact, reduce the association between Smo and β-arrestin 2 by more than twofold, as reported previously (6). Taken together, in accordance with previously published data, these data suggest that active Smo associates with β-arrestin 2. Furthermore, we find that at least one function of GRK2 in this pathway is to facilitate the interaction between Smo and β-arrestin 2.
GRK2 synergy with Smo is dependent on β-arrestin 2.
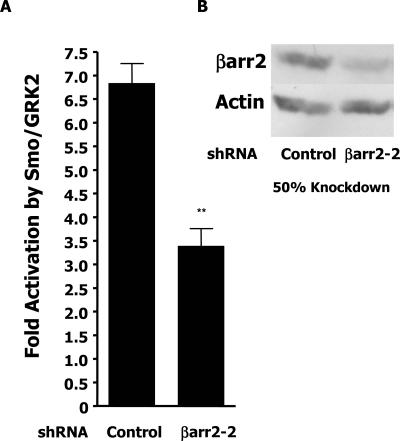
Recent data indicating that β-arrestin 2 is necessary for Shh pathway function in zebra fish development suggests that GRK2 might mediate the observed synergistic effects on Smo signaling by promoting the recruitment of β-arrestin 2 to further transmit the signal (46). The overexpression of β-arrestin 2 in our signaling system has no further effect on the Gli reporter (Fig. 1A), indicating that perhaps β-arrestin 2 is not limiting in C3H10T1/2 cells. We therefore used an RNA interference approach to reduce levels of cellular β-arrestin 2 to assess its importance in the observed synergistic activation of Gli activity by Smo and GRK2. An shRNA, βarr2-2, was generated to reduce β-arrestin 2 expression. C3H10T1/2 cells were transfected with Smo and GRK to induce Gli-dependent signaling as measured by the cotransfected Gli reporter. Figure 6A shows that, in the presence of the control shRNA, Smo/GRK2 can induce the Gli reporter approximately sevenfold relative to cells transfected with the control shRNA and the reporter alone. This induction of the Gli reporter through Smo/GRK2 is significantly inhibited (P < 0.001) when the β-arrestin 2 shRNA is coexpressed, suggesting that β-arrestin 2 contributes to the observed synergy between Smo and GRK2. The representative Western blot assay in Fig. 6B shows that the βarr2-2 shRNA reduces the expression of β-arrestin 2 in C3H10T1/2 cells by approximately 50%. Thus, there is a correlation between the extent of reduction in signaling and the reduction in β-arrestin 2 expression levels. Indeed, other βarr2 shRNAs and small interfering RNAs that reduce the expression of endogenous β-arrestin 2 by less than 40% also modestly but significantly inhibit the ability of Smo/GRK2 to activate the Gli reporter (data not shown).
FIG. 6.
Smo/GRK2 synergy is β-arrestin 2 dependent. (A) C3H10T1/2 cells were transfected with the indicated shRNAs and the Gli reporter with either Smo and GRK2 expression plasmids or a pCDNA3 control plasmid. Fold activation refers to the induction of luciferase activity in samples transfected with Smo and GRK2 relative to that of samples with pCDNA3. Luciferase values were adjusted for transfection efficiency with a cotransfected βgal vector. The data shown are the results of three independent experiments performed in triplicate. Significance (**, P < 0.001) was measured with the Student t test (Microsoft Excel). (B) Cells were transfected as for panel A and sorted by flow cytometry for a cotransfected GFP vector. Extracts from equal numbers of cells were immunoblotted with β-arrestin (A1CT) and actin antibodies.
DISCUSSION
Smo is a 7TM protein with structural similarities to classical 7TMR proteins. The paucity of data linking Smo to G-protein-dependent signaling raises the possibility that Smo may signal through less-well-appreciated pathways of 7TMRs that utilize GRKs and β-arrestins as positive signaling molecules (26, 27). In support of this possibility, recent work has shown that GRK2 is the predominant kinase involved in the phosphorylation of active Smo in a mammalian cell line (HEK 293) and that β-arrestin 2 is necessary for Shh-mediated signaling during zebra fish development (6, 46). Here we provide evidence supporting a positive role for GRK2/β-arrestin 2 in Smo-mediated signaling in a cellular system. Furthermore, unpublished work from this laboratory also demonstrates that GRK2 plays a positive role in Smo-dependent zebra fish development (G. B. Fralish, unpublished data). Taken together, these findings suggest that GRK2 functions in this pathway as a mediator of signaling, at least in part, by facilitating the recruitment of β-arrestin 2 to Smo.
Mice with a targeted deletion of GRK2 were generated 10 years ago (18). These mice die at embryonic day 15.5 with hypoplasia of the ventricular myocardium. While at the time no connections were made that GRK2 may be important for Shh-mediated signaling, recent literature suggests that Shh signaling plays an important role in cardiomyogenesis and heart situs, offering a possible explanation for the observed phenotype of GRK2 knockout mice (14, 28). Interestingly, mice with a targeted deletion of β-arrestin 2 show no developmental defects that might be expected of mice with an Shh pathway mutation. However, β-arrestin 1 may be able to compensate for the loss of β-arrestin 2 in these mice. Mice with disruptions of both β-arrestin 1 and β-arrestin 2 are embryonic lethal, but the developmental defects of these mice have yet to be examined (F.-T. Lin and R. J. Lefkowitz, unpublished observations). Thus, the phenotype of at least the GRK2 knockout mice further suggests a possible role for GRK2 in Shh signaling.
We find that the kinase domain of GRK2 is essential for the observed synergy between GRK2 and Smo. What, then, might GRK2 do to promote Smo signaling? One possibility is that GRK2 phosphorylates other components in the Smo signaling cascade such as Fused, SuFu, or Kif27/Kif7, which in Drosophila are phosphorylated upon Hh pathway activation (for a review, see reference 48). This phosphorylation may then activate or inhibit the function of these signaling proteins. Interestingly, GRK2 has been shown to phosphorylate beta-tubulin, a component of microtubules (37a). As microtubules are part of the large cytoplasmic complex containing Fused, Costal2, and Ci in Drosophila, perhaps GRK2 facilitates the disruption of this complex, promoting entry of Ci/Gli into the nucleus to activate transcription. Alternatively, as microtubules are the main component of cilia, a cellular organelle recently touted as the location of Smo signaling function, it is also possible that GRK2 may aid in ciliary function (9, 15).
A second possibility is that GRK2 mediates the observed positive effects by facilitating the recruitment of other cellular factors to Smo. The most obvious such factor is the β-arrestin protein. Several recent papers have shown that PKA and casein kinase I (CKI) can directly phosphorylate Drosophila Smo (20, 48). It has been proposed that this phosphorylation promotes Smo signaling by inducing a conformational change that allows the binding of Costal 2 or other cellular factors that facilitate signaling (48). As these phosphorylation sites are not conserved between Drosophila and mammals, it is possible that, in mammalian systems, GRK2 plays the role of PKA and CKI by directly phosphorylating the C terminus of Smo. This might, then, similarly induce the conformational changes necessary for the recruitment of other cellular factors, such as β-arrestin, that then mediate signaling.
In classical 7TMR biology, the phosphorylation of a 7TMR by a GRK facilitates the recruitment of β-arrestins to the receptor. Indeed, β-arrestin 2 is similarly recruited to active Smo and this recruitment is enhanced, in both β-arrestin 2 translocation assays and in coimmunoprecipitation assays, by GRK2. We also find that reducing β-arrestin 2 expression through shRNAs reduces the synergistic activation of the Gli-dependent reporter by GRK2 and Smo. Perhaps one reason for a less-than-complete inhibition of reporter activation by the β-arrestin 2 hairpin is the remaining cellular β-arrestin 2 protein levels. As β-arrestin 2 is likely not limiting in C3H10T1/2 cells, a reduction of protein expression by 50%, such as that achieved with one shRNA, may only be sufficient to reduce signaling but not abolish it. Similar findings were obtained when β-arrestin 2 expression was reduced by small interfering RNAs. Unfortunately, attempts to rescue the Smo/GRK2 synergy with an amino acid conservation mutant form of β-arrestin 2 have been unsuccessful. However, this may well be due to a “squelching effect,” where overexpressed β-arrestin 2, which likely acts as a scaffold rather than as an enzyme, sequesters essential signaling components in unproductive complexes. In addition, these shRNAs have no effect on pathway activation mediated by SAG. This may reflect the difference between the amounts of β-arrestin 2 required for recruitment to endogenous Smo versus the amounts of β-arrestin2 required under conditions in which Smo is overexpressed.
Although there are many possibilities for how GRK2 may act to induce Smo signaling, we favor the hypothesis that GRK2 facilitates the recruitment of other cellular factors to Smo, as has been hypothesized for Drosophila Smo phosphorylation by PKA and CKI (48). We show here that GRK2 facilitates the recruitment of β-arrestin 2 to active Smo, likely through direct phosphorylation of Smo. There are several possible ways in which β-arrestin might then transmit the signal. First, the recruitment of β-arrestin 2 to Smo has been shown previously to promote Smo internalization (6). This internalization may be necessary for Smo trafficking to other cellular locales or environments that favor signaling. For example, it has recently been appreciated that the primary cilium functions as a specialized organelle for Smo signal transduction (9, 15, 32). Indeed, Smo mutants that inhibit ciliary localization also inhibit Smo signaling. It has also been observed that β-arrestins are concentrated in the cilia, consistent with the possibility of a role for β-arrestin 2 in this process (9, 10). Another way in which β-arrestin may function to transmit the signal is that upon recruitment to Smo, β-arrestin 2 may scaffold other cellular signaling components, resulting in the activation of signaling cascades (4, 27, 41). Indeed, several other 7TM proteins mediate the activation of mitogen-activated protein kinases through β-arrestin 2-dependent “signalosomes.” Moreover, recent studies have found that Shh and Smo agonists activate ERK (29, 37). While the exact mechanisms by which β-arrestin/GRK2 functions in the Smo pathway remain to be established, these findings suggest that GRK2 inhibitors may offer a novel avenue for investigation of therapeutics for cancers caused by Shh pathway deregulation.
Acknowledgments
This work was supported by the SPORE program in the Pediatric Brain Tumor Foundation Institute at Duke University. This work was also supported in part by NIH grants N519576 and MH40159 to M.G.C. and HL706031 and HL16037 to R.J.L. M.G.C. is the NARSAD Lattner Foundation Distinguished Investigator. R.J.L. is an investigator for the Howard Hughes Medical Institute. A.R.M. was supported by the National Institute of General Medical Sciences (GM74349-01) and the Raychem/Rogers/Morris postdoctoral fellowship award. J.B.F. was supported by the National Institute of General Medical Sciences (GM069086).
We also thank Wei Chen, Trudy Oliver, and Xiao Dong Zhang for reagents; Martin Beaulieu and Ivan Medvedev for help with statistical analysis; Lynn Martinek for core fluorescence-activated cell sorter analysis support; and Amy Ramsey for helpful scientific discussions.
Footnotes
Published ahead of print on 14 August 2006.
REFERENCES
- 1.Ahumada, A., D. C. Slusarski, X. Liu, R. T. Moon, C. C. Malbon, and H. Y. Wang. 2002. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science 298:2006-2010. [DOI] [PubMed] [Google Scholar]
- 2.Attramadal, H., J. L. Arriza, C. Aoki, T. M. Dawson, J. Codina, M. M. Kwatra, S. H. Snyder, M. G. Caron, and R. J. Lefkowitz. 1992. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J. Biol. Chem. 267:17882-17890. [PubMed] [Google Scholar]
- 3.Barak, L. S., S. S. Ferguson, J. Zhang, and M. G. Caron. 1997. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J. Biol. Chem. 272:27497-27500. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu, J. M., T. D. Sotnikova, S. Marion, R. J. Lefkowitz, R. R. Gainetdinov, and M. G. Caron. 2005. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261-273. [DOI] [PubMed] [Google Scholar]
- 5.Chen, J. K., J. Taipale, K. E. Young, T. Maiti, and P. A. Beachy. 2002. Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. USA 99:14071-14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W., X. R. Ren, C. D. Nelson, L. S. Barak, J. K. Chen, P. A. Beachy, F. de Sauvage, and R. J. Lefkowitz. 2004. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science 306:2257-2260. [DOI] [PubMed] [Google Scholar]
- 7.Claing, A., S. A. Laporte, M. G. Caron, and R. J. Lefkowitz. 2002. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog. Neurobiol. 66:61-79. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, M. M., Jr. 2003. The hedgehog signaling network. Am. J. Med. Genet. A 123:5-28. [DOI] [PubMed] [Google Scholar]
- 9.Corbit, K. C., P. Aanstad, V. Singla, A. R. Norman, D. Y. Stainier, and J. F. Reiter. 2005. Vertebrate Smoothened functions at the primary cilium. Nature 437:1018-1021. [DOI] [PubMed] [Google Scholar]
- 10.Dawson, T. M., J. L. Arriza, D. E. Jaworsky, F. F. Borisy, H. Attramadal, R. J. Lefkowitz, and G. V. Ronnett. 1993. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science 259:825-829. [DOI] [PubMed] [Google Scholar]
- 11.DeCamp, D. L., T. M. Thompson, F. J. de Sauvage, and M. R. Lerner. 2000. Smoothened activates Gαi-mediated signaling in frog melanophores. J. Biol. Chem. 275:26322-26327. [DOI] [PubMed] [Google Scholar]
- 12.Denef, N., D. Neubuser, L. Perez, and S. M. Cohen. 2000. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell 102:521-531. [DOI] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Gianakopoulos, P. J., and I. S. Skerjanc. 2005. Hedgehog signaling induces cardiomyogenesis in P19 cells. J. Biol. Chem. 280:21022-21028. [DOI] [PubMed] [Google Scholar]
- 15.Huangfu, D., and K. V. Anderson. 2005. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 102:11325-11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huangfu, D., and K. V. Anderson. 2006. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development 133:3-14. [DOI] [PubMed] [Google Scholar]
- 17.Ingham, P. W., and A. P. McMahon. 2001. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15:3059-3087. [DOI] [PubMed] [Google Scholar]
- 18.Jaber, M., W. J. Koch, H. Rockman, B. Smith, R. A. Bond, K. K. Sulik, J. Ross, Jr., R. J. Lefkowitz, M. G. Caron, and B. Giros. 1996. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc. Natl. Acad. Sci. USA 93:12974-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia, J., C. Tong, and J. Jiang. 2003. Smoothened transduces Hedgehog signal by physically interacting with Costal2/Fused complex through its C-terminal tail. Genes Dev. 17:2709-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia, J., C. Tong, B. Wang, L. Luo, and J. Jiang. 2004. Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature 432:1045-1050. [DOI] [PubMed] [Google Scholar]
- 21.Kalderon, D. 2000. Transducing the hedgehog signal. Cell 103:371-374. [DOI] [PubMed] [Google Scholar]
- 22.Kasai, K., M. Takahashi, N. Osumi, S. Sinnarajah, T. Takeo, H. Ikeda, J. H. Kehrl, G. Itoh, and H. Arnheiter. 2004. The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells 9:49-58. [DOI] [PubMed] [Google Scholar]
- 23.Katoh, Y., and M. Katoh. 2004. KIF27 is one of orthologs for Drosophila Costal-2. Int. J. Oncol. 25:1875-1880. [PubMed] [Google Scholar]
- 24.Kinto, N., M. Iwamoto, M. Enomoto-Iwamoto, S. Noji, H. Ohuchi, H. Yoshioka, H. Kataoka, Y. Wada, G. Yuhao, H. E. Takahashi, S. Yoshiki, and A. Yamaguchi. 1997. Fibroblasts expressing Sonic hedgehog induce osteoblast differentiation and ectopic bone formation. FEBS Lett. 404:319-323. [DOI] [PubMed] [Google Scholar]
- 25.Kong, G., R. Penn, and J. L. Benovic. 1994. A beta-adrenergic receptor kinase dominant negative mutant attenuates desensitization of the beta 2-adrenergic receptor. J. Biol. Chem. 269:13084-13087. [PubMed] [Google Scholar]
- 26.Lefkowitz, R. J., and S. K. Shenoy. 2005. Transduction of receptor signals by beta-arrestins. Science 308:512-517. [DOI] [PubMed] [Google Scholar]
- 27.Lefkowitz, R. J., and E. J. Whalen. 2004. Beta-arrestins: traffic cops of cell signaling. Curr. Opin. Cell Biol. 16:162-168. [DOI] [PubMed] [Google Scholar]
- 28.Levin, M., R. L. Johnson, C. D. Stern, M. Kuehn, and C. Tabin. 1995. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 82:803-814. [DOI] [PubMed] [Google Scholar]
- 29.Li, C., S. Chi, N. He, X. Zhang, O. Guicherit, R. Wagner, S. Tyring, and J. Xie. 2004. IFNα induces Fas expression and apoptosis in hedgehog pathway activated BCC cells through inhibiting Ras-Erk signaling. Oncogene 23:1608-1617. [DOI] [PubMed] [Google Scholar]
- 30.Liu, T., A. J. DeCostanzo, X. Liu, H. Wang, S. Hallagan, R. T. Moon, and C. C. Malbon. 2001. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science 292:1718-1722. [DOI] [PubMed] [Google Scholar]
- 31.Lum, L., C. Zhang, S. Oh, R. K. Mann, D. P. von Kessler, J. Taipale, F. Weis-Garcia, R. Gong, B. Wang, and P. A. Beachy. 2003. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol. Cell 12:1261-1274. [DOI] [PubMed] [Google Scholar]
- 32.May, S. R., A. M. Ashique, M. Karlen, B. Wang, Y. Shen, K. Zarbalis, J. Reiter, J. Ericson, and A. S. Peterson. 2005. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 287:378-389. [DOI] [PubMed] [Google Scholar]
- 33.Merchant, M., F. F. Vajdos, M. Ultsch, H. R. Maun, U. Wendt, J. Cannon, W. Desmarais, R. A. Lazarus, A. M. de Vos, and F. J. de Sauvage. 2004. Suppressor of fused regulates Gli activity through a dual binding mechanism. Mol. Cell. Biol. 24:8627-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura, T., T. Aikawa, M. Iwamoto-Enomoto, M. Iwamoto, Y. Higuchi, M. Pacifici, N. Kinto, A. Yamaguchi, S. Noji, K. Kurisu, T. Matsuya, and P. Maurizio. 1997. Induction of osteogenic differentiation by hedgehog proteins. Biochem. Biophys. Res. Commun. 237:465-469. [DOI] [PubMed] [Google Scholar]
- 35.Nusslein-Volhard, C., and E. Wieschaus. 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287:795-801. [DOI] [PubMed] [Google Scholar]
- 36.Ogden, S. K., M. Ascano, Jr., M. A. Stegman, L. M. Suber, J. E. Hooper, and D. J. Robbins. 2003. Identification of a functional interaction between the transmembrane protein Smoothened and the kinesin-related protein Costal2. Curr. Biol. 13:1998-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osawa, H., H. Ohnishi, K. Takano, T. Noguti, H. Mashima, H. Hoshino, H. Kita, K. Sato, H. Matsui, and K. Sugano. 2006. Sonic hedgehog stimulates the proliferation of rat gastric mucosal cells through ERK activation by elevating intracellular calcium concentration. Biochem. Biophys. Res. Commun. 344:680-687. [DOI] [PubMed] [Google Scholar]
- 37a.Pitcher, J. A., R. A. Hall, Y. Daaka, J. Zhang, S. S. Ferguson, S. Hester, S. Miller, M. G. Caron, R. J. Lefkowitz, and L. S. Barak. 1998. The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J. Biol. Chem. 273:12316-12324. [DOI] [PubMed]
- 38.Rahnama, F., R. Toftgard, and P. G. Zaphiropoulos. 2004. Distinct roles of PTCH2 splice variants in Hedgehog signalling. Biochem. J. 378:325-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins, D. J., K. E. Nybakken, R. Kobayashi, J. C. Sisson, J. M. Bishop, and P. P. Therond. 1997. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 90:225-234. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki, H., C. Hui, M. Nakafuku, and H. Kondoh. 1997. A binding site for Gli proteins is essential for HNF-3β floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development 124:1313-1322. [DOI] [PubMed] [Google Scholar]
- 41.Shenoy, S. K., and R. J. Lefkowitz. 1. November 2005. Seven-transmembrane receptor signaling through beta-arrestin. Science STKE 2005(308):cm10. [DOI] [PubMed] [Google Scholar]
- 42.Taipale, J., J. K. Chen, M. K. Cooper, B. Wang, R. K. Mann, L. Milenkovic, M. P. Scott, and P. A. Beachy. 2000. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406:1005-1009. [DOI] [PubMed] [Google Scholar]
- 43.Therond, P. P., J. D. Knight, T. B. Kornberg, and J. M. Bishop. 1996. Phosphorylation of the fused protein kinase in response to signaling from hedgehog. Proc. Natl. Acad. Sci. USA 93:4224-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, G., K. Amanai, B. Wang, and J. Jiang. 2000. Interactions with Costal2 and suppressor of fused regulate nuclear translocation and activity of cubitus interruptus. Genes Dev. 14:2893-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, H. Y., and C. C. Malbon. 2003. Wnt signaling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science 300:1529-1530. [DOI] [PubMed] [Google Scholar]
- 46.Wilbanks, A. M., G. B. Fralish, M. L. Kirby, L. S. Barak, Y. X. Li, and M. G. Caron. 2004. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science 306:2264-2267. [DOI] [PubMed] [Google Scholar]
- 47.Xie, J., M. Murone, S. M. Luoh, A. Ryan, Q. Gu, C. Zhang, J. M. Bonifas, C. W. Lam, M. Hynes, A. Goddard, A. Rosenthal, E. H. Epstein, Jr., and F. J. de Sauvage. 1998. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391:90-92. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, C., E. H. Williams, Y. Guo, L. Lum, and P. A. Beachy. 2004. Extensive phosphorylation of Smoothened in Hedgehog pathway activation. Proc. Natl. Acad. Sci. USA 101:17900-17907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu, A. J., L. Zheng, K. Suyama, and M. P. Scott. 2003. Altered localization of Drosophila Smoothened protein activates Hedgehog signal transduction. Genes Dev. 17:1240-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]