Abstract
The sex steroid progesterone is essential for the proliferation and differentiation of the mammary gland epithelium during pregnancy. In relation to this, in vitro studies using breast carcinoma T47D cells have demonstrated a biphasic progesterone response, consisting of an initial proliferative burst followed by a sustained growth arrest. However, the transcriptional factors acting with the progesterone receptor (PR) to mediate the progesterone effects on mammary cell growth and differentiation remain to be determined. Recently, it has been demonstrated that the transcriptional regulating protein of 132 kDa (TReP-132), initially identified as a regulator of steroidogenesis, is also a cell growth suppressor. Similar to progesterone-bound PR, TReP-132 acts by inducing the gene expression of the G1 cyclin-dependent kinase inhibitors p21WAF1/Cip1 (p21) and p27Kip1 (p27). The putative interaction between TReP-132 and progesterone pathways in mammary cells was therefore analyzed in the present study. Our results show that TReP-132 interacts in vitro and in T47D cells with progesterone-activated PR. TReP-132 synergizes with progesterone-bound PR to trans activate the p21 and p27 gene promoters at proximal Sp1-binding sites. Moreover, TReP-132 overexpression and knockdown, respectively, increased or prevented the induction of p21 and p27 gene expression by progesterone. As a consequence, TReP-132 knockdown also resulted in the loss of the inhibitory effects of progesterone on pRB phosphorylation, G1/S cell cycle progression, and cell proliferation. Furthermore, the knockdown of TReP-132 expression also prevented the induction of both early and terminal markers of breast cell differentiation which had been previously identified as progesterone target genes. As well, the progesterone-induced accumulation of lipid vacuoles was inhibited in the TReP-132-depleted cells. Finally, TReP-132 gene expression levels increased following progesterone treatment, indicating the existence of a positive auto-regulatory loop between PR and TReP-132. Taken together, these data identify TReP-132 as a coactivator of PR mediating the growth-inhibitory and differentiation effects of progesterone on breast cancer cells.
The steroid hormones estrogen and progesterone play key roles in the growth of the mammary gland (49). Estrogens appear to be the main drive for proliferation of the mammary gland epithelium, whereas progesterone is required for its terminal growth and differentiation (27). The induction of mammary epithelial development during pregnancy is mediated by a rise in progesterone levels (51). The physiological effects of progesterone occur mainly via interaction with specific intracellular progesterone receptors (PRs), PR-A and PR-B, which are products of a single gene and members of the nuclear receptor (NR) family (11). Studies performed on mice in which the expression of both PRs was ablated have demonstrated that progesterone is necessary for ductal branching and the lobulo-alveolar development of the mammary gland (45). More recently, selective ablation of each receptor isoform has indicated that PR-B is specifically required for the progesterone-dependent development of the mammary gland during pregnancy (11).
In relation to the function of progesterone in breast development, both growth-stimulatory and -inhibitory effects have been previously reported on breast epithelium cells and cancer development in tumor animal models (11, 42, 46). Moreover, in vitro studies using the PR-positive mammary carcinoma T47D cell line as a model have demonstrated a biphasic cellular response to either progesterone or its derivatives (R5020 or ORG 2058), with an immediate proliferative burst followed by a sustained growth arrest (28, 38, 50). As with many hormones and growth factors, the regulation of retinoblastoma gene product (pRB) phosphorylation—a critical checkpoint of the G1/S transition—plays a major role in the control of proliferation by progesterone (18, 39, 71). The initial pRB phosphorylation provoked by progesterone is catalyzed by constitutively expressed cyclin-dependent kinases (CDKs), which are activated through interaction with specific cyclins induced by progesterone (39). The ensuing growth arrest is associated, at least in part, with the transitory induction of the cyclin-dependent kinase inhibitors (CDKIs) p21Cip1/WAF1 (p21) and p18INK4c (p18), followed by a sustained induction of p27Kip1 (p27) (28, 50, 69). Associations of these CDKIs with the different G1 CDK complexes lead to an inhibition of their activity and a decrease in pRB phosphorylation, resulting in an arrest in the late G1 phase of the cell cycle. It is known that progesterone induces both p21 and p27 expression through a transcriptional mechanism which involves interaction between progesterone-bound PR, the general coactivator CBP/p300, and the transcription factor Sp1 at proximal Sp1-binding sites (25, 52). However, given the fact that PR is expressed during both phases of the progesterone response (28, 50), it is likely that yet-unidentified PR target genes and/or cofactors of PR are involved with PR in the delayed growth-inhibitory effects of progesterone.
The transcriptional regulating protein of 132 kDa (TReP-132) was isolated as a novel factor implicated in the regulation of various steroidogenic genes (21, 22, 26). Notably, in human adrenal carcinoma NCI-H295 cells, TReP-132 acts as a coactivator of the nuclear receptor steroidogenic factor 1 to enhance cytochrome P450 side chain cleavage (P450scc) and c17 (P450c17) gene promoter activity (23). Recently, we identified a new function for TReP-132 as a growth suppressor protein in HeLa and mammary cancer cells (24). As with PR, the antiproliferative activity of TReP-132 involves its interaction with Sp1 to activate the p21 and p27 promoters, resulting in the inhibition of G1 CDK-mediated phosphorylation of pRB and histone H1. Considering the ability of TReP-132 to function as a nuclear receptor coactivator, in this study we tested whether TReP-132 interacts with PR and influence the progesterone-dependent regulation of mammary cancer cell growth. Using the T47D cell line as a model, we show that TReP-132 does indeed act as a PR coactivator to activate the p21 and p27 gene promoters. Furthermore, we show that TReP-132 gene expression itself is steadily induced by progesterone, which is necessary for the long-term growth-inhibitory and differentiating effects of the hormone. Thus, TReP-132 is likely to mediate a positive feedback loop in the progesterone response which is crucial for the delayed and sustained action of progesterone in breast cancer cells.
MATERIALS AND METHODS
Plasmids.
The TReP-132 expression vector subcloned in pcDNA3 and the wild-type or mutated glutathione S-transferase (GST)-TReP-132 fusion protein were constructed as previously described (24). The p21WAF1/Cip1 and the p27Kip1 promoter luciferase reporter constructs were kindly provided by X. Wang (Dept. of Pharmacology, Duke University Medical Center, Durham, NC) (13) and T. Sakai (Kyoto Prefectural University of Medicine, Japan) (34), respectively.
Cell culture assays.
Human T47D ductal carcinoma cells, a commonly used model to study progesterone signaling in breast cancer cells (e.g., references 28, 38, and 50), were obtained from the American Type Culture Collection (Rockville, MD) and were cultured as monolayers as previously described (25). In all assays, cells were first synchronized in G0/G1 phase by serum starvation for at least 48 h in medium containing 0.4% fetal calf serum. Progesterone (30 nM) or ethanol (vehicle) was added daily when cells resumed proliferation by reincubation in the routine growth medium (corresponding to time zero of the experiments). Each experiment was repeated at least three times, and values represent the means ± standard deviations (SD) of a representative experiment carried out in triplicate.
Cellular DNA content and flow cytometry profiles were, respectively, performed by the staining of nuclear DNA using the fluorochrome 3,5-diaminobenzoicacid free acid and propidium iodide as described previously (24).
Transient transfections were performed with FuGENE 6 (Roche Molecular Biochemicals) as previously described (25). The efficiency of transfection, which was verified using a green fluorescent protein expression plasmid, was ≥60%.
Chromatin immunoprecipitation assays were performed as described elsewhere (24) by using 4 μg of anti-TReP-132, anti-PR (MS-298; NeoMarkers), and anti-p300 and anti-Sp1 (sc-584X and sc-59, respectively; Santa Cruz Biotechnology) antibodies for the immunoprecipitation of cell lysates.
Immunocytological staining.
T47D cells, subconfluently grown on glass coverslips, were transfected with small interfering RNA (siRNA), treated with progesterone or ethanol (vehicle) for 48 h, and then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS). For fluorescent immunocytochemistry, cells were first permeabilized by boiling in 10 mM citrate buffer. The rabbit polyclonal TReP-132 antibody (24) or a rabbit nonimmune serum (as control) was then detected with an AlexaFluor 488-conjugated goat anti-rabbit immunoglobulin G (IgG; Molecular Probes). For lipid detection, cells were stained using Oil Red O and counterstained with hematoxylin. After final washes, the coverslips and their attached cells were mounted on glass microscope slides using Mowiol mountant (Hoechst, Frankfurt, Germany). Specimens were visualized and photographed using a Leitz DMRB microscope and Lhesa NB (for fluorescent immunocytochemistry) or Leica DC480 video (for Oil Red O staining) camera. Oil Red O staining intensity was quantified with Adobe Photoshop using the public program from the National Institutes of Health (http://rsb.info.nih.gov/nih-image/). Results represent the means ± SD of values from a single experiment (n = 6 fields/point) which was repeated four times with similar results.
Reverse transcription and quantitative PCR.
Transcript levels in extracted total RNA were assessed by quantitative reverse transcription-PCR (RT-PCR) by using the oligonucleotide primers specific for human TReP-132, p21, and p27 as described previously (24). In addition, the following primer pairs were used: desmoplakin, 5′-TGATAAACTCAGACAGCGCC-3′ and 5′-CATCAAACACCAGCTTGGAG-3′ (GenBank identification number gi 58530839 ); Na+/K+-ATPase α1, 5′-CTGGCTTGAGGCTGTCATCTTCCTC-3′ and 5′-TTCCTTGCCATGCGTTTGGC-3′ (GenBank identification number gi 48762680 ); fatty acid synthase (FAS), 5′-ATCGTGGACGGAGGCATCAACC-3′ and 5′-TTGGCCATCATCGCTCGCTG-3′ (GenBank identification number gi 41872630 ); tissue-nonspecific alkaline phosphatase (ALP), 5′-TCACTCTCCGAGATGGTGGTGGTGG-3′ and 5′-TTCCTTCATGGTGCCCGTGG-3′ (GenBank identification number gi 13787192 ). Because of their stability during cell cycle progression, 28S RNA levels were simultaneously quantified for normalization. Each figure indicates mRNA levels as means ± SD (n = 3) of one experiment representative of at least three independent experiments performed in triplicate.
Knockdown of TReP-132 expression.
Knockdown of TReP-132 expression using siRNA was performed as described elsewhere (24) with slight modifications. Briefly, oligonucleotides used for the generation of two TReP-132 siRNAs targeting two distinct regions of TReP-132 cDNA (siRNA-1, nucleotides 298 to 318; siRNA-2, nucleotides 362 to 382) were synthesized by Eurogentec. The nonsilencing siRNA oligonucleotide, which does not target any known mammalian gene and is used as a negative control, was from QIAGEN. Transfections of siRNA duplexes (700 ng) were performed at 0 and 3 days using jetSI (Polyplus Transfection, Illkirch, France). The efficiency of the transfection was ascertained by fluorescence-activated cell sorter (FACS) analysis (see below) following control transfection of a fluorescein-coupled nonsilencing siRNA. The loss of endogenous TReP-132 gene expression after transfection with TReP-132 siRNA was verified by quantitative PCR and immunofluorescence using an anti-TReP-132 antibody as described previously (24). The nonsilencing siRNA control did not significantly influence TReP-132 expression (data not shown). Experiments performed with TReP-132 siRNA-1 and -2 led to a decrease in TReP-132 mRNA levels by ∼77% and 62%, respectively, and comparable effects were obtained. Representative experiments have been performed with TReP-132 siRNA-1.
Protein assays.
In vitro GST pull-down analyses were performed as described in reference 24. In-cell protein assays were performed as previously described (23) according to the Santa Cruz Biotechnology (California) protocol. Cells were washed twice with ice-cold PBS and collected with ice-cold RIPA buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, phosphatase, and protease inhibitors). After removing cell debris by centrifugation, extracts were aliquoted and either used immediately (e.g., for coimmunoprecipitation assays) or stored at −80°C. For coimmunoprecipitation assays, whole-cell lysates in RIPA buffer were first cleared with 1.0 μg nonimmune rabbit IgG (sc-2027; Santa Cruz), together with 30 μl of protein A-Sepharose beads (Amersham Biosciences, Uppsala, Sweden). After centrifugation, cell lysates were immunoprecipitated for 1 h at 4°C with 1 μg of the anti-TReP-132 antibody or nonimmune rabbit IgG and then incubated overnight at 4°C with protein A-Sepharose. The immunoprecipitated proteins were washed three times with lysis buffer and once with PBS and then resuspended in electrophoresis sample buffer. Samples of immunoprecipitated or total proteins (30 μg) were analyzed by Western blotting using as antibodies the anti-ppRB-Ser807/811 antibody (9308; Cell Signaling) raised against a pRB peptide phosphorylated on the Ser807/811 residue, which is phosphorylated by both CDK2 and CDK4/6 kinases (2), or the anti-pRB raised against underphosphorylated pRB (554164; BD Pharmingen), the anti-PR antibody specific for the B-form of PR (MS-192; Microm France, Francheville, France), anti-p21, anti-p27, and anti-β-actin (as control antibody) as previously described (24, 25). The blots represent typical results of at least three independent experiments.
Statistical analyses.
Statistical analyses were done using the nonparametric Mann-Whitney test.
RESULTS
TReP-132 enhances the progesterone response of the CDKI p21 and p27 gene promoters.
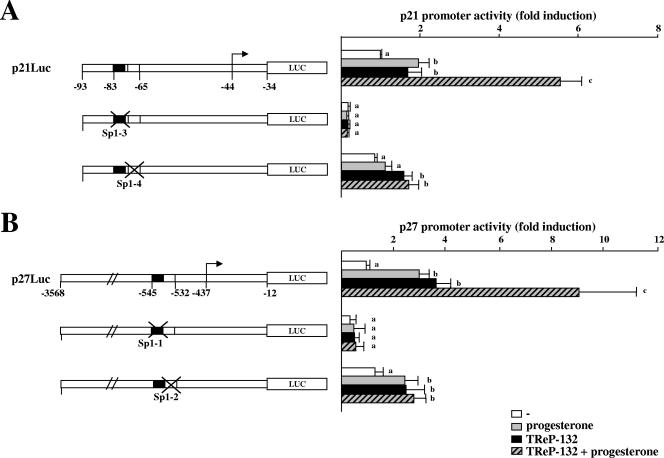
TReP-132 and progesterone-bound PR have been shown to activate the p21 and p27 genes by interacting with Sp1 through the same proximal Sp1-binding elements (composed of the Sp1-3 and Sp1-4 sites for p21 [25, 52] and the Sp1-1 and Sp1-2 sites for p27 [24, 25]). Therefore, it was hypothesized that TReP-132 and PR may functionally interact at these proximal Sp1 response elements to activate transcription of both promoters. To test this, wild-type p21 or p27 promoter reporter constructs (denoted as p21Luc and p27Luc, respectively) were cotransfected with the TReP-132 expression plasmid in PR-positive breast carcinoma T47D cells (21, 22, 24), and cells were treated or not with progesterone (Fig. 1). Confirming the results of previous studies (24, 25, 52), TReP-132 or progesterone treatment alone stimulated both p21 and p27 gene promoter activities. Interestingly, a synergistic effect of TReP-132 and progesterone was observed on both CDKI promoters. To further assess the roles of the Sp1 sites in this response, the p21 and p27 reporter constructs mutated at each Sp1 site were transiently cotransfected with TReP-132 in cells incubated or not with progesterone (Fig. 1). As previously reported, the mutation of the Sp1-3 and Sp1-1 sites diminished the basal activity and abolished the responses of the p21 and p27 promoters to TReP-132 or progesterone alone (24, 25, 52). Moreover, the mutation of either the Sp1-4 or the Sp1-2 sites resulted in the reduction of progesterone-dependent trans activation of the p21 or p27 promoters, respectively. However, mutation of each of these sites prevented the synergistic effects of TReP-132 and progesterone on both promoters. These results indicate that TReP-132 cooperates with the progesterone pathway to trans activate proximal Sp1 response elements of p21 and p27 gene promoters.
FIG. 1.
TReP-132 enhances p21 and p27 promoter activities induced by progesterone. T47D cells were transiently cotransfected with the reporter constructs containing the indicated p21 (A) or p27 (B) promoter fragments. Twelve hours after transfection, cells were incubated with progesterone (30 nM) or vehicle (ethanol) for 34 h and then harvested for the luciferase activity assay. Results are expressed as increase (mean ± SD) over luciferase activity levels of control (−) p21Luc or p27Luc, arbitrarily set as 1. The arrows represent the transcription start sites; the crosses indicate the mutated Sp1-binding sites. For each promoter construct, columns followed by different symbols are statistically significantly different from each other.
TReP-132 is recruited by progesterone-activated PR at proximal Sp1-binding sites of p21 and p27 gene promoters.
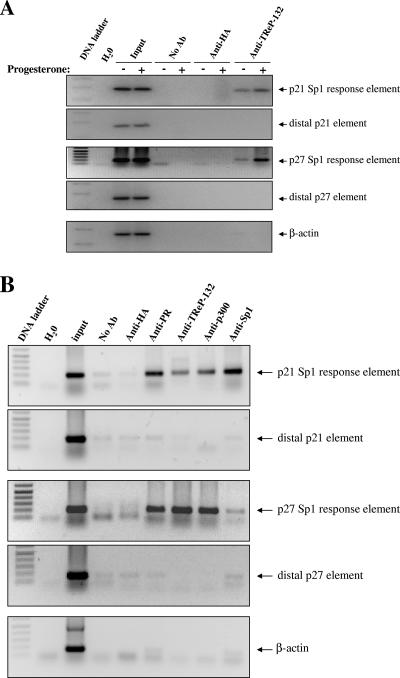
To investigate the in-cell occupancy of these Sp1-binding sites by TReP-132 and the influence of progesterone on this, chromatin immunoprecipitation assays were performed on DNA isolated from T47D cells treated or not with progesterone (Fig. 2A). In good correlation with our previous findings in HeLa cells (24), TReP-132 was found to be associated by immunoprecipitation with the proximal Sp1-binding elements of the p21 and p27 genes. Interestingly, this association was greater in progesterone-treated than in vehicle-incubated cells.
FIG. 2.
Progesterone enhances TReP-132 recruitment at multiprotein complexes formed with PR and CBP/p300 at proximal Sp1 elements of the p21 and p27 promoters. Soluble chromatin was prepared from T47D cells incubated with progesterone (30 nM) or ethanol (vehicle) (A) or with progesterone (30 nM) alone (B) for 2 h 30 min before lysis. Immunoprecipitations were then performed using antibodies as indicated (top). Controls included PCRs done without DNA (H2O) or with nonprecipitated genomic DNA (input) or immunoprecipitation assays performed without antibody (no Ab) or with an irrelevant antibody (anti-HA). The extracted DNA was amplified using the primer pairs covering either the −83/−65 progesterone-responsive Sp1-binding region of the p21 gene promoter (upper panel), a distal region of the p21 gene located ∼1 kb from this element, the −545/−532 progesterone-responsive Sp1-binding region of the p27 gene promoter, a distal region of the p27 gene located ∼1 kb from this element, or a β-actin gene region (lower panel).
Previous reports have indicated that the CBP/p300 protein functions as a coactivator of PR (44, 65) and cooperates with PR at the proximal Sp1-binding sites of the p21 and p27 gene promoters to increase their activities (25, 52). Concurring with these data, PR, TReP-132, and Sp1 were found to be present together with CBP/p300 at proximal Sp1 elements of the p21 and p27 promoters in progesterone-treated T47D cells (Fig. 2B). As a control of specificity, amplification using primers covering regions ∼1 kb upstream of these sites or oligonucleotides specific for the β-actin gene resulted in nonrelevant background products.
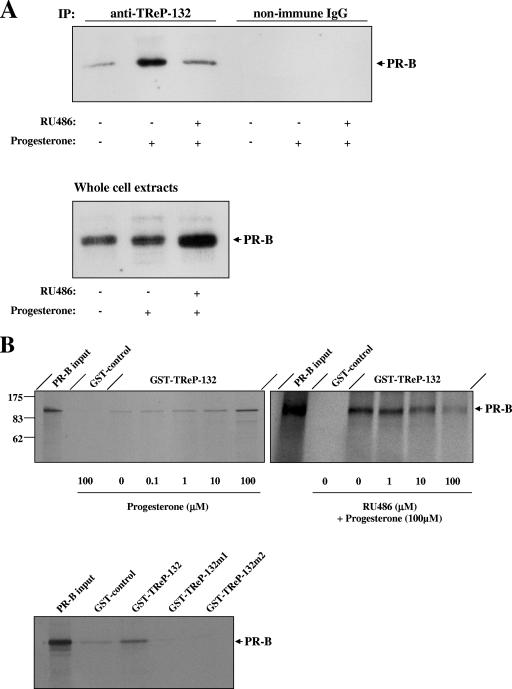
The putative interaction between TReP-132 and progesterone-activated PR was then analyzed. To this end, we first determined in coimmunoprecipitation assays whether endogenous TReP-132 and PR interact in T47D cells treated or not for 12 h with progesterone and the partial PR antagonist RU486 (Fig. 3A). Low levels of endogenous PR were found in the complex immunoprecipitated by the anti-TReP-132 antibody in untreated T47D cells. However, the amount of PR coimmunoprecipitated with TReP-132 drastically increased in cells treated with progesterone alone. This effect was attenuated by cotreatment with RU486. To further assess whether TReP-132 interacts in vitro with PR, GST pull-down assays were performed with GST-TReP-132 and PR proteins (Fig. 3B). The results indicated that TReP-132 interacts physically with PR and that this interaction is enhanced in a dose-dependent manner by progesterone (Fig. 3B, top left panel). In good correlation with Fig. 3A, the binding observed between TReP-132 and progesterone-activated PR diminished in the presence of RU486 (Fig. 3B, top right panel). We previously demonstrated that TReP-132 contains two putative nuclear receptor box (NR-box) LXXLL motifs at amino acids 181 (LRQLL) and 863 (LEMLL) (22), which are nuclear receptor interaction domains of numerous transcriptional coregulatory proteins (30). Interestingly, the mutation of either LXXLL motif drastically reduced the binding of TReP-132 to PR. This indicates the importance of both LXXLL motifs in the in vitro interaction between TReP-132 and PR (Fig. 3B, lower panel).
FIG. 3.
TReP-132 interacts directly with progesterone-bound PR via its LXXLL NR-boxes. A. Coimmunoprecipitation assays. T47D cells were treated or not with progesterone (30 nM) and RU486 (10 nM) for 12 h. Total protein extracts (30 μg) were then submitted to Western blot analyses using a PR antibody either after immunoprecipitation with anti-TReP-132 or nonimmune rabbit IgG (negative control) antibodies (upper panel) or directly for control of the in-cell PR levels (lower panel). B. GST pull-down assays. Top panel: GST-TReP-132 immobilized on glutathione-coupled Sepharose was incubated with [35S]methionine-labeled PR (−) with increasing concentrations of progesterone (0 to 100 μM; left panel) or with 100 μM progesterone together with increasing doses of RU486 (0 to 100 μM; right panel). Specificity of interaction was assessed by comparison with background levels obtained by incubating GST alone with [35S]methionine-labeled PR in the presence of 100 μM progesterone (GST-control). Lower panel: GST-TReP-132, either wild type or mutated in the LRQLL (GST-TReP-132m1) or LEMLL (GST-TReP-132m2) motifs, was assayed for interaction with [35S]PR as described above in the presence of progesterone (10 μM). PR input contains 1/10 the amount of radioactive proteins used in the incubations.
Taken together, these results suggest that TReP-132 is recruited by progesterone-activated PR through its NR-box LXXLL motifs in the regulatory complexes formed with Sp1 at proximal Sp1-binding sites of the p21 and p27 gene promoters.
TReP-132 expression is required for the progesterone-induced increase of p21 and p27 expression and inhibition of G1/S T47D cell cycle progression.
The next question we addressed was whether TReP-132 is required for the induction of p21 and p27 expression as well as the regulation of cell proliferation by progesterone.
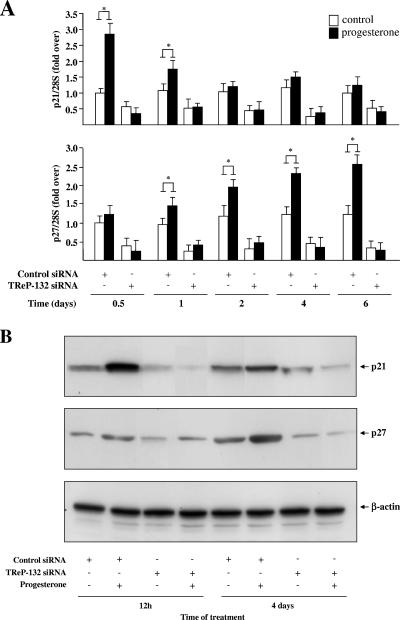
First, the expression of both genes in response to progesterone was assessed in T47D cells in which TReP-132 expression was silenced using an siRNA strategy (24) (Fig. 4). Concurring with reference 24, the decrease of TReP-132 expression resulted in a significant down-regulation of both p21 and p27 mRNA (Fig. 4A) and protein levels (Fig. 4B). Moreover, in good agreement with previous observations (28, 50), progesterone treatment resulted in an early and transient up-regulation of p21, followed by a delayed and sustained up-regulation of p27. Strikingly, this progesterone-dependent modulation of p21 and p27 gene expression was completely abolished upon siRNA-mediated specific silencing of TReP-132.
FIG. 4.
TReP-132 mediates the induction of p21 and p27 gene expression by progesterone. T47D cells, treated with or without progesterone (30 nM) and transfected with control or TReP-132 siRNAs (700 ng) at 0 and 3 days, were harvested at the indicated times for RNA (A) and protein (B) extraction. (A) Quantitative RT-PCR analyses. mRNA levels are expressed relative to levels in vehicle-treated control siRNA-transfected cells harvested at 0.5 days (12 h), arbitrarily set as 1. *, P ≤ 0.05 versus control. (B) Western blot analyses of p21 and p27 as well as β-actin as control.
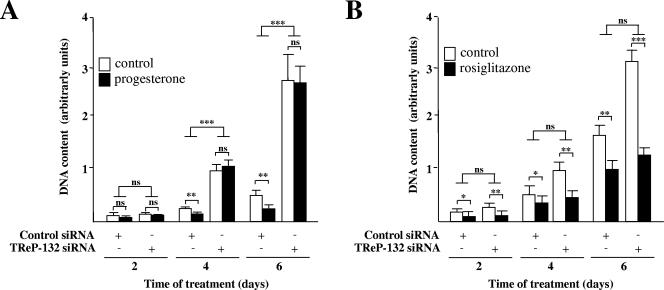
Next, the influence of TReP-132 silencing on the growth-inhibitory effects of progesterone was tested. In good correlation with previous reports, cell growth of control-transfected cells was inhibited by progesterone after 4 and 6 days of treatment as a consequence of p21 and p27 up-regulation (28, 50), and silencing of TReP-132 provoked an increased proliferation of untreated cells (24). However, siRNA silencing of TReP-132 completely prevented the growth-inhibitory effects of progesterone (Fig. 5A). To assess the specificity of this effect, the role of TReP-132 in the response to rosiglitazone, a ligand of peroxisome proliferator-activated receptor γ (PPARγ) which has been previously reported to also inhibit mammary cancer cell growth (19, 48), was also analyzed. In contrast to progesterone, silencing of TReP-132 did not alleviate the growth-inhibitory effects of rosiglitazone on T47D cells (Fig. 5B). This therefore indicates that TReP-132 specifically mediates the inhibition of breast cancer cell proliferation by PR.
FIG. 5.
siRNA silencing of TReP-132 abolishes inhibition of T47D cell proliferation by progesterone but not by rosiglitazone. T47D cells, treated with or without progesterone (A) (30 nM) or rosiglitazone (B) (0.5 μM) and transfected with control or TReP-132 siRNAs (700 ng) at 0 and 3 days, were harvested at 2, 4, or 6 days for measure of DNA content. ns, not significant; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 versus control.
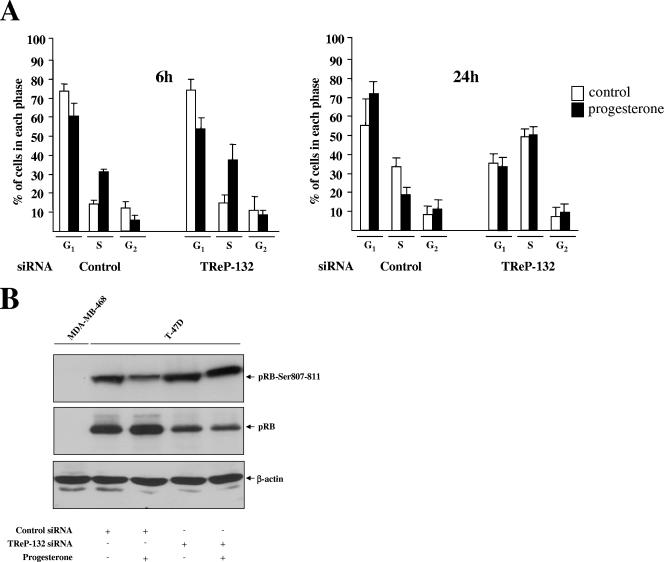
Finally, the cell cycle phase distribution and in-cell pRB phosphorylation status were analyzed (Fig. 6). As previously reported (28, 50), progesterone induced an initial acceleration of cell cycle progression (Fig. 6A, left panel; performed after 6 h of progesterone treatment), followed by an inhibition of the G1/S transition (Fig. 6A, right panel; performed after 24 h of progesterone treatment) which was associated with an inhibition of pRB phosphorylation (Fig. 6B; performed 24 h after progesterone treatment). Interestingly, siRNA-induced TReP-132 silencing did not affect the early cell mitogenic response to progesterone. By contrast, TReP-132 knockdown prevented the subsequent decreases in the percentage of cells in the S phase as well as the regulation of phosphorylated and dephosphorylated pRB levels (Fig. 6A, right panel, and B).
FIG. 6.
TReP-132 is required for the inhibition of G1/S cell cycle progression by progesterone. Synchronized T47D cells, transfected with control or TReP-132 siRNAs (700 ng) and treated with or without progesterone, were harvested after 6 h or 24 h for determination of cell cycle phase distribution (A) or Western blot analysis using either an antibody raised against pRB phosphorylated at Ser807/811 or an antibody recognizing specifically the underphosphorylated form of pRB (B). ppRB-Ser807-811, pRB phosphorylated on the Ser807/811 residue; pRB, hypophosphorylated pRB. The MDA-MB-468 cell line, deficient for pRB expression (40, 70), was used as a negative control of pRB staining.
Collectively, these data indicate a specific role for TReP-132 as a coactivator of PR in the regulation of the progesterone-induced G1-phase cell cycle arrest of breast cancer cells.
TReP-132 mediates the differentiation-enhancing activities of progesterone in breast cancer cells.
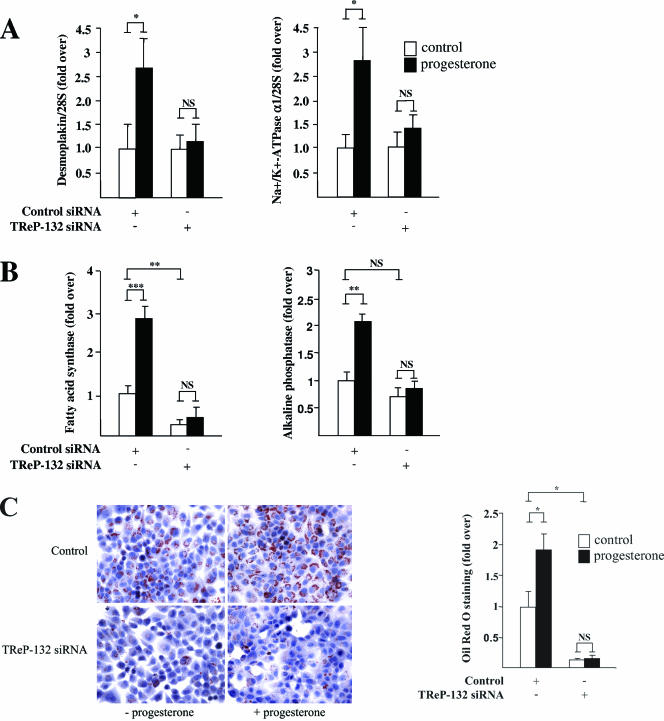
Recent in vitro reports have associated the ability of progesterone and its derivatives to negatively control mammary cancer cell proliferation with the induction of a cell differentiation program (3, 43, 50), which leads to the acquisition of a secretory phenotype (15, 29). Therefore, we tested whether siRNA silencing of TReP-132 also influences the effects of progesterone on T47D cell differentiation. To this end, the expression levels of a panel of previously identified markers of early and terminal differentiation in breast cancer cells (15, 35, 37) were analyzed (Fig. 7A and B). As reported, progesterone induced the early gene expression of desmoplakin and Na+/K+-ATPase α1 (37), which are markers for epithelial differentiation and glandular development, respectively (56, 67) (Fig. 7A). Moreover, concurring with previous data (16, 35), progesterone increased the expression of FAS and ALP, which are markers of differentiation correlating with lipid storage in breast cancer cells (9, 15) (Fig. 7B). Interestingly, the induction of each of these mRNA levels was abolished by TReP-132 knockdown (Fig. 7A and B). Concurring with these RNA data, progesterone treatment induced an accumulation of lipid droplets as visualized by Oil Red O staining, and this effect was significantly decreased by ∼87% in TReP-132-deficient cells (Fig. 7C).
FIG. 7.
TReP-132 silencing prevents the induction of differentiation by progesterone. Synchronized T47D cells transfected with control or TReP-132 or siRNA (700 ng) and treated with progesterone (30 nM) or vehicle were harvested after 12 h (A) or 6 days (B and C) for quantitative RT-PCR analyses (A and B) and Oil Red O staining of lipid droplets (C). In panels A and B, mRNA levels are expressed relative to vehicle-incubated control siRNA-transfected cells, which were arbitrarily set as 1. NS, not significant; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001 versus control. C. Lipid detection. Left panel: representative Oil Red O-stained cellular sections. Right panel: quantification of the lipid staining intensity.
Therefore, the induction of a differentiated secretory phenotype of breast cancer cells by progesterone requires the expression of TReP-132.
TReP-132 gene expression is induced by progesterone.
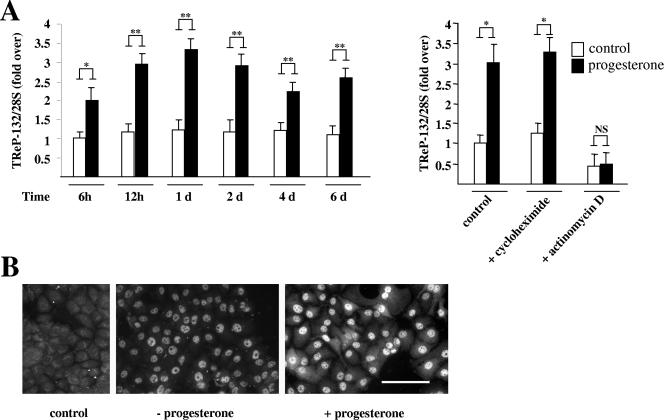
Since progesterone exerts biphasic effects on mammary cancer cell proliferation despite the continuous presence of transcriptionally competent PR, it has been proposed that the long-term growth arrest provoked by progesterone requires the induction of additional factors (28, 50). To determine whether TReP-132 expression is regulated by progesterone, TReP-132 mRNA levels were measured by quantitative RT-PCR analysis in T47D cells following progesterone treatment (Fig. 8). Interestingly, treatment with progesterone provoked a long-lasting increase in TReP-132 mRNA levels which was already obvious within 12 h of treatment (Fig. 8A, left panel). Induction of TReP-132 mRNA levels by progesterone was inhibited by actinomycin D (an inhibitor of RNA polymerase II), but not by cycloheximide (an inhibitor of protein synthesis) (Fig. 8A, right panel), indicating that progesterone-bound PR may directly induce TReP-132 gene transcription. Correlating with mRNA data, TReP-132 protein levels increased in progesterone-treated T47D cells, as shown by fluorescence immunocytochemistry after 24 h of progesterone treatment (Fig. 8B). Together, these results indicate that TReP-132 is a progesterone-responsive gene acting with PR in a positive feedback loop that inhibits mammary cell proliferation and stimulates differentiation.
FIG. 8.
TReP-132 gene expression is induced by progesterone. Synchronized T47D cells were treated with or without progesterone (30 nM) for the indicated periods of time (A, left panel) or for 24 h (A, right panel, and B). A. Quantitative PCR analysis. mRNA levels are expressed relative to levels in vehicle-treated control cells harvested at 0.5 days (12 h), arbitrarily set as 1. Right panel. Cycloheximide (5 μg/ml) or actinomycin D (10 μg/ml) was added to the medium 1 h 30 min before the addition of progesterone or ethanol (vehicle). d, day; NS, not significant; *, P ≤ 0.05; **, P ≤ 0.01 versus control. B. Fluorescence immunocytochemistry of TReP-132 after 24-h progesterone treatment. Representative immunocytochemistry signals for each condition are shown.
DISCUSSION
It has been proposed that the delayed growth arrest provoked by sustained progesterone treatment requires the presence and/or activation of other transcription factors and/or coregulators acting with PR (28). Both TReP-132 and progesterone have been previously shown to act at the G1/S transition checkpoint through similar mechanisms, i.e., the transcriptional induction of the p21 and p27 CDKI gene promoters via their proximal Sp1-binding sites (25, 28, 50, 52). Knockdown of p21 and p27 expression using an siRNA approach prevented the growth-inhibitory response to either progesterone or TReP-132 (25, 28, 50, 52; data not shown), highlighting their requirements for both inhibitory pathways. Therefore, in the present study, we analyzed whether PR and TReP-132 also cross talk in the control of these cell cycle-regulating genes in the TReP-132- and PR-expressing mammary carcinoma T47D cell line (21, 22, 24). Our results indicate that TReP-132 is rapidly recruited following progesterone treatment to the multiprotein complex formed with PR and CBP/p300 at proximal Sp1-binding elements of the p21 and p27 gene promoters. Moreover, TReP-132 and progesterone synergize to trans activate the p21 and p27 gene promoters through these proximal Sp1-binding sites. Coimmunoprecipitation on intact cells and GST pull-down assays further revealed a physical interaction between PR and TReP-132 that was enhanced by progesterone and mediated by the two LXXLL NR-boxes of TReP-132. These observations thus identify a novel function for TReP-132 as a coactivator of PR implicated in the progesterone-dependent regulation of the p21 and p27 genes.
Basal TReP-132 levels may be sufficient to initiate the growth-inhibitory progesterone response via a direct transcriptional effect on the p21 and p27 gene promoters. Indeed, the induction of p21 and p27 observed after 24-h progesterone treatment was inhibited by the RNA polymerase II inhibitor actinomycin D but was not affected by the protein synthesis inhibitor cycloheximide (data not shown). However, it was noteworthy that progesterone treatment also provoked an early and steady induction of TReP-132 gene expression itself. Although a positive control by progesterone of the expression of PR coactivators such as SRC-1 and CBP/p300 has been observed in normal human endometrium (60), to our knowledge such molecular pathways have not been shown in breast tissue yet. Moreover, TReP-132 knockdown experiments demonstrated that TReP-132 expression is necessary for the consecutive progesterone-induced transitory p21 and long-lasting p27 increases and the consequent inhibitory effects on G1/S cell cycle progression. Concurring with this, we have previously shown that TReP-132 and p27 gene expression levels correlate with PR levels in human primary breast tumor biopsies (24, 25). These data indicate that TReP-132 mediates, as both a PR cofactor and a target gene, a positive feedback loop participating in the growth-inhibitory response to progesterone (28, 50).
TReP-132 deficiency did not affect the previously reported rosiglitazone-induced inhibition of breast cancer cell growth (19, 48). Interestingly, TReP-132 siRNA did not affect either the transcriptional up-regulation by progesterone of metallothionein IIA and pepsinogen C, which are genes not directly related to cell cycle control and are regulated by progesterone via a canonical PR-responsive element (5, 63) (data not shown). Thus, specific interactions between PR, TReP-132, and other proteins included in the multiprotein complexes formed at proximal Sp1-binding elements of the cell cycle regulatory p21 and p27 genes appear to exist and underlie the growth-inhibitory response to progesterone. Importantly, lowering of Sp1 levels by ∼60% using an siRNA approach resulted in a significant decrease of p21 and p27 mRNA levels in both the absence and presence of progesterone (data not shown). This indicates the requirement of Sp1 for basal and progesterone-stimulated p21 and p27 promoter activities. However, as shown for the trans activation of the p21 gene by PPARγ (31), it is obvious that the contribution of Sp3 and/or Sp4 protein in the regulation of the p21 and/or p27 promoters by TReP-132 and progesterone cannot be excluded. Besides, in addition to the two NR-box LXXLL motifs, the primary structure of TReP-132 also contains several other putative interaction domains with coregulatory proteins (22). Significantly, TReP-132 interacts in vitro and in NCI-H295 cells with CBP/p300 (22, 23), which is, to date, the only known PR coactivator that synergizes with progesterone-bound PR to trans activate the p21 and p27 promoters (25, 52). Whether additional transcriptional regulatory factors such as CBP/p300 interact also with TReP-132 to mediate the progesterone response therefore remains to be analyzed.
It is, however, possible that TReP-132 binds to nuclear receptors or cofactors at Sp1 sites of cell cycle regulatory genes to regulate their expression in contexts other than the progesterone response. In fact, the proximal Sp1-binding regions of the p21 and p27 gene promoters integrate different growth-modulatory signals. For instance, the Sp1 sites are involved in the trans activation of the p21 promoter by transforming growth factor β (13) and BRCA1 (66, 72), which is also a corepressor for the estrogen receptor, the androgen receptor, and the PR (72). The proximal Sp1 sites of the p27 promoter are required for the response to vitamin D3 (33) and tamoxifen (41). Furthermore, the Sp1-binding sites have a common important role in the modulation of the cell cycle (53). Notably, they also mediate the induction of cyclin D1 by estrogens (7). Thus, further studies are warranted in order to analyze whether TReP-132 could play a more general role in the cross talk of different growth signaling pathways.
We have recently shown that TReP-132 expression levels are positively associated with markers of cell differentiation in human breast tumor biopsies (24). Our present data further demonstrate that TReP-132 itself acts as a differentiation factor in breast epithelial cancer cells, notably in the mediation of progesterone effects. Indeed, the presence of TReP-132 was a prerequisite for the early induction by progesterone of certain common differentiation markers (37), as well as for the progesterone-dependent increase in the synthesis of lipid droplets which is associated with a differentiated secretory phenotype in mammary cells (15). It is tempting to propose that p21 and p27 are involved in the TReP-132-dependent effects on breast cancer cell differentiation. In fact, the induction of p21 and p27 has been shown to constitute a molecular switch that facilitates hormone-induced differentiation in numerous cell systems (34). In breast tissue, high levels of p21 and p27 are found in intermediately differentiated and well-differentiated ductal carcinoma in situ, respectively (47). Furthermore, p27 up-regulation is thought to be the molecular basis for the blockage in the alveolar differentiated state (58). Thus, the molecular mechanisms underlying the function of TReP-132 in mammary cell differentiation and the putative role of p21 and p27 induction therein remain to be investigated.
The role of PR activity on breast tumorigenesis has been the subject of controversy. In epidemiological studies, progesterone levels during a first pregnancy at an early age are thought to confer protective effects against future breast tumor development (62). However, the possible deleterious effect of certain progestins used in hormone replacement therapies (HRTs) after menopause and as contraceptives to counteract the proliferative action of unopposed estrogens on the uterus recently resurfaced in the Women's Health Initiative and the Million Women studies (6, 57). These studies showed that a combined estrogen plus progestin regimen as HRT is associated with an increased risk of breast cancer compared to estrogen alone or placebo. In spite of these data, different reports have also indicated that the effects of progesterone and its derivatives may depend on several different factors, including the family history of breast cancer, the mode of administration (e.g., episodical or cyclical versus continuous), and the dose and type of progestins (e.g., estrane or pregnane derivatives) (for reviews, see references 17, 36, and 61). The in vitro demonstration of the delayed action of progesterone derivatives on breast cancer cell proliferation could explain their potential differential stimulatory or growth-inhibitory effects, depending on whether the treatment is transitory or continuous, respectively (28, 50, 68). Interestingly, siRNA knockdown of TReP-132 did not modulate the influence of progesterone on the initial proliferative burst, as shown by FACS analysis (Fig. 6A, left panel) or bromodeoxyuridine proliferation assays (data not shown). In correlation with this, the induction after 6 h of progesterone treatment of cyclin D1, D3, A, B1, or E gene expression, which participate in the immediate acceleration of cell cycle progression (28, 50, 68), was not affected by TReP-132 siRNA transfection (data not shown). These data, indicating a role for TReP-132 specifically in the growth-inhibitory response to progesterone, suggest that the effects of each progestin treatment may also depend on levels of TReP-132 expression in breast epithelium and/or of TReP-132 recruitment to the PR.
In clinical practice, the expression of PR in breast tumor biopsies is assessed as a predictive marker for favorable disease prognosis, with the absence of PR reflecting a nonfunctional estrogen receptor and resistance to hormone therapy (12, 32). Expression levels of PR correlate with p27 and differentiation status in large populations of primary mammary tumors (25, 55). p27 mRNA and protein levels in breast cancer biopsies correlate positively with a favorable outcome in human breast tumors, whereas a loss of p27 gene expression is associated with a shorter overall survival (8, 25, 54). However, a subgroup of steroid receptor-positive tumors with low levels of p27, which are of poor prognosis and are resistant to antiestrogen therapy, exists (4). It is tempting to speculate that an alteration of the activity of transcriptional factors—such TReP-132—involved with PR in the (dys)regulation of the p27 gene promoter could play a role in p27 loss and mammary tumor development. It is noteworthy that breast tumor formation is associated with a decrease in TReP-132 gene expression (24). Moreover, the human TReP-132 gene lies on the chromosome 6p21.1-p12.1 locus (14, 21, 59), for which allelic loss has been shown to accompany the acquisition of tamoxifen resistance by MCF-7 cells (1). Analyses of potential correlations between genetic alterations of the TReP-132 gene and p27 expression could be decisive in clarifying the role of TReP-132 in familial predisposition to certain cancers and/or failure of hormonal therapy.
Following tamoxifen, the first identified selective estrogen receptor modulator, a number of other antiestrogens have been developed (20). The notion that selective nuclear receptor modulators can exhibit cell- and tissue-specific effects has also been extended to a panel of other nuclear receptors (64). Selective progesterone receptor modulators, such as Asoprinil, are now under investigation in the treatment of uterine fibroids and endometriosis (10). However, to date, they have been mostly identified by empirical and in vivo studies (10). The characterization of TReP-132 as a PR coregulator molecule involved in the antiproliferative and differentiation effects of progesterone in breast tissue may have the potential to explain the biological effects of different progestins on breast cancer and to guide the future discovery of drugs. In fact, selective progesterone receptor modulators inducing TReP-132 cofactor recruitment to PR may prevent breast cancer development when used in HRTs, contraceptives, or treatment of uterine diseases. Further studies are thus warranted to evaluate the putative tissue-specific roles of TReP-132 in progesterone pathways.
Acknowledgments
Grants were provided by Genfit, the Cancéropôle Nord-Ouest, and the Fondation de France. R. Robillard was supported by a 1-year ARC fellowship.
We thank D. Monté (IBL, France) for the kind gift of the pcDNA3-Sp1 expression vector. We thank P. Lefebvre for critical reading of the manuscript.
REFERENCES
- 1.Achuthan, R., S. M. Bell, P. Roberts, J. P. Leek, K. Horgan, A. F. Markham, K. A. MacLennan, and V. Speirs. 2001. Genetic events during the transformation of a tamoxifen-sensitive human breast cancer cell line into a drug-resistant clone. Cancer Genet. Cytogenet. 130:166-172. [DOI] [PubMed] [Google Scholar]
- 2.Adams, P. D., X. Li, W. R. Sellers, K. B. Baker, X. Leng, J. W. Harper, Y. Taya, and W. G. Kaelin, Jr. 1999. Retinoblastoma protein contains a C-terminal motif that targets it for phosphorylation by cyclin-CDK complexes. Mol. Cell. Biol. 19:1068-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhalaf, M., A. El-Mowafy, and S. Karam. 2002. Growth inhibition of MCF-7 human breast cancer cells by progesterone is associated with cell differentiation and phosphorylation of Akt protein. Eur. J. Cancer Prev. 11:481-488. [DOI] [PubMed] [Google Scholar]
- 4.Arteaga, C. L. 2004. Cdk inhibitor p27Kip1 and hormone dependence in breast cancer. Clin. Cancer Res. 10:368S-371S. [DOI] [PubMed] [Google Scholar]
- 5.Balbin, M., and C. Lopez-Otin. 1996. Hormonal regulation of the human pepsinogen C gene in breast cancer cells. Identification of a cis-acting element mediating its induction by androgens, glucocorticoids, and progesterone. J. Biol. Chem. 271:15175-15181. [DOI] [PubMed] [Google Scholar]
- 6.Beral, V. 2003. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362:419-427. [DOI] [PubMed] [Google Scholar]
- 7.Castro-Rivera, E., I. Samudio, and S. Safe. 2001. Estrogen regulation of cyclin D1 gene expression in ZR-75 breast cancer cells involves multiple enhancer elements. J. Biol. Chem. 276:30853-30861. [DOI] [PubMed] [Google Scholar]
- 8.Catzavelos, C., N. Bhattacharya, Y. C. Ung, J. A. Wilson, L. Roncari, C. Sandhu, P. Shaw, H. Yeger, I. Morava-Protzner, L. Kapusta, E. Franssen, K. I. Pritchard, and J. M. Slingerland. 1997. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat. Med. 3:227-230. [DOI] [PubMed] [Google Scholar]
- 9.Chalbos, D., C. Joyeux, F. Galtier, and H. Rochefort. 1992. Progestin-induced fatty acid synthetase in human mammary tumors: from molecular to clinical studies. J. Steroid Biochem. Mol. Biol. 43:223-228. [DOI] [PubMed] [Google Scholar]
- 10.Chwalisz, K., M. C. Perez, D. Demanno, C. Winkel, G. Schubert, and W. Elger. 2005. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr. Rev. 26:423-438. [DOI] [PubMed] [Google Scholar]
- 11.Conneely, O. M., B. Mulac-Jericevic, and J. P. Lydon. 2003. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 68:771-778. [DOI] [PubMed] [Google Scholar]
- 12.Cui, X., R. Schiff, G. Arpino, C. K. Osborne, and A. V. Lee. 2005. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J. Clin. Oncol. 23:7721-7735. [DOI] [PubMed] [Google Scholar]
- 13.Datto, M. B., Y. Yu, and X. F. Wang. 1995. Functional analysis of the transforming growth factor beta responsive elements in the WAF1/Cip1/p21 promoter. J. Biol. Chem. 270:28623-28628. [DOI] [PubMed] [Google Scholar]
- 14.Dib, C., S. Faure, C. Fizames, D. Samson, N. Drouot, A. Vignal, P. Millasseau, S. Marc, J. Hazan, E. Seboun, M. Lathrop, G. Gyapay, J. Morissette, and J. Weissenbach. 1996. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152-154. [DOI] [PubMed] [Google Scholar]
- 15.Di Lorenzo, D., M. Gianni, G. F. Savoldi, F. Ferrari, A. Albertini, and E. Garattini. 1993. Progesterone induced expression of alkaline phosphatase is associated with a secretory phenotype in T47D breast cancer cells. Biochem. Biophys. Res. Commun. 192:1066-1072. [DOI] [PubMed] [Google Scholar]
- 16.Di Lorenzo, D., A. Albertini, and D. Zava. 1991. Progestin regulation of alkaline phosphatase in the human breast cancer cell line T47D. Cancer Res. 51:4470-4475. [PubMed] [Google Scholar]
- 17.Eden, J. 2003. Progestins and breast cancer. Am. J. Obstet. Gynecol. 188:1123-1131. [DOI] [PubMed] [Google Scholar]
- 18.Ekholm, S. V., and S. I. Reed. 2000. Regulation of G1 cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 12:676-684. [DOI] [PubMed] [Google Scholar]
- 19.Elstner, E., C. Muller, K. Koshizuka, E. A. Williamson, D. Park, H. Asou, P. Shintaku, J. W. Said, D. Heber, and H. P. Koeffler. 1998. Ligands for peroxisome proliferator-activated receptor gamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc. Natl. Acad. Sci. USA 95:8806-8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabian, C. J., and B. F. Kimler. 2005. Selective estrogen-receptor modulators for primary prevention of breast cancer. J. Clin. Oncol. 23:1644-1655. [DOI] [PubMed] [Google Scholar]
- 21.Gizard, F., M. El-Alfy, Y. Duguay, B. Lavallee, F. DeWitte, B. Staels, B. G. Beatty, and D. W. Hum. 2002. Function of the transcriptional regulating protein of 132 kDa (TReP- 132) on human P450scc gene expression. Endocr. Res. 28:559-574. [DOI] [PubMed] [Google Scholar]
- 22.Gizard, F., B. Lavallee, F. DeWitte, and D. W. Hum. 2001. A novel zinc finger protein TReP-132 interacts with CBP/p300 to regulate human CYP11A1 gene expression. J. Biol. Chem. 276:33881-33892. [DOI] [PubMed] [Google Scholar]
- 23.Gizard, F., B. Lavallee, F. DeWitte, E. Teissier, B. Staels, and D. W. Hum. 2002. The transcriptional regulating protein of 132 kDa (TReP-132) enhances P450scc gene transcription through interaction with steroidogenic factor-1 in human adrenal cells. J. Biol. Chem. 277:39144-39155. [DOI] [PubMed] [Google Scholar]
- 24.Gizard, F., R. Robillard, O. Barbier, B. Quatannens, A. Faucompre, F. Revillion, J. P. Peyrat, B. Staels, and D. W. Hum. 2005. TReP-132 controls cell proliferation by regulating the expression of the cyclin-dependent kinase inhibitors p21WAF1/Cip1 and p27Kip1. Mol. Cell. Biol. 25:4335-4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gizard, F., R. Robillard, P. Gervois, A. Faucompre, F. Revillion, J. P. Peyrat, W. D. Hum, and B. Staels. 2005. Progesterone inhibits human breast cancer cell growth through transcriptional upregulation of the cyclin-dependent kinase inhibitor p27Kip1 gene. FEBS Lett. 579:5535-5541. [DOI] [PubMed] [Google Scholar]
- 26.Gizard, F., E. Teissier, I. Dufort, G. Luc, V. Luu-The, B. Staels, and D. W. Hum. 2004. The transcriptional regulating protein of 132 kDa (TReP-132) differentially influences steroidogenic pathways in human adrenal NCI-H295 cells. J. Mol. Endocrinol. 32:557-569. [DOI] [PubMed] [Google Scholar]
- 27.Graham, J. D., and C. L. Clarke. 1997. Physiological action of progesterone in target tissues. Endocr. Rev. 18:502-519. [DOI] [PubMed] [Google Scholar]
- 28.Groshong, S. D., G. I. Owen, B. Grimison, I. E. Schauer, M. C. Todd, T. A. Langan, R. A. Sclafani, C. A. Lange, and K. B. Horwitz. 1997. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27Kip1. Mol. Endocrinol. 11:1593-1607. [DOI] [PubMed] [Google Scholar]
- 29.Guiochon-Mantel, A., and E. Milgrom. 1999. Role of progestins and progesterone receptors in breast cancer biology, p. 245-259. In A. Manni (ed.), Contemporary endocrinology: endocrinology of breast cancer. Humana Press Inc., Totowa, N.J.
- 30.Heery, D. M., E. Kalkhoven, S. Hoare, and M. G. Parker. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733-736. [DOI] [PubMed] [Google Scholar]
- 31.Hong, J., I. Samudio, S. Liu, M. Abdelrahim, and S. Safe. 2004. Peroxisome proliferator-activated receptor gamma-dependent activation of p21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology 145:5774-5785. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz, K. B., W. L. McGuire, O. H. Pearson, and A. Segaloff. 1975. Predicting response to endocrine therapy in human breast cancer: a hypothesis. Science 189:726-727. [DOI] [PubMed] [Google Scholar]
- 33.Huang, Y. C., J. Y. Chen, and W. C. Hung. 2004. Vitamin D3 receptor/Sp1 complex is required for the induction of p27Kip1 expression by vitamin D3. Oncogene 23:4856-4861. [DOI] [PubMed] [Google Scholar]
- 34.Inoue, T., J. Kamiyama, and T. Sakai. 1999. Sp1 and NF-Y synergistically mediate the effect of vitamin D3 in the p27Kip1 gene promoter that lacks vitamin D response elements. J. Biol. Chem. 274:32309-32317. [DOI] [PubMed] [Google Scholar]
- 35.Joyeux, C., H. Rochefort, and D. Chalbos. 1989. Progestin increases gene transcription and messenger ribonucleic acid stability of fatty acid synthetase in breast cancer cells. Mol. Endocrinol. 3:681-686. [DOI] [PubMed] [Google Scholar]
- 36.Kenemans, P., and A. Bosman. 2003. Breast cancer and post-menopausal hormone therapy. Best Pract. Res. Clin. Endocrinol. Metab. 17:123-137. [DOI] [PubMed] [Google Scholar]
- 37.Kester, H. A., B. M. van der Leede, P. T. van der Saag, and B. van der Burg. 1997. Novel progesterone target genes identified by an improved differential display technique suggest that progestin-induced growth inhibition of breast cancer cells coincides with enhancement of differentiation. J. Biol. Chem. 272:16637-16643. [DOI] [PubMed] [Google Scholar]
- 38.Lacroix, M., and G. Leclercq. 2004. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res. Treat. 83:249-289. [DOI] [PubMed] [Google Scholar]
- 39.Lange, C. A., J. K. Richer, and K. B. Horwitz. 1999. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol. Endocrinol. 13:829-836. [DOI] [PubMed] [Google Scholar]
- 40.Lee, E. Y., H. To, J. Y. Shew, R. Bookstein, P. Scully, and W. H. Lee. 1988. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science 241:218-221. [DOI] [PubMed] [Google Scholar]
- 41.Lee, T. H., H. C. Chang, L. Y. Chuang, and W. C. Hung. 2003. Involvement of PKA and Sp1 in the induction of p27Kip1 by tamoxifen. Biochem. Pharmacol. 66:371-377. [DOI] [PubMed] [Google Scholar]
- 42.Lin, V. C., A. S. Eng, N. E. Hen, E. H. Ng, and S. H. Chowdhury. 2001. Effect of progesterone on the invasive properties and tumor growth of progesterone receptor-transfected breast cancer cells MDA-MB-231. Clin. Cancer Res. 7:2880-2886. [PubMed] [Google Scholar]
- 43.Lin, V. C., R. Jin, P. H. Tan, S. E. Aw, C. T. Woon, and B. H. Bay. 2003. Progesterone induces cellular differentiation in MDA-MB-231 breast cancer cells transfected with progesterone receptor complementary DNA. Am. J. Pathol. 162:1781-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, Z., J. Wong, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2001. Sequential recruitment of steroid receptor coactivator-1 (SRC-1) and p300 enhances progesterone receptor-dependent initiation and reinitiation of transcription from chromatin. Proc. Natl. Acad. Sci. USA 98:12426-12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lydon, J. P., F. J. DeMayo, C. R. Funk, S. K. Mani, A. R. Hughes, C. A. Montgomery, Jr., G. Shyamala, O. M. Conneely, and B. W. O'Malley. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9:2266-2278. [DOI] [PubMed] [Google Scholar]
- 46.Michna, H., Y. Nishino, G. Neef, W. L. McGuire, and M. R. Schneider. 1992. Progesterone antagonists: tumor-inhibiting potential and mechanism of action. J. Steroid Biochem. Mol. Biol. 41:339-348. [DOI] [PubMed] [Google Scholar]
- 47.Mommers, E. C., A. M. Leonhart, F. Falix, R. Michalides, C. J. Meijer, J. P. Baak, and P. J. Diest. 2001. Similarity in expression of cell cycle proteins between in situ and invasive ductal breast lesions of same differentiation grade. J. Pathol. 194:327-333. [DOI] [PubMed] [Google Scholar]
- 48.Mueller, E., P. Sarraf, P. Tontonoz, R. M. Evans, K. J. Martin, M. Zhang, C. Fletcher, S. Singer, and B. M. Spiegelman. 1998. Terminal differentiation of human breast cancer through PPAR gamma. Mol. Cell 1:465-470. [DOI] [PubMed] [Google Scholar]
- 49.Musgrove, E. A., and R. L. Sutherland. 1994. Cell cycle control by steroid hormones. Semin. Cancer Biol. 5:381-389. [PubMed] [Google Scholar]
- 50.Musgrove, E. A., A. Swarbrick, C. S. Lee, A. L. Cornish, and R. L. Sutherland. 1998. Mechanisms of cyclin-dependent kinase inactivation by progestins. Mol. Cell. Biol. 18:1812-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neville, M. C., T. B. McFadden, and I. Forsyth. 2002. Hormonal regulation of mammary differentiation and milk secretion. J. Mammary Gland Biol. Neoplasia 7:49-66. [DOI] [PubMed] [Google Scholar]
- 52.Owen, G. I., J. K. Richer, L. Tung, G. Takimoto, and K. B. Horwitz. 1998. Progesterone regulates transcription of the p21WAF1 cyclin- dependent kinase inhibitor gene through Sp1 and CBP/p300. J. Biol. Chem. 273:10696-10701. [DOI] [PubMed] [Google Scholar]
- 53.Pagliuca, A., P. Gallo, and L. Lania. 2000. Differential role for Sp1/Sp3 transcription factors in the regulation of the promoter activity of multiple cyclin-dependent kinase inhibitor genes. J. Cell Biochem. 76:360-367. [DOI] [PubMed] [Google Scholar]
- 54.Porter, P. L., K. E. Malone, P. J. Heagerty, G. M. Alexander, L. A. Gatti, E. J. Firpo, J. R. Daling, and J. M. Roberts. 1997. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat. Med. 3:222-225. [DOI] [PubMed] [Google Scholar]
- 55.Reed, W., V. A. Florems, R. Holm, E. Hannisdal, and J. M. Nesland. 1999. Elevated levels of p27, p21 and cyclin D1 correlate with positive oestrogen and progesterone receptor status in node-negative breast carcinoma patients. Virchows Arch. 435:116-124. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez-Boulan, E., and W. J. Nelson. 1989. Morphogenesis of the polarized epithelial cell phenotype. Science 245:718-725. [DOI] [PubMed] [Google Scholar]
- 57.Rossouw, J. E., G. L. Anderson, R. L. Prentice, A. Z. LaCroix, C. Kooperberg, M. L. Stefanick, R. D. Jackson, S. A. Beresford, B. V. Howard, K. C. Johnson, J. M. Kotchen, and J. Ockene. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288:321-333. [DOI] [PubMed] [Google Scholar]
- 58.Said, T. K., R. C. Moraes, U. Singh, F. S. Kittrell, and D. Medina. 2001. Cyclin-dependent kinase (cdk) inhibitors/cdk4/cdk2 complexes in early stages of mouse mammary preneoplasia. Cell Growth Differ. 12:285-295. [PubMed] [Google Scholar]
- 59.Schuler, G. D., M. S. Boguski, E. A. Stewart, L. D. Stein, G. Gyapay, K. Rice, R. E. White, P. Rodriguez-Tome, A. Aggarwal, E. Bajorek, S. Bentolila, B. B. Birren, A. Butler, A. B. Castle, N. Chiannilkulchai, A. Chu, C. Clee, S. Cowles, P. J. Day, T. Dibling, N. Drouot, I. Dunham, S. Duprat, C. East, T. J. Hudson, et al. 1996. A gene map of the human genome. Science 274:540-546. [PubMed] [Google Scholar]
- 60.Shiozawa, T., H. C. Shih, T. Miyamoto, Y. Z. Feng, J. Uchikawa, K. Itoh, and I. Konishi. 2003. Cyclic changes in the expression of steroid receptor coactivators and corepressors in the normal human endometrium. J. Clin. Endocrinol. Metab. 88:871-878. [DOI] [PubMed] [Google Scholar]
- 61.Sitruk-Ware, R., and G. Plu-Bureau. 1999. Progestins and cancer. Gynecol. Endocrinol. 13(Suppl. 4):3-9. [DOI] [PubMed] [Google Scholar]
- 62.Sivaraman, L., and D. Medina. 2002. Hormone-induced protection against breast cancer. J. Mammary Gland Biol. Neoplasia 7:77-92. [DOI] [PubMed] [Google Scholar]
- 63.Slater, E. P., A. C. Cato, M. Karin, J. D. Baxter, and M. Beato. 1988. Progesterone induction of metallothionein-IIA gene expression. Mol. Endocrinol. 2:485-491. [DOI] [PubMed] [Google Scholar]
- 64.Smith, C. L., and B. W. O'Malley. 2004. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 25:45-71. [DOI] [PubMed] [Google Scholar]
- 65.Smith, C. L., S. A. Onate, M. J. Tsai, and B. W. O'Malley. 1996. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc. Natl. Acad. Sci. USA 93:8884-8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Somasundaram, K., H. Zhang, Y. X. Zeng, Y. Houvras, Y. Peng, G. S. Wu, J. D. Licht, B. L. Weber, and W. S. El-Deiry. 1997. Arrest of the cell cycle by the tumour-suppressor BRCA1 requires the CDK-inhibitor p21WAF1/CiP1. Nature 389:187-190. [DOI] [PubMed] [Google Scholar]
- 67.Sommers, C. L., S. W. Byers, E. W. Thompson, J. A. Torri, and E. P. Gelmann. 1994. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res. Treat. 31:325-335. [DOI] [PubMed] [Google Scholar]
- 68.Stahlberg, C., A. T. Pederson, E. Lynge, and B. Ottesen. 2003. Hormone replacement therapy and risk of breast cancer: the role of progestins. Acta Obstet. Gynecol. Scand. 82:335-344. [PubMed] [Google Scholar]
- 69.Swarbrick, A., C. S. Lee, R. L. Sutherland, and E. A. Musgrove. 2000. Cooperation of p27Kip1 and p18INKc in progestin-mediated cell cycle arrest in T-47D breast cancer cells. Mol. Cell. Biol. 20:2581-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.T'Ang, A., J. M. Varley, S. Chakraborty, A. L. Murphree, and Y. K. Fung. 1988. Structural rearrangement of the retinoblastoma gene in human breast carcinoma. Science 242:263-266. [DOI] [PubMed] [Google Scholar]
- 71.Weinberg, R. A. 1995. The retinoblastoma protein and cell cycle control. Cell 81:323-330. [DOI] [PubMed] [Google Scholar]
- 72.Zheng, L., L. A. Annab, C. A. Afshari, W. H. Lee, and T. G. Boyer. 2001. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc. Natl. Acad. Sci. USA 98:9587-9592. [DOI] [PMC free article] [PubMed] [Google Scholar]