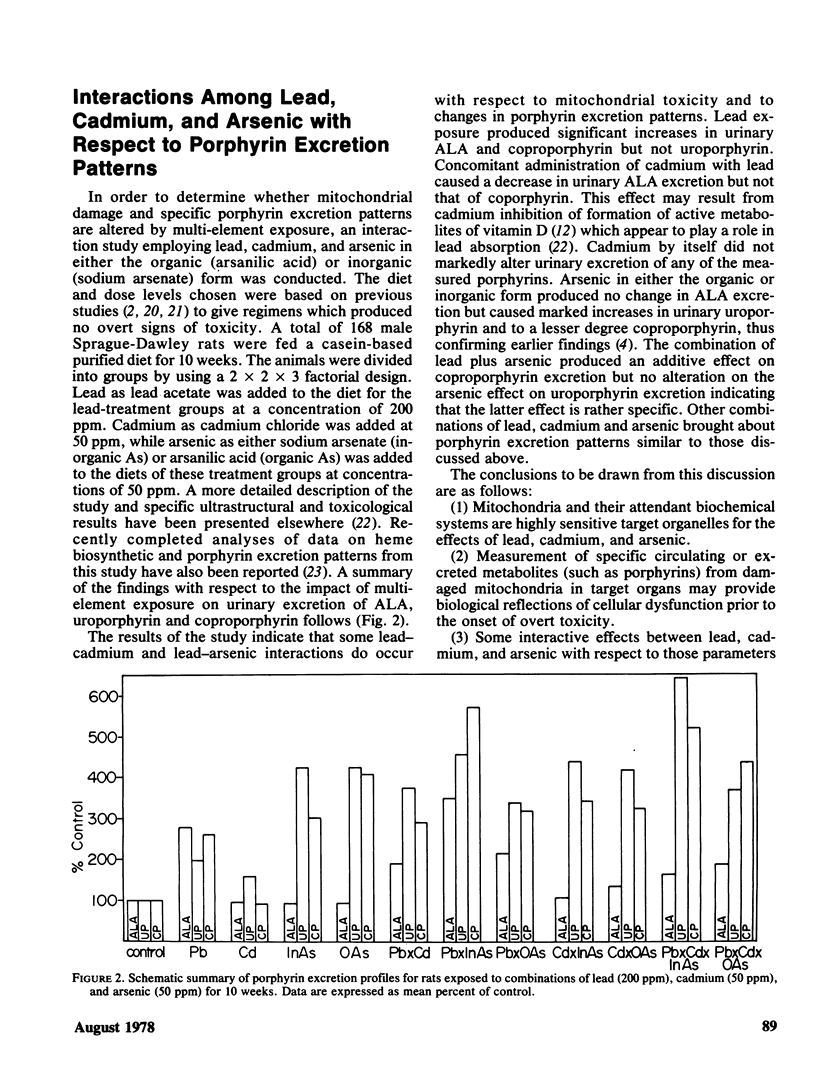
Abstract
This paper reviews the effects of lead (Pb), cadmium (Cd), and arsenic (As) on the mitrochondrion with emphasis on alteration of mitochondrial heme biosynthetic pathway. The information was used to examine results of a Pb x Cd x As interaction study which employed urinary porphyrin excretion patterns as one assessment criterion. Data from the study showed that dietary Pb produced increased urinary excretion of aminolevulinic acid (ALA) and coproporphyrin. Dietary exposure to organic or inorganic As caused increased excretion of uroporphyrin and to a lesser extent coproporphyrin, while dietary Cd caused no significant changes in urinary levels of any of the porphyrins measured. The combination of Pb plus As produced an additive effect on coproporphyrin excretion but not that of either ALA or uroporphyrin. These data are discussed in relation to utilization of urinary porphyrins for assessing toxicity and elemental interactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZZONE G. F., ERNSTER L. Compartmentation of mitochondrial phosphorylations as disclosed by studies with arsenate. J Biol Chem. 1961 May;236:1510–1517. [PubMed] [Google Scholar]
- BRADLEY L. B., JACOB M., JACOBS E. E., SANADI D. R. Uncoupling of oxidative phosphorylation by cadmium ion. J Biol Chem. 1956 Nov;223(1):147–156. [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- ESTABROOK R. W. Effect of oligomycin on the arsenate and DNP stimulation of mitochondrial oxidations. Biochem Biophys Res Commun. 1961 Feb 24;4:89–91. doi: 10.1016/0006-291x(61)90352-7. [DOI] [PubMed] [Google Scholar]
- Fowler B. A. General subcellular effects of lead, mercury, cadmium, and arsenic. Environ Health Perspect. 1978 Feb;22:37–41. doi: 10.1289/ehp.782237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler B. A., Jones H. S., Brown H. W., Haseman J. K. The morphological effects of chronic cadmium administration on the renal vasculature of rats given low and normal calcium diets. Toxicol Appl Pharmacol. 1975 Nov;34(2):233–252. doi: 10.1016/0041-008x(75)90028-9. [DOI] [PubMed] [Google Scholar]
- Fowler B. A., Woods J. S., Schiller C. M. Ultrastructural and biochemical effects of prolonged oral arsenic exposure on liver mitochondria of rats. Environ Health Perspect. 1977 Aug;19:197–204. doi: 10.1289/ehp.7719197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler B. A., Woods J. S. The transplacental toxicity of methyl mercury to fetal rat liver mitochondria. Morphometric and biochemical studies. Lab Invest. 1977 Feb;36(2):122–130. [PubMed] [Google Scholar]
- Goyer R. A., Rhyne B. C. Pathological effects of lead. Int Rev Exp Pathol. 1973;12:1–77. [PubMed] [Google Scholar]
- Lauwerys R. R., Buchet J. P., Roels H. A. Comparative study of effect of inorganic lead and cadmium on blood delta-aminolevulinate dehydratase in man. Br J Ind Med. 1973 Oct;30(4):359–364. doi: 10.1136/oem.30.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegren C. C., Lindegren G. Mitochondrial modification and respiratory deficiency in the yeast cell caused by cadmium poisoning. Mutat Res. 1973 Dec;21(6):315–322. [PubMed] [Google Scholar]
- Mahaffey K. R., Fowler B. A. Effects of concurrent administration of lead, cadmium, and arsenic in the rat. Environ Health Perspect. 1977 Aug;19:165–171. doi: 10.1289/ehp.7719165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyne B. C., Goyer R. A. Cytochrome content of kidney mitochondria in experimental lead poisoning. Exp Mol Pathol. 1971 Jun;14(3):386–391. doi: 10.1016/0014-4800(71)90009-8. [DOI] [PubMed] [Google Scholar]
- Schiller C. M., Fowler B. A., Woods J. S. Effects of arsenic on pyruvate dehydrogenase activation. Environ Health Perspect. 1977 Aug;19:205–207. doi: 10.1289/ehp.7719205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six K. M., Goyer R. A. Experimental enhancement of lead toxicity by low dietary calcium. J Lab Clin Med. 1970 Dec;76(6):933–942. [PubMed] [Google Scholar]
- WADKINS C. L. Stimulation of adenosine triphosphatase activity of mitochondria and submitochondrial particles by arsenate. J Biol Chem. 1960 Nov;235:3300–3303. [PubMed] [Google Scholar]
- Woods J. S., Fowler B. A. Effects of chronic arsenic exposure on hematopoietic function in adult mammalian liver. Environ Health Perspect. 1977 Aug;19:209–213. doi: 10.1289/ehp.7719209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. S., Fowler B. A. Renal porphyrinuria during chronic methyl mercury exposure. J Lab Clin Med. 1977 Aug;90(2):266–272. [PubMed] [Google Scholar]


