Abstract
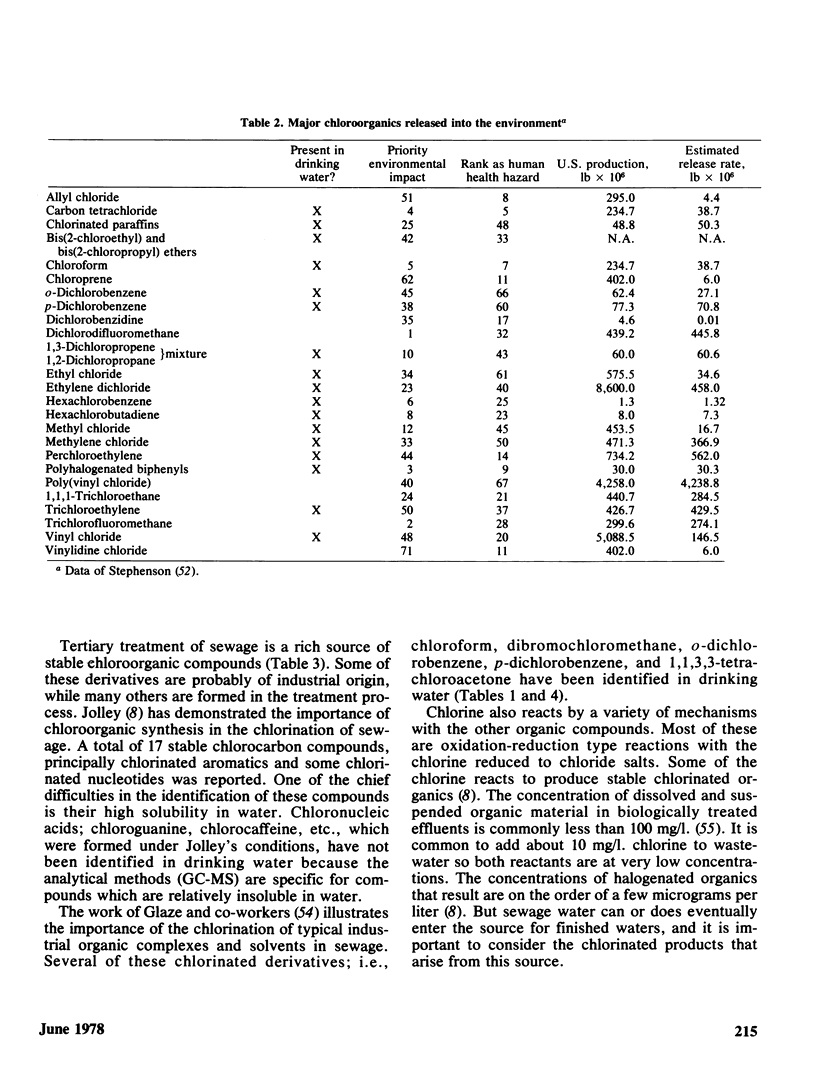
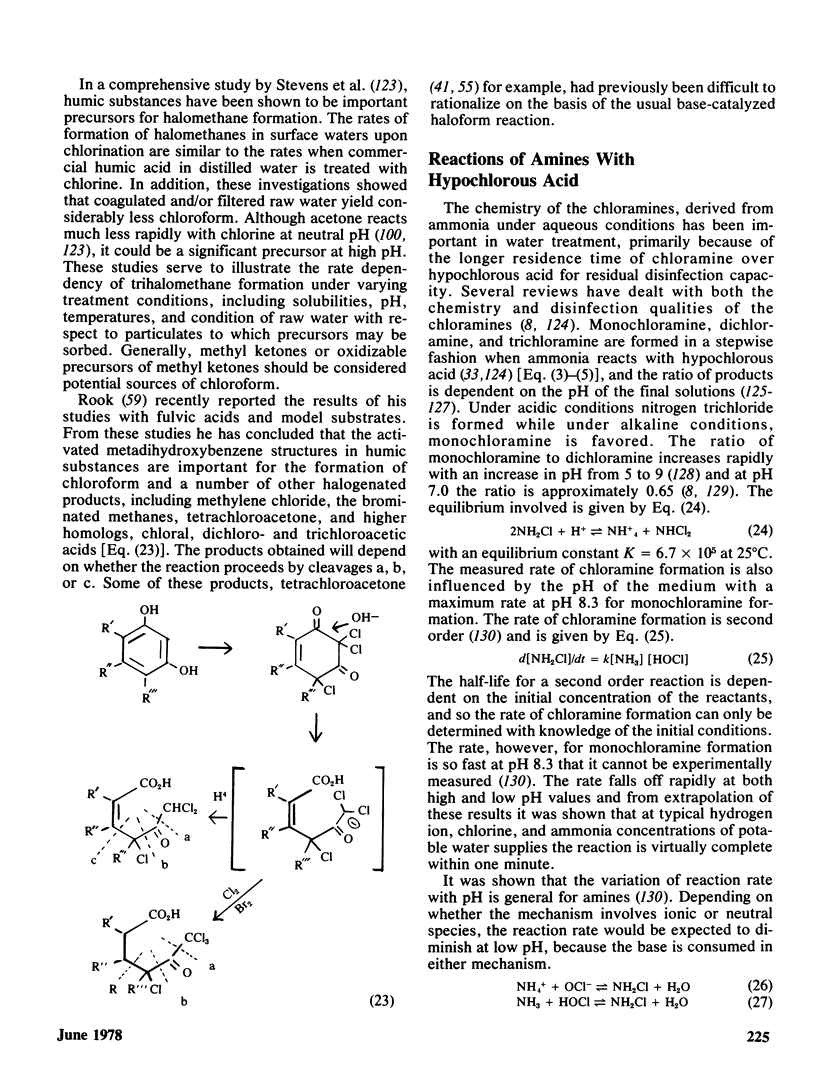
The disinfection of drinking water by chlorination has in recent years come under closer scrutiny because of the potential hazards associated with the production of stable chlorinated organic chemicals. Organic chemical contaminants are common to all water supplies and it is now well-established that chlorinated by-products are obtained under conditions of disinfection, or during tertiary treatment of sewage whose products can ultimately find their way into drinking water supplies. Naturally occurring humic substances which are invariably present in drinking waters are probably the source of chloroform and other halogenated methanes, and chloroform has shown up in every water supply investigated thus far.
The Environmental Protection Agency is charged with the responsibility of assessing the public health effects resulting from the consumption of contaminated drinking water. It has specifically undertaken the task of determining whether organic contaminants or their chlorinated derivatives have a special impact, and if so, what alternatives there are to protect the consumer against bacterial and viral diseases that are transmitted through infected drinking waters. The impetus to look at these chemicals is not entirely without some prima facie evidence of potential trouble. Epidemiological studies suggested a higher incidence of cancer along the lower Mississippi River where the contamination from organic chemicals is particularly high. The conclusions from these studies have, to be sure, not gone unchallenged.
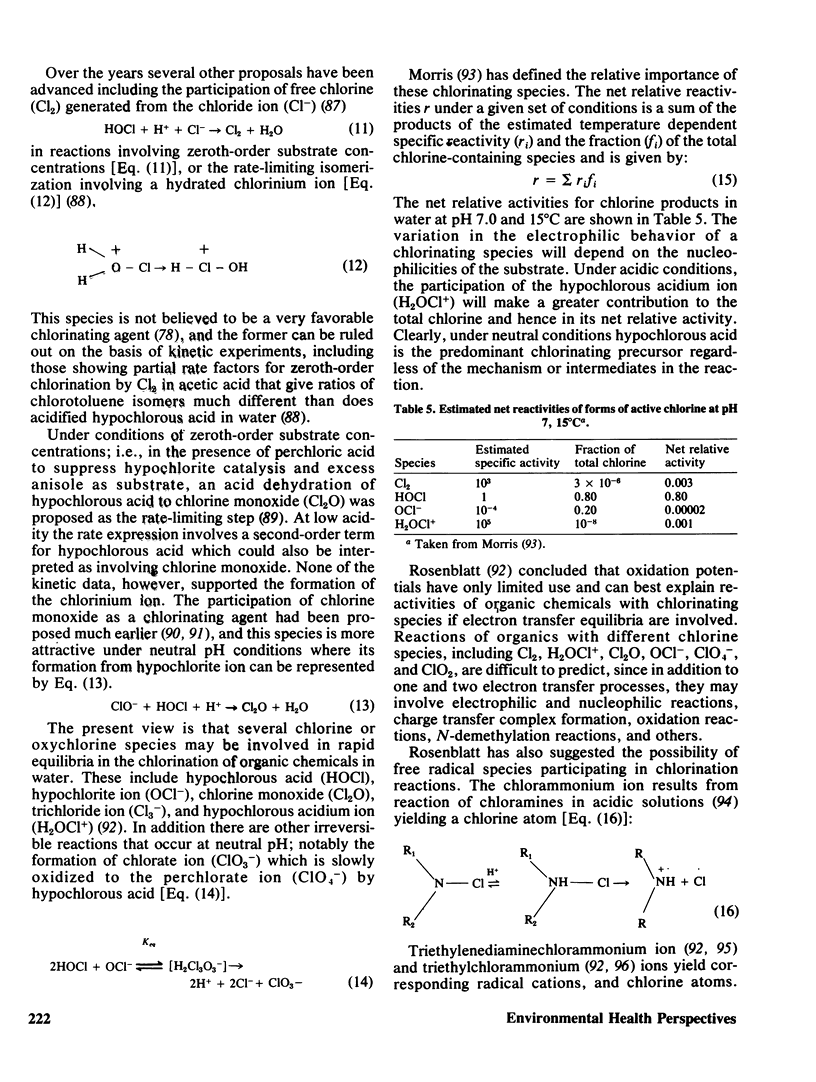
The task of assessing the effects of chemicals in the drinking water is a difficult one. It includes many variables, including differences in water supplies and the temporal relationship between contamination and consumption of the finished product. It must also take into account the relative importance of the effects from these chemicals in comparison to those from occupational exposure, ingestion of contaminated foods, inhalation of polluted air, and many others. The susceptibility of different age, genetic, and ethnic groups within the population must also be carefully considered. The present review discusses: the reasons for disinfection; the general occurrence of chlorinated organics in drinking water; the chemistry in the synthesis of chlorinated organics under aqueous conditions; and alternatives to chlorine for disinfection.
Full text
PDF
























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benarde M. A., Snow W. B., Olivieri V. P., Davidson B. Kinetics and mechanism of bacterial disinfection by chlorine dioxide. Appl Microbiol. 1967 Mar;15(2):257–265. doi: 10.1128/am.15.2.257-265.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G. Removal of viruses from sewage, effluents, and waters. I. A review. Bull World Health Organ. 1973;49(5):451–460. [PMC free article] [PubMed] [Google Scholar]
- Brungs W. A. Effects of residual chlorine on aquatic life. J Water Pollut Control Fed. 1973 Oct;45(10):2180–2193. [PubMed] [Google Scholar]
- DE LA MARE P. B. D., HUGHES E. D., VERNON C. A. Reaction of chlorine cations with olefins. Research. 1950 May;3(5):242–243. [PubMed] [Google Scholar]
- Dakin H. D. The Oxidation of Amino-Acids to Cyanides. Biochem J. 1916 Jun;10(2):319–323. doi: 10.1042/bj0100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawn H. S., Pitman I. H., Higuchi T., Young S. N-chlorosaccharin as a possible chlorinating reagent: structure, chlorine potential, and stability in water and organic solvents. J Pharm Sci. 1970 Jul;59(7):955–959. doi: 10.1002/jps.2600590707. [DOI] [PubMed] [Google Scholar]
- FRIBERG L. Quantitative studies on the reaction of chlorine with bacteria in water disinfection; an experimental investigation with radioactive chlorine (C136). Acta Pathol Microbiol Scand. 1956;38(2):135–144. doi: 10.1111/j.1699-0463.1956.tb00987.x. [DOI] [PubMed] [Google Scholar]
- FUKUHARA T. K., VISSER D. W. Pyrimidine nucleoside antagonists. J Biol Chem. 1951 May;190(1):95–101. [PubMed] [Google Scholar]
- Gehrs C. W., Eyman L. D., Jolley R. J., Thompson J. E. Effects of stable chlorine-containing organics on aquatic environments. Nature. 1974 Jun 14;249(458):675–676. doi: 10.1038/249675b0. [DOI] [PubMed] [Google Scholar]
- Grob K. Organic substances in potable water and in its precursor. I. Methods for their determination by gas-liquid chromatography. J Chromatogr. 1973 Sep 26;84(2):255–273. doi: 10.1016/s0021-9673(01)91705-4. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Pan S., Ukita T. Reaction of sodium hypochlorite with nucleic acids and their constituents. Chem Pharm Bull (Tokyo) 1971 Oct;19(10):2189–2192. doi: 10.1248/cpb.19.2189. [DOI] [PubMed] [Google Scholar]
- Malina J. F., Jr, Ranganathan K. R., Sagik B. P., Moore B. E. Poliovirus inactivation by activated sludge. J Water Pollut Control Fed. 1975 Aug;47(8):2178–2183. [PubMed] [Google Scholar]
- Miller R. W. Carcinogens in drinking water. Committee on environmental hazards. Pediatrics. 1976 Apr;57(4):462–464. [PubMed] [Google Scholar]
- Norman M. F. The oxidation of amino-acids by hypochlorite: Glycine. Biochem J. 1936 Mar;30(3):484–496. doi: 10.1042/bj0300484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton W., Bacon V., Duffield A. M., Halpern B., Hoyano Y., Pereira W., Lederberg J. Chlorination studies. I. The reaction of aqueous hypochlorous acid with cytosine. Biochem Biophys Res Commun. 1972 Aug 21;48(4):880–884. doi: 10.1016/0006-291x(72)90690-0. [DOI] [PubMed] [Google Scholar]
- Pereira W. E., Hoyano Y., Summons R. E., Bacon V. A., Duffield A. M. Chlorination studies. II. The reaction of aqueous hypochlorous acid with alpha-amino acids and dipeptides. Biochim Biophys Acta. 1973 Jun 20;313(1):170–180. doi: 10.1016/0304-4165(73)90198-0. [DOI] [PubMed] [Google Scholar]
- Prat R., Nofre C., Cier A. Effet de l'hypochlorite de sodium sur les constituants pyrimidiques des bactéries. C R Acad Sci Hebd Seances Acad Sci D. 1965 May 3;260(18):4859–4861. [PubMed] [Google Scholar]
- Sandford P. A., Nafziger A. J., Jeanes A. Reaction of sodium hypochlorite with amines and amides: a new method for quantitating amino sugars in monomeric form. Anal Biochem. 1971 Aug;42(2):422–436. doi: 10.1016/0003-2697(71)90056-x. [DOI] [PubMed] [Google Scholar]
- WEST I. PESTICIDES AS CONTAMINANTS. Arch Environ Health. 1964 Nov;9:626–633. doi: 10.1080/00039896.1964.10663894. [DOI] [PubMed] [Google Scholar]
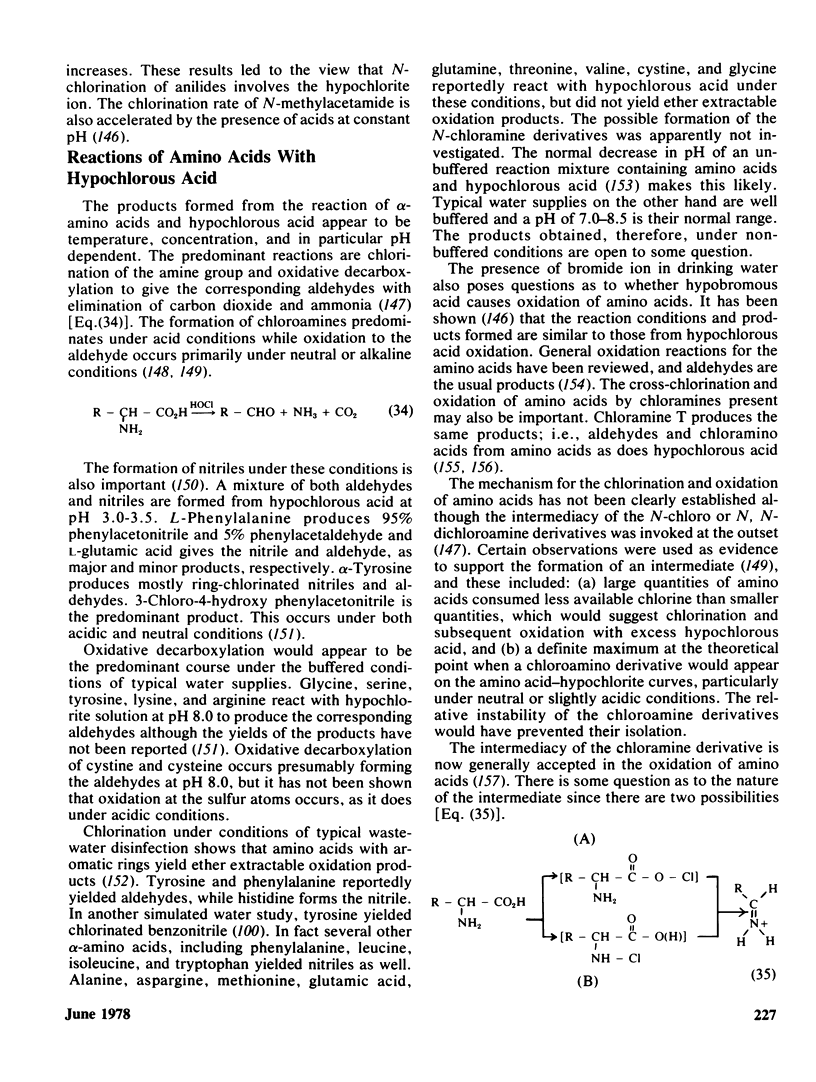
- Wright N. C. The Action of Hypochlorites on Amino-Acids and Proteins. Biochem J. 1926;20(3):524–532. doi: 10.1042/bj0200524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N. C. The action of hypochlorites on amino-acids and proteins. The effect of acidity and alkalinity. Biochem J. 1936 Sep;30(9):1661–1667. doi: 10.1042/bj0301661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tamelen E. E., Haarstad V. B., Orvis R. L. Hypohalite-induced oxidative decarboxylation of alpha-amino acids. Tetrahedron. 1968 Jan;24(2):687–704. doi: 10.1016/0040-4020(68)88018-4. [DOI] [PubMed] [Google Scholar]


