Abstract
Virulence of Plasmodium falciparum is associated with the expression of variant surface antigens designated PfEMP1 (P. falciparum erythrocyte membrane protein 1) that are encoded by a family of var genes. Data presented show that the transmission stages of P. falciparum also express PfEMP1 variants. Virulence in this host–parasite system can be considered a variable outcome of optimizing the production of sexual transmission stages from the population of disease-inducing asexual stages. Immunity to PfEMP1 will contribute to the regulation of this trade-off by controlling the parasite population with potential to produce mature transmission stages.
Conventional wisdom holds that successful parasites will evolve to become less virulent to their hosts, and that only maladapted, novel parasites are harmful. Garnham (1), for example, stated that Plasmodium falciparum was the most virulent of the human malarias because of its recent phylogenetic origin.
Evolutionary ecologists have an alternative view of the evolution of virulence (2–7). Both theoretical and recent empirical data demonstrate that pathogenicity can be maintained when it is a direct or indirect consequence of the parasite’s exploitation of the host during production of transmission stages. Parasite reproduction is balanced against host survival. In an attempt to better understand the processes that influence the evolution of virulence of P. falciparum in the human host, we have explored the molecular relationship between virulence and transmissibility in this host–parasite system.
During its life cycle P. falciparum develops and multiplies asexually within host erythrocytes (RBCs). RBCs infected with mature parasites, called trophozoites, sequester from the peripheral circulation by adhesion to capillary endothelium in a variety of tissues (8–10). Under conditions of blood flow, this adhesion is facilitated by the expression of knob-like protrusions under the infected RBC membrane (11).
Virulence of P. falciparum, measured by the clinical outcome of infection, has been associated with parasite density, which, in turn, is influenced by this ability to adhere to endothelium (12). A number of host receptors involved in the parasite adhesion process have been identified (reviewed in ref. 13). These include the leukocyte differentiation antigen CD36, thrombospondin, intercellular adhesion molecule 1, and chondroitin sulfate A. Trophozoite-infected RBCs from the majority of wild-type parasites tested so far can bind to CD36, whereas adhesion to other receptors occurs less frequently (14–16). The parasite ligand for the host receptors CD36 and intercellular adhesion molecule 1 is a high Mr protein known as P. falciparum erythrocyte membrane protein 1 (PfEMP1) (17, 18). This protein is expressed on the surface of trophozoite-infected RBCs (19) and is highly immunogenic (20). PfEMP1 also undergoes clonal antigenic variation, with variant forms differing both antigenically and in adhesion characteristics (21, 22). As in other host–parasite systems, the ability to adhere to different host endothelial receptors may also determine the virulence of a P. falciparum parasite (16, 23, 24), as the life-threatening condition known as cerebral malaria is associated with expression of intercellular adhesion molecule 1 and the accumulation of large numbers of parasites in the brain capillaries (10, 25–27).
A multigene family called var encodes PfEMP1 proteins (28–30). Var genes have two exons: exon II is predicted to code for a conserved cytoplasmic domain, and exon I encodes a large, highly variable, extracellular region of the protein consisting of between two and four domains known as Duffy-binding-like (DBL) domains because of their similarity to proteins involved in the binding of Plasmodium knowlesi and Plasmodium vivax malarial parasites to host Duffy blood group antigen before invasion of an RBC. Also, encoded by exon I is a relatively conserved cysteine-rich region that has recently been identified as a CD36-binding domain (31).
Transmission of malaria from the human host to the anopheline vector requires the production of transmission stages (gametocytes). During the asexual proliferation of P. falciparum, some parasites commit to sexual development and become gametocytes. Developing gametocyte-infected RBCs sequester from the peripheral circulation to the blood spaces of the bone marrow and the spleen (32–34). In these organs, they pass through a series of morphologically distinct forms, categorized I–IV (35). This takes 8–10 days, after which “mature” gametocytes are released into the circulation and become infectious to the mosquito vector after a further 2–3 days.
We have found that early gametocyte-infected RBCs (stages I–IIA), but not later stages of development (stages IIB–V), can adhere to CD36 expressed on C32MC and that these early gametocytes express knobs (36). We report here that the parasite ligand for this binding is PfEMP1.
MATERIALS AND METHODS
P. falciparum Culture and Purification of mRNA.
Papua, New Guinean isolate 1776 was maintained in continuous culture and gametocytogenesis was induced as reported (5). Trophozoites were enriched by Plasmagel flotation (37) and yielded 107 infected RBCs. Gametocyte cultures were processed as before to remove asexual parasites (36) and examined for purity as described below. In brief, gametocytes were treated with 5% sorbitol on 3 consecutive days to kill trophozoites (38). Early gametocytes (2 × 106 infected RBCs) were then purified from any remaining ring stage parasites by adhesion to C32MC, thoroughly washed, and released from the target cells by the addition of 25 mM NaHCO3. Late gametocytes (2 × 106 infected RBCs) were enriched from the same sorbitol-purified cultures by a seven-step discontinuous Percoll gradient (Pharmacia; as described in ref. 36). To ascertain that gametocytes were free from asexual parasites, samples of the infected RBCs were smeared onto glass slides and indirect immunofluorescence was performed, with an mAb designated 2G7, which is specific for gametocyte protein Pfg16 (39), as described below. Of 10,000 infected RBCs (counted randomly in 10 areas of the slide) 100% were positive for reactivity with the mAb.
Reverse Transcription (RT)–PCR.
All parasite stages were lysed in 0.1% saponin in PBS and mRNA was isolated directly by using Dynabeads oligo(dT)25 according to the manufacturer’s instructions. The mRNA was eluted from the beads and treated with DNase I (Boehringer Mannheim). Superscriptase II (GIBCO/BRL) was used to synthesize cDNA with the primer X1R2 [5′-TCTTCIGCCCATTC(G/C)TCGGACCA], specific for the 3′ end of the DBL1-encoding region of the var genes. Reactions without the addition of Superscriptase II were carried out in parallel as a control for DNA contamination. The resulting cDNA was used in a PCR reaction with the primers X1R2 and X1F2 [5′-GCACG(A/C)AGTTTTGC(A/G)GA(C/T)AT(A/T)GG], specific for a region approximately 450 bp 5′ to X1R2. The reaction contained 50 mM KCl, 10 mM Tris⋅HCl, 1% Triton X-100, 2 mM MgCl2, 0.15 mM dNTPs, 0.05 unit/liter Taq DNA polymerase (Promega), and 2 ng/μl each of oligonucleotide primers. After an initial 1.5 min at 94°C, there were 38 cycles of: 58°C for 50 s, 68°C for 70 s, and 94°C for 25 s. This was followed by a final 5 min at 58°C and 10 min at 68°C. The resulting product was stained with ethidium bromide and run on a 0.8% agarose gel in 0.5× buffer (1× is 90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3).
Adhesion and Immunofluorescence Assays.
Adhesion assays were carried out as described (40). Various dilutions of recombinant PfEMP1–glutathione S-transferase fusion protein rC1–2 (20–200 μg/ml), which binds CD36 (31), were used for inhibition curves of early gametocyte and trophozoite adhesion. Gametocyte adhesion was verified by indirect immunofluorescence assay (IFA) of dishes (36) with gametocyte-specific mAb 2G7 (1:100; ref. 39). IFA of gametocyte-infected blood films for PfEMP1 was based on a previous protocol (40) modified to include methanol fixation of air-dried films for 45 min at −20°C followed by incubation of the slides with human tonicity PBS (HTPBS)/10% normal human serum for 45 min. Staining was then carried out with a rat antiserum G1 (17), which was shown to react specifically with PfEMP1 by Western blot and immunoprecipitation (D.I.B., unpublished observation). A fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG antibody (1:50; Sigma) was used as a secondary antibody, and the slides were viewed on an Olympus BX50 microscope. Direct fluorescence assays were adapted from original methods (42). Trophozoites were stained as reported. Early and late gametocytes were enriched for by Percoll gradient (36) and incubated sequentially with human hyperimmune serum (HIS; 1:10), a biotin-conjugated sheep anti-human IgG antibody (1:35) and then FITC-conjugated streptavidin (1:100) with washing in HTPBS in between. The gametocytes were fixed overnight in HTPBS/2% paraformaldehyde at 4°C and permeabilized in HTPBS/0.01% Triton X-100 for 15 min. Cells were then incubated with mAb 2G7 (1:250) and phycoerthyrin-conjugated goat anti-mouse IgG (1:40) for 30 min. Samples were analyzed with a Becton Dickinson flow cytometer.
Coagglutination Assays.
Agglutination assays were adapted from previous methods (43–45). Trophozoites and early gametocytes were enriched as usual, and the parasitemias were equalized at 10%. Trophozoites were incubated with 50 μg/ml ethidium bromide and early gametocytes (sorbitol treated) were incubated with 1 mg/ml 4,6-diamidino-2-phenylindole for 10 min before thorough washing. Trophozoite (25 μl) and early gametocyte suspensions were mixed together and incubated with HIS (1:10), and the assay was carried out as usual.
Dot Blots.
Genomic DNA (25 ng) was isolated from the parasite isolate 1776 as described (46) and was used in a PCR reaction with the primers X1F2 and X1R2 for 27 cycles by using the conditions described for RT-PCR. The amplification products were cloned into the plasmid, pCR2.1 (Invitrogen), and sequenced with the Sequenase II kit (United States Biochemical). Thirty-seven segments of DBL1-encoding regions of var genes, as identified by sequence comparison (FASTA), were identified. Each plasmid containing a unique fragment (3 μg) was spotted onto a nylon membrane (Hybond-N, Amersham) as described (47). The blots were prehybridized in 50% formamide, 6× standard saline citrate (SSC; 1× = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7), 5× Denhardt’s solution (1× = 0.02% polyvinylpyrrolidone/0.02% Ficoll/0.02% BSA), and 0.5% SDS with 10 mg/ml boiled salmon sperm (GIBCO/BRL) at 57°C for 40 min. RT-PCR products were labeled with ([32P]dATP, from ICN) by random priming as described (47) and hybridized with the dot blots in 50% formamide, 6× SSC, and 0.5% SDS with 10 mg/ml boiled salmon sperm (GIBCO/BRL) at 57°C overnight. Filters were washed in 2× SSC and 0.1% SDS at room temperature for 20 min, 1× SSC, and 0.1% SDS at 45°C for 20 min, and 0.1× SSC and 0.1% SDS at 70°C for 25 min. The membrane was then exposed to autoradiographic film.
RESULTS
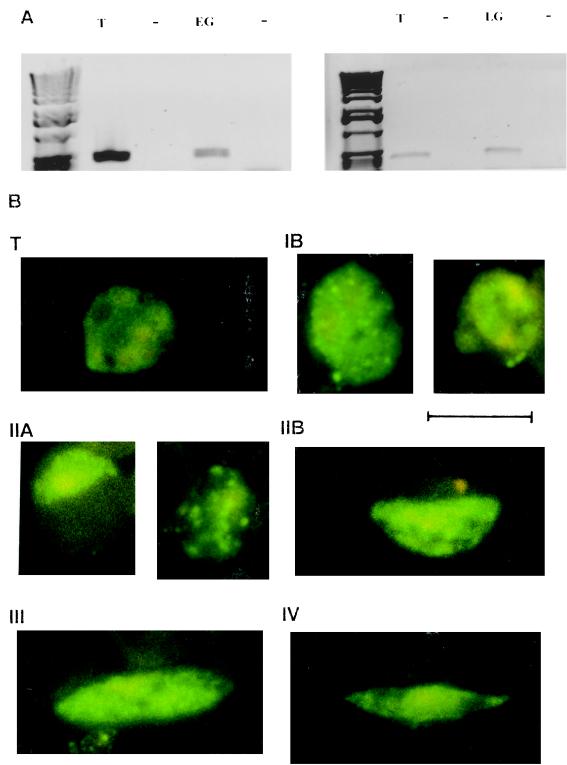
We first investigated whether var genes are expressed by gametocytes. mRNA was isolated from purified early gametocytes (stages I–IIA), late gametocytes (stages IIB–V), and trophozoites, which were cultured in parallel. These mRNA samples were reverse transcribed, and a product of approximately 450 bp from the DBL1-encoding region of the var genes was amplified by PCR. Transcript was detected by RT-PCR in both early and late gametocyte populations, as well as in trophozoites (Fig. 1A).
Figure 1.
Gametocytes express var genes and produce PfEMP1 protein. (A) RT-PCR of DBL1-encoding fragments from var genes run on agarose gels. Products were amplified with isolate 1776 mRNA isolated from trophozoites (T), early gametocytes (EG), and late gametocytes (LG) grown in parallel. Lanes designated − contain control reactions testing for genomic DNA contamination of the samples. (B) Sorbitol-purified gametocyte-infected RBCs of the isolate 1776 were permeabilized with methanol and reacted with a rat antiserum specific for the cytoplasmic region of the PfEMP1and then with FITC-conjugated goat anti-rat IgG. T, Mature trophozoite; IB, IIA, IIB, III, and IV, gametocyte stages. The antiserum was not reactive with uninfected RBCs. Infected RBCs were not detected by FITC-conjugated IgG alone. Gametocytes of cloned lines ITO4 and 3D7 and the isolate MUZ12 showed the same reactivity (data not shown). (Bar = 10 μm.)
We detected the PfEMP1 protein in gametocyte-infected RBCs with an antiserum against the conserved, cytoplasmic domain of the molecule (encoded by exon 2 of the var gene) by IFA (Fig. 1B). Trophozoites reacted with a pattern of fluorescence similar to that previously described (29). All stages of gametocyte-infected RBCs reacted with these antibodies, but there were stage-specific patterns of localization. Early gametocytes (stages I and IIA) showed expression of PfEMP1 in the parasite cytoplasm and also diffuse, or occasionally punctate, staining at the RBC membrane. In contrast, late gametocytes (stages IIB–V) reacted with the anti-PfEMP1 antiserum in a diffuse pattern, but there was no staining at the infected RBC surface, as observed for early gametocyte- and trophozoite-infected RBCs.
PfEMP1 mediates the binding of trophozoite-infected RBCs to CD36 (31) and appears (by the use of a anti-CD36 mAb) to play the same role for early gametocytes (36). Incubation of trophozoite-infected RBC adhesion assays with glutathione S-transferase–PfEMP1 fusion protein inhibits binding of the RBCs to CD36 expressed on C32MC (31). Similarly, adhesion of early gametocyte-infected RBCs to C32MC was abrogated by the addition of the fusion protein at comparable dilutions (data not shown). Total inhibition of gametocyte binding was achieved by incubation with 50 μg/ml. Glutathione S-transferase alone did not inhibit binding of early gametocyte-infected RBCs to C32MC. These data suggest a functional role for PfEMP1 in adhesion of early gametocyte-infected RBCs.
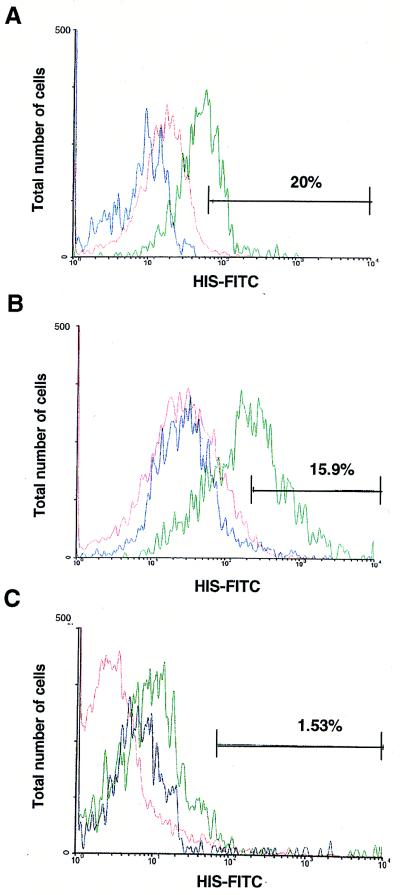
To further investigate whether the PfEMP1 molecule is exposed on the extracellular side of the gametocyte-infected RBC, we carried out direct immunofluorescence assays (Fig. 2). Variant specific antibodies reactive with the surface of trophozoite-infected RBCs are detectable in the serum of individuals after infection with P. falciparum (43). We have demonstrated that such antibodies are specific to PfEMP1 (42), and other data clearly show a direct correlation between antigenic phenotype on the trophozoite-infected RBC surface and expression of different var genes (28). We tested whether early gametocyte-infected RBCs were also recognized by such antibodies. Pooled HIS was screened for reactivity with a double-staining technique. The gametocyte-specific mAb 2G7 (raised against Pfg16; ref. 39) was used to differentiate between RBCs containing trophozoites and those containing gametocytes after surface labeling was complete. Analysis by flow cytometry confirmed that some, but not all, early gametocyte-infected RBCs at any point in continuous culture were recognized by HIS, suggesting expression of variant antigenic types. Later stages of gametocyte-infected cells were not recognized by HIS.
Figure 2.
Naturally acquired antibodies react with the surface of RBCs infected with early gametocytes but not late gametocytes. Samples of isolate 1776. Trophozoite-infected (A), early gametocyte-infected (stages I–IIA; B), and late gametocyte-infected (stages III–V; C) RBCs were analyzed by flow cytometry for reactivity with pooled HIS as detected by FITC-conjugated anti-human secondary antibodies. Trophozoites were then stained with ethidium bromide to distinguish infected from uninfected RBCs, and the samples were sorted so that reactivity with HIS was scored only in the population of cells positive for ethidium bromide staining. Samples containing early gametocytes were permeabilized and reacted with gametocyte-specific mAb 2G7 and phycoerythrin-conjugated goat anti-mouse IgG to distinguish gametocytes from asexual parasites. Similarly, the samples were sorted so that reactivity with HIS was scored only in the population of cells positive for phycoerythrin staining. Cells were scored for intensity of surface labeling with the HIS–FITC conjugate. The percentage of infected cells in each sample positive for reactivity with pooled serum (green) was calculated as described (44) to account for any nonspecific fluorescence from controls and is reported in each figure. Uninfected RBC-HIS–FITC (red) and infected RBC-normal human serum–FITC (blue) control reactions are shown for each sample.
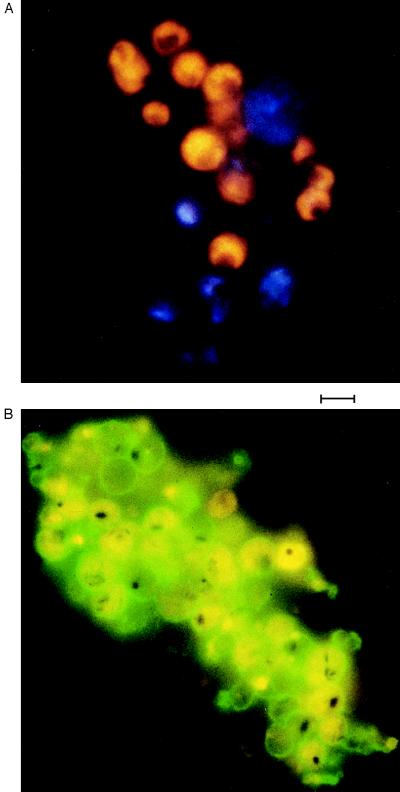
We compared the antigenic types of PfEMP1 that were expressed by sexual and asexual stage parasites derived from a given culture by coagglutination assay (45). In this experiment trophozoite and early gametocyte-infected RBCs were mixed, and variant-specific HIS was added. Coagglutination of the different parasite stages indicated that the same antigenic types were being expressed by both trophozoites and gametocytes. Mixed agglutinates, i.e., clumps containing both asexual and sexual stage parasite-infected cells, were observed (Fig. 3).
Figure 3.
Gametocytes and trophozoites of the isolate 1776 express the same var genes. (A) Sorbitol-purified early gametocytes (stained blue with 4,6-diamidino-2-phenylindole) and a separate culture of trophozoites (stained yellow with ethidium bromide) were both enriched to 10% parasitemias before combining and reacting with HIS in coagglutination assays. Agglutinates containing both parasite stages were also obtained with gametocytes and trophozoites from isolates MUZ12 and MUZ37 (data not shown). (Bar = 10 μm.) (B) Mixed agglutinates were observed in a direct fluorescence assay with cultures containing both parasite stages. Trophozoites and gametocytes could be distinguished by light microscopy; the distinctive granular pigment of the early gametocytes and the single spot of pigment of trophozoites were clearly visible. (Bar = 10 μm.)
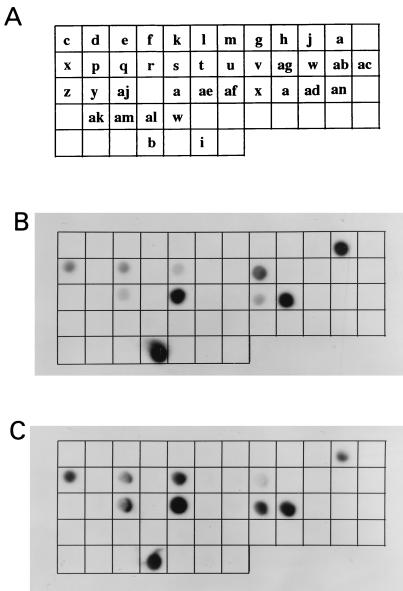
To discover whether the PfEMP1 molecules being expressed by trophozoites and gametocytes were identical, we compared the var genes being transcribed by these different stages. Dot blots were made. Each sample spotted on the membrane was a unique DBL1-encoding region from a different var gene present in the parasite genome. The RT-PCR products described earlier (Fig. 1A) were radiolabeled and hybridized with the dot blots. The dots that can be visualized by autoradiography are those that correspond in sequence to the var genes being transcribed in the parasite population. The patterns observed (Fig. 4) clearly indicate that the same var types are being transcribed by both asexual and sexual parasites. It is likely that a single gametocyte expresses the same PfEMP1 type as the parental trophozoite from which it originated; however, it is impossible to demonstrate this at the single-cell level because the parental trophozoite is destroyed during the production of progeny parasites.
Figure 4.
(A) A schematic diagram of dot blots containing 37 unique DBL1 fragments obtained by genomic PCR. Each letter name represents a unique sequence type in the genome of the parasite isolate 1776. Trophozoite (B) and gametocyte (C) amplification products of expressed var gene fragments obtained by RT-PCR were radiolabeled and hybridized with the dot blots. Dots that were positive contained sequence types being expressed in the parasite population.
DISCUSSION
Our data demonstrate stage-specific, functional expression of PfEMP1 in the gametocyte. Although all stages of gametocytes transcribe and translate var genes, PfEMP1 is translocated to the infected RBC membrane only in the early stages of development. Our data demonstrate the functional, surface expression of PfEMP1 on early gametocyte-infected RBCs because their binding to this host receptor is trypsin sensitive (36) and completely blocked by recombinant PfEMP1. Characteristic of PfEMP1 expression, these early gametocytes also express an antigenically variable molecule on the infected RBC surface, and although we cannot exclude the possibility of another variant molecule being expressed on the early gametocyte-infected RBC (48), the evidence is considerable for PfEMP1.
More mature transmission stages are not detected by a variant antigen-specific immune response. Late gametocytes (stages IIB–V), although they make PfEMP1 protein, do not express it at or near the infected RBC surface, a finding that correlates with their inability to bind to CD36 (36). An additional plasma membrane, which encircles the gametocyte at stage II of development (49), may account for this stage-specific change in the trafficking of proteins. Stage specificity of adhesion in vitro, at which only stage I–IIA gametocytes bind to CD36, cannot explain the in vivo observation that stages I–IV sequester outside of the main circulation. This suggests that stage IIB–IV gametocytes may adhere to another host receptor by using a molecule other than PfEMP1. This model of gametocyte adhesion differs from that proposed by Rogers et al. (50). These authors suggested that PfEMP1 mediates adhesion of stage I–IV gametocytes and that altered band 3 may act as a second adhesion ligand for these sexual stage parasites. Data from this paper and ref. 36 demonstrate that this is unlikely to be the case.
Exposure to the rapidly reproducing asexual parasite population stimulates PfEMP1 variant-specific antibodies, which results in a clearing of parasites expressing those antigenic types. As this variant antigen-specific immunity limits the numbers of parasites, so the potential to create gametocytes may be reduced. Our data suggest that, in addition, this immunity might also affect the maturation of early sexual stages by recognizing them during stages I and IIA of development and reducing transmission in a second way through immunity to PfEMP1.
Theoretical and empirical data (2, 3, 6) have demonstrated that, if the transmission success of a parasite is coupled to its virulence, then a level of virulence will be selected that optimizes transmissibility. Virulence in the case of P. falciparum is associated with the adhesion of infected RBCs to endothelial tissue (12, 23, 24), which is mediated by PfEMP1 (17, 18). Because the antibody response to this variant antigen has a direct effect on the survival and development of the parasite transmission stages as described above, we propose that PfEMP1 is a molecular link between virulence and transmission in this host–parasite system. As such, virulence of P. falciparum could be considered a variable outcome of balancing the parasite’s need to produce transmission stages with the host’s survival, as influenced by the density of disease-inducing asexual stages. Variant-specific immunity to PfEMP1 will contribute to regulation of this trade-off by controlling the densities of asexual stages with the potential to become transmission stages. If this immunity is too effective, it will prevent disease at the cost of transmission, and, therefore, this selection for maximal transmission will drive the evolution of PfEMP1 and regulate virulence.
Acknowledgments
We are grateful to Richard Carter (University of Edinburgh) for providing mAb 2G7 and to Karl Hoffmann (National Institute of Allergy and Infectious Diseases/National Institutes of Health) for control glutathione S-transferase protein. We thank Gloria Rudenko (The Netherlands Cancer Institute) for useful comments concerning the manuscript in preparation. R.E.H. was funded by a Medical Research Council studentship, B.T. was funded by a Wellcome Trust Prize Studentship, K.P.P. and K.P.D. were funded by a Wellcome Trust Program grant, and D.I.B. is supported by an National Institutes of Health grant.
ABBREVIATIONS
- RBC
erythrocyte
- PfEMP1
Plasmodium falciparum erythrocyte membrane protein 1
- C32MC
human amelanotic melanoma cell line C32
- DBL
Duffy-binding-like
- ITC
fluorescein isothiocyanate
- IFA
indirect immunofluorescence assay
- HTPBS
human tonicity PBS
- HIS
human hyperimmune serum
References
- 1.Garnham P C C. Malaria Parasites and Other Haemosporidia. Oxford: Blackwell; 1966. [Google Scholar]
- 2.Levin S, Pimental D. Am Nat. 1981;117:308–311. [Google Scholar]
- 3.Anderson R M, May R M. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 4.Herre E A. Science. 1993;259:1442–1445. doi: 10.1126/science.259.5100.1442. [DOI] [PubMed] [Google Scholar]
- 5.Day K P, Karamalis F, Thompson J, Barnes D A, Peterson C, Brown H, Brown G V, Kemp D J. Proc Natl Acad Sci USA. 1993;90:8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebert D. Science. 1994;265:1084–1086. doi: 10.1126/science.265.5175.1084. [DOI] [PubMed] [Google Scholar]
- 7.Ewald P W. J Parasitol. 1995;81:659–669. [PubMed] [Google Scholar]
- 8.Miller L H. Am J Trop Med Hyg. 1969;18:860–865. doi: 10.4269/ajtmh.1969.18.860. [DOI] [PubMed] [Google Scholar]
- 9.MacPherson G G, Warrell M J, White N J, Looareesuwan S, Warrell D A. Am J Pathol. 1985;119:384–401. [PMC free article] [PubMed] [Google Scholar]
- 10.Turner G D, Morrison H, Jones M, Davis M E, Looareesuwan S, Buley I D, Gatter K C, Newbold C I, Pukritayakamee S, Nagachinta B, et al. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 11.Crabb B S, Cooke B M, Reeder J C, Waller R F, Caruana S R, Davern K M, Wickham M E, Brown G V, Coppel R L, Cowman A F. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 12.Langreth S G, Peterson E. Infect Immun. 1985;47:760–766. doi: 10.1128/iai.47.3.760-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deitsch K W, Wellems T E. Mol Biochem Parasitol. 1996;76:1–10. doi: 10.1016/0166-6851(95)02575-8. [DOI] [PubMed] [Google Scholar]
- 14.Hasler T, Handunnetti S M, Aguiar J C, van Schravendijk M R, Greenwood B M, Lallinger G, Cegielski P, Howard R J. Blood. 1990;76:1845–1852. [PubMed] [Google Scholar]
- 15.Ockenhouse C F, Ho M, Tandon N N, Van Seventer G A, Shaw S, White N J, Jamieson G A, Chulay J D, Webster H K. J Infect Dis. 1991;164:163–169. doi: 10.1093/infdis/164.1.163. [DOI] [PubMed] [Google Scholar]
- 16.Newbold C I. Am J Trop Med Hyg. 1997;57:389–392. doi: 10.4269/ajtmh.1997.57.389. [DOI] [PubMed] [Google Scholar]
- 17.Baruch D I, Gormley J A, Howard R J, Pasloske B L. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner J P, Pinches R A, Roberts D J, Newbold C I. Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leech J H, Barnwell J W, Miller L H, Howard R J. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard R J, Barnwell J W, Rock E P, Neequave J, Ofiri-Adjei D, Maloy W L, Lyon J A, Saul A. Mol Biochem Parasitol. 1988;27:207–224. doi: 10.1016/0166-6851(88)90040-0. [DOI] [PubMed] [Google Scholar]
- 21.Biggs B A, Gooze L, Wycherley K, Wollish W, Southwell B, Leech J H, Brown G V. Proc Natl Acad Sci USA. 1991;88:9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Nature (London) 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Nature (London) 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 24.Ho M, Singh B, Looareesuwan S, Davis T M, Bunnag D, White N J. Infect Immun. 1991;59:873–878. doi: 10.1128/iai.59.3.873-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard R J, Gilladoga A D. Blood. 1989;74:2603–2618. [PubMed] [Google Scholar]
- 26.Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 27.Aikawa M, Brown A, Smith C D, Tegoshi T, Howard R J, Hasler T H, Ito Y, Perry G, Collins W E, Webster K. Am J Trop Med Hyg. 1992;46:391–397. doi: 10.4269/ajtmh.1992.46.391. [DOI] [PubMed] [Google Scholar]
- 28.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 30.Su X, Heatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 31.Baruch D I, Ma X C, Singh H B, Bi X, Pasloske B L, Howard R J. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 32.Garnham P C C. Kenya East Afr Med J. 1931;8:2–19. [Google Scholar]
- 33.Thomson J G, Robertson A. Trans R Soc Trop Med Hyg. 1935;29:31–40. [Google Scholar]
- 34.Smalley M E, Abdalla S, Brown J. Trans R Soc Trop Med Hyg. 1980;75:103–105. doi: 10.1016/0035-9203(81)90019-5. [DOI] [PubMed] [Google Scholar]
- 35.Field J W, Shute P G. The Microscopic Diagnosis of Human Malaria. A Morphilogical Study of Erythrocytic Parasites. Kuala Lumpar: Government Press; 1956. [Google Scholar]
- 36.Day K P, Hayward R E, Smith D, Culvenor J. Mol Biochem Parasitol. 1998;93:167–177. doi: 10.1016/s0166-6851(98)00040-1. [DOI] [PubMed] [Google Scholar]
- 37.Pasvol G, Wilson R J M, Smalley M E, Brown J. Ann Trop Med. 1978;72:82–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 38.Lambros C, Vanderberg J P. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 39.Bruce M C, Alano P, Carter R, Nakamura K, Aikawa M, Carter R. Mol Biochem Parasitol. 1994;65:11–22. doi: 10.1016/0166-6851(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 40.Biggs B A, Gooze L, Wycherley K, Wilkinson D, Boyd A W, Forsyth K P, Edelman L, Brown G V, Leech J H. J Exp Med. 1990;171:1883–1892. doi: 10.1084/jem.171.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowman A F, Karcz S, Galatis D, Culvenor J G. Cell Biol. 1991;114:1033–1042. doi: 10.1083/jcb.113.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piper, K. P., Roberts, D. J. & Day, K. P. Exp. Parasitol., in press [DOI] [PubMed]
- 43.Marsh K, Howard R J. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 44.Forsyth K P, Philip G, Smith T, Kum E, Southwell B, Brown G V. Am J Trop Med Hyg. 1989;41:259–265. [PubMed] [Google Scholar]
- 45.Newbold C I, Pinches R, Roberts D J, Marsh K. Exp Parasitol. 1992;75:281–292. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 46.Robson R. Proc R Soc London Ser B. 1990;242:205–211. [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning. A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 48.Weber J L. Mol Biochem Parasitol. 1988;29:117–121. doi: 10.1016/0166-6851(88)90066-7. [DOI] [PubMed] [Google Scholar]
- 49.Sinden R E. Parasitology. 1982;84:1–11. doi: 10.1017/s003118200005160x. [DOI] [PubMed] [Google Scholar]
- 50.Rogers N J, Daramola O, Targett G A T, Hall B S. Infect Immun. 1996;64:1480–1483. doi: 10.1128/iai.64.4.1480-1483.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]