Abstract
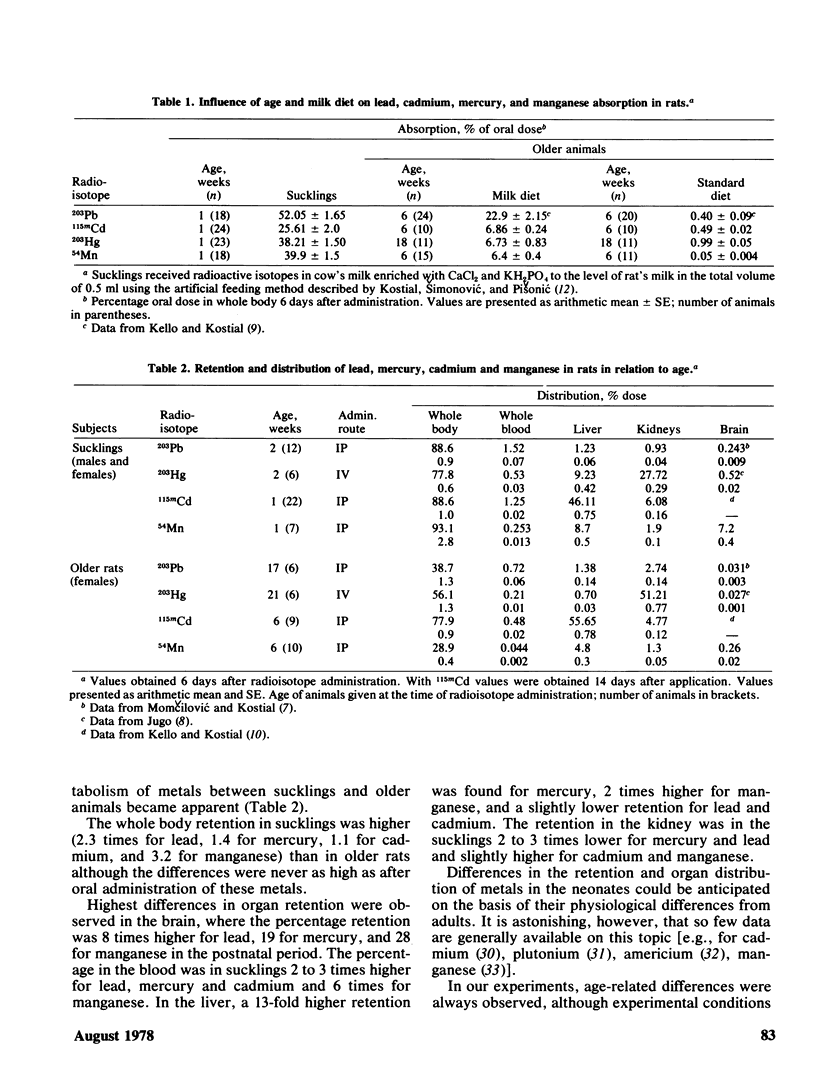
The metabolism and toxicity of lead, cadmium, mercury, and manganese in the postnatal period was studied in rats. Absorption, whole body retention, and organ distribution of 203Pb, 115mCd, 203Hg, and 54Mn were determined after oral and parenteral administration of these radioisotopes. The acute oral toxicity (LD50) was determined after a single application of metal chlorides. The results obtained in sucklings show a very high intestinal absorption of all metals which is partly attributed to milk diet; a higher whole body retention, higher blood levels and a much higher accumulation in the brain; and a higher oral toxicity. These results indicate age specific differences in the pharmacokinetics of metals in sucklings. It seems reasonable to consider the early neonatal age as a critical period for metal accumulation and therefore for metal toxicity. The results are interpreted on the basis of current concepts of developmental physiology and pharmacology and suggestions for future research trends are made.
Full text
PDF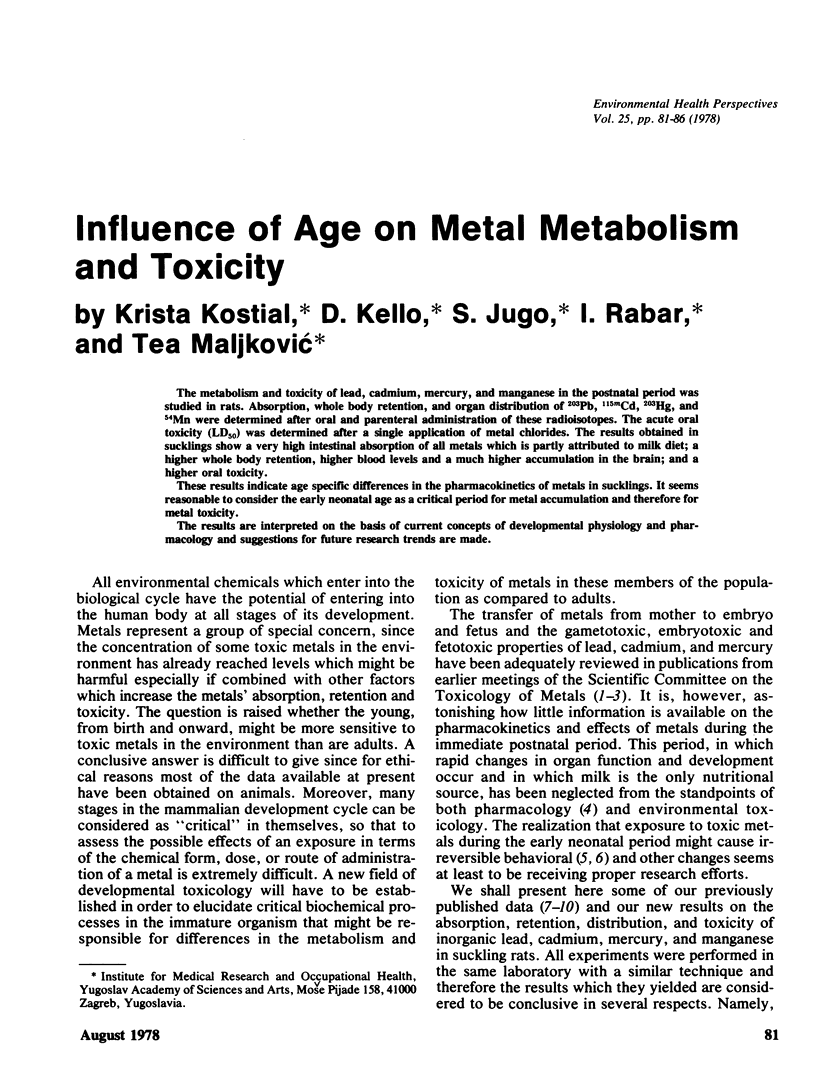




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander F. W., Clayton B. E., Delves H. T. Mineral and trace-metal balances in children receiving normal and synthetic diets. Q J Med. 1974 Jan;43(169):89–111. [PubMed] [Google Scholar]
- BELCHER E. H., HARRISS E. B. Studies of plasma volume, red cell volume and total blood volume in young growing rats. J Physiol. 1957 Nov 14;139(1):64–78. doi: 10.1113/jphysiol.1957.sp005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. C., Jr, Miller W. J., Neathery M. W., Gentry R. P., Stake P. E., Blackmon D. M. Manganese metabolism with oral and intravenous 54Mn young calves as influenced by supplemental manganese. J Anim Sci. 1974 Jun;38(6):1284–1290. doi: 10.2527/jas1974.3861284x. [DOI] [PubMed] [Google Scholar]
- Chisolm J. J., Jr The susceptibility of the fetus and child to chemical pollutants. Heavy metal exposures: toxicity from metal-metal interactions, and behavioral effects. Pediatrics. 1974 May;53(5):841–843. [PubMed] [Google Scholar]
- DOBBING J. The blood-brain barrier. Physiol Rev. 1961 Jan;41:130–188. doi: 10.1152/physrev.1961.41.1.130. [DOI] [PubMed] [Google Scholar]
- Durakovic A. B., Hollins J. G., Storr M. C. The influence of age and sex on the metabolism of americium by rats. Health Phys. 1973 May;24(5):541–546. doi: 10.1097/00004032-197305000-00009. [DOI] [PubMed] [Google Scholar]
- Finberg L. The susceptibility of the fetus and child to chemical pollutants. Interaction of the chemical environment with the infant and young child. Pediatrics. 1974 May;53(5):831–837. [PubMed] [Google Scholar]
- Forbes G. B., Reina J. C. Effect of age on gastrointestinal absorption (Fe, Sr, Pb) in the rat. J Nutr. 1972 May;102(5):647–652. doi: 10.1093/jn/102.5.647. [DOI] [PubMed] [Google Scholar]
- GUNN S. A., GOULD T. C. Selective accumulation of Cd115 by cortex of rat kidney. Proc Soc Exp Biol Med. 1957 Dec;96(3):820–823. doi: 10.3181/00379727-96-23619. [DOI] [PubMed] [Google Scholar]
- Goldenthal E. I. A compilation of LD50 values in newborn and adult animals. Toxicol Appl Pharmacol. 1971 Jan;18(1):185–207. doi: 10.1016/0041-008x(71)90328-0. [DOI] [PubMed] [Google Scholar]
- Hamilton D. L., Valberg L. S. Relationship between cadmium and iron absorption. Am J Physiol. 1974 Nov;227(5):1033–1037. doi: 10.1152/ajplegacy.1974.227.5.1033. [DOI] [PubMed] [Google Scholar]
- Inaba J., Lengemann F. W. Intestinal uptake and whole-body retention of 141 Ce by suckling rats. Health Phys. 1972 Feb;22(2):169–175. doi: 10.1097/00004032-197202000-00008. [DOI] [PubMed] [Google Scholar]
- Jugo S., Maljković T., Kostial K. The effect of chelating agents on lead excretion in rats in relation to age. Environ Res. 1975 Oct;10(2):271–279. doi: 10.1016/0013-9351(75)90089-4. [DOI] [PubMed] [Google Scholar]
- Jugo S. Metabolism of toxic heavy metals in growing organisms: a review. Environ Res. 1977 Feb;13(1):36–46. doi: 10.1016/0013-9351(77)90002-0. [DOI] [PubMed] [Google Scholar]
- Jugo S. Retention and distribution of 203HgCl2 in suckling and adult rats. Health Phys. 1976 Feb;30(2):240–241. [PubMed] [Google Scholar]
- Kello D., Kostial K. The effect of milk diet on lead metabolism in rats. Environ Res. 1973 Sep;6(3):355–360. doi: 10.1016/0013-9351(73)90048-0. [DOI] [PubMed] [Google Scholar]
- Kostial K., Maljković T., Jugo S. Lead acetate toxicity in rats in relation to age and sex. Arch Toxikol. 1974 Feb 28;31(3):265–269. doi: 10.1007/BF00311058. [DOI] [PubMed] [Google Scholar]
- Kostial K., Simonović I., Pisonić M. Lead absorption from the intestine in newborn rats. Nature. 1971 Oct 22;233(5321):564–564. doi: 10.1038/233564a0. [DOI] [PubMed] [Google Scholar]
- Kostial K., Simonović I., Pisonić M. Reduction of lead absorption from the intestine in newborn rats. Environ Res. 1971 Oct;4(4):360–363. doi: 10.1016/0013-9351(71)90035-1. [DOI] [PubMed] [Google Scholar]
- Lecce J. G. Selective absorption of macromolecules into intestinal epithelium and blood by neonatal mice. J Nutr. 1972 Jan;102(1):69–75. doi: 10.1093/jn/102.1.69. [DOI] [PubMed] [Google Scholar]
- Lin F. M., Malaiyandi M., Sierra C. R. Toxicity of methylmercury: effects on different ages of rats. Bull Environ Contam Toxicol. 1975 Aug;14(2):140–148. doi: 10.1007/BF01701304. [DOI] [PubMed] [Google Scholar]
- Lowe C. U. The susceptibility of the fetus and child to chemical pollutants. Pediatric aspects: from the embryo through adolescence: special susceptibility and exposure. Pediatrics. 1974 May;53(5):779–782. [PubMed] [Google Scholar]
- Mahlum D. D., Sikov M. R. Distribution and toxicity of monomeric and polymeric 239Pu in immature and adult rats. Radiat Res. 1974 Oct;60(1):75–88. [PubMed] [Google Scholar]
- Martinsson K., Jönsson L. On the mechanism of intestinal absorption of macromolecules in piglets studied with dextran blue. Zentralbl Veterinarmed A. 1975 May;22(4):276–282. doi: 10.1111/j.1439-0442.1975.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Miller S. T., Cotzias G. C., Evert H. A. Control of tissue manganese: initial absence and sudden emergence of excretion in the neonatal mouse. Am J Physiol. 1975 Oct;229(4):1080–1084. doi: 10.1152/ajplegacy.1975.229.4.1080. [DOI] [PubMed] [Google Scholar]
- Momcilović B., Kostial K. Kinetics of lead retention and distribution in suckling and adult rats. Environ Res. 1974 Oct;8(2):214–220. doi: 10.1016/0013-9351(74)90053-x. [DOI] [PubMed] [Google Scholar]
- Moore W., Jr, hysell D., Crocker W., Stara J. Biological fate of -103Pd in rats following different routes of exposure. Environ Res. 1974 Oct;8(2):234–240. doi: 10.1016/0013-9351(74)90055-3. [DOI] [PubMed] [Google Scholar]
- Roth G. S., Adelman R. C. Age related changes in hormone binding by target cells and tissues; possible role in altered adaptive responsiveness. Exp Gerontol. 1975 Feb;10(1):1–11. doi: 10.1016/0531-5565(75)90009-1. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y., Ichikawa R. Absorption and retention of 144 Ce and 95 Zr- 95 Nb in newborn, juvenile and adult rats. Health Phys. 1972 Apr;22(4):373–378. doi: 10.1097/00004032-197204000-00009. [DOI] [PubMed] [Google Scholar]
- Sikov M. R., Mahlum D. D. Plutonium in the developing animal. Health Phys. 1972 Jun;22(6):707–712. doi: 10.1097/00004032-197206000-00027. [DOI] [PubMed] [Google Scholar]
- Six K. M., Goyer R. A. The influence of iron deficiency on tissue content and toxicity of ingested lead in the rat. J Lab Clin Med. 1972 Jan;79(1):128–136. [PubMed] [Google Scholar]
- TAYLOR D. M., BLIGH P. H., DUGGAN M. H. The absorption of calcium, strontium, barium and radium from the gastrointestinal tract of the rat. Biochem J. 1962 Apr;83:25–29. doi: 10.1042/bj0830025. [DOI] [PMC free article] [PubMed] [Google Scholar]


