Abstract
Pesticide chemicals are an important component of modern agriculture. Through their use, plants and animals are exposed to pesticides directly and indirectly from transport through soil, water, and other environmental components. Pesticide chemicals which are absorbed by plants and animals undergo extensive biotransformation. Lipophilic compounds are converted to polar metabolites through a variety of microsomal and extramicrosomal reactions in plants and animals. Generally, biotransformations are qualitatively similar in both systems. However, there are important quantitative rate differences in metabolism which often determine the balance between activation and deactivation of a pesticide. Furthermore, there are qualitative differences in conjugative mechanisms in plants and animals. Animals through an efficient excretory system eliminate transformation products via the urine and feces. Since efficient excretory systems are absent in plants, terminal degradation products are stored as conjugates and/or derivatives which may be incorporated into the plants themselves. Metabolic transformations of selected pesticides illustrating various types of reactions in plants and animals are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bull D. L., Stokes R. A. Metabolism of dimethyl p-(methylthio)phenyl phosphate in animals and plants. J Agric Food Chem. 1970 Nov-Dec;18(6):1134–1138. doi: 10.1021/jf60172a021. [DOI] [PubMed] [Google Scholar]
- Casida J. E., Gray R. A., Tilles H. Thiocarbamate sulfoxides: potent, selective, and biodegradable herbicides. Science. 1974 May 3;184(4136):573–574. doi: 10.1126/science.184.4136.573. [DOI] [PubMed] [Google Scholar]
- Conney A. H., Burns J. J. Metabolic interactions among environmental chemicals and drugs. Science. 1972 Nov 10;178(4061):576–586. doi: 10.1126/science.178.4061.576. [DOI] [PubMed] [Google Scholar]
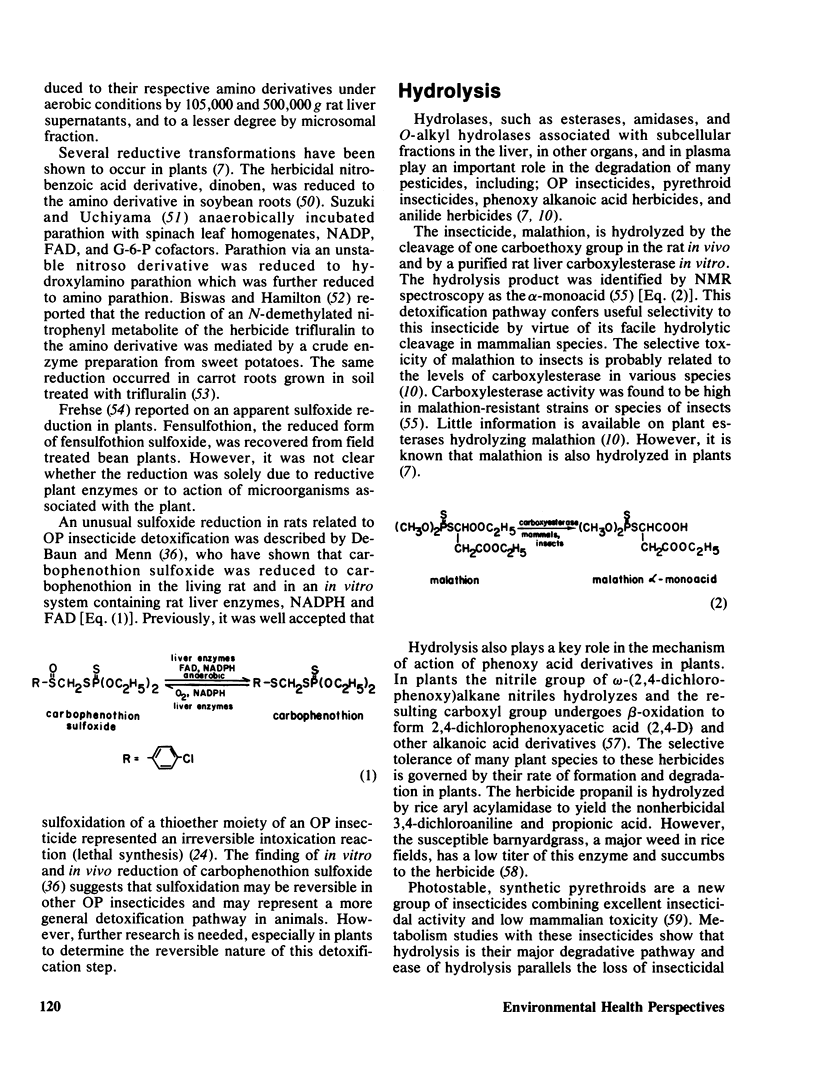
- DeBaun J. R., Menn J. J. Sulfoxide reduction in relation to organophosphorus insecticide detoxification. Science. 1976 Jan 16;191(4223):187–188. doi: 10.1126/science.1246606. [DOI] [PubMed] [Google Scholar]
- FINLAYSON D. G., MACCARTHY H. R. THE MOVEMENT AND PERSISTENCE OF INSECTICIDES IN PLANT TISSUE. Residue Rev. 1965;9:114–152. doi: 10.1007/978-1-4615-8395-0_3. [DOI] [PubMed] [Google Scholar]
- Gill S. S., Hammock B. D., Casida J. E. Mammalian metabolism and environmental degradation of the juvenoid 1-(4'-ethylphenoxy)-3,7-dimethyl-6,7-epoxy-trans-2-octene and related compounds. J Agric Food Chem. 1974 May-Jun;22(3):386–395. doi: 10.1021/jf60193a058. [DOI] [PubMed] [Google Scholar]
- Hammock B. D., Gill S. S., Casida J. E. Synthesis and morphogenetic activity of derivaties and analogs of aryl geranyl ether juvenoids. J Agric Food Chem. 1974 May-Jun;22(3):379–385. doi: 10.1021/jf60193a043. [DOI] [PubMed] [Google Scholar]
- Harrison R. B., Holmes D. C., Roburn J., Tatton J. O. The fate of some organochlorine pesticides on leaves. J Sci Food Agric. 1967 Jan;18(1):10–15. doi: 10.1002/jsfa.2740180104. [DOI] [PubMed] [Google Scholar]
- Hoffman L. J., Ross J. H., Menn J. J. Metabolism of 1-(4'-ethylphenoxy)-6,7-epoxy-3,7-dimethyl-2-octene (R 20458) in the rat. J Agric Food Chem. 1973 Mar-Apr;21(2):156–163. doi: 10.1021/jf60186a034. [DOI] [PubMed] [Google Scholar]
- Hubbell J. P., Casida J. E. Metabolic fate of the N,N-dialkylcarbamoyl moiety of thiocarbamate herbicides in rats and corn. J Agric Food Chem. 1977 Mar-Apr;25(2):404–413. doi: 10.1021/jf60210a006. [DOI] [PubMed] [Google Scholar]
- Hull H. M. Leaf structure as related to absorption of pesticides and other compounds. Residue Rev. 1970;31:1–150. [PubMed] [Google Scholar]
- Lay M., Hubbell J. P., Casida J. E. Dichloroacetamide antidotes for thiocarbamate herbicides: mode of action. Science. 1975 Jul 25;189(4199):287–289. doi: 10.1126/science.1145201. [DOI] [PubMed] [Google Scholar]
- Lichtenstein E. P., Fuhremann T. W., Hochberg A. A., Zahlten R. N., Stratman F. W. Metabolism of ( 14 C)parathion and ( 14 C)paraoxon with fractions and subfractions of rat liver cells. J Agric Food Chem. 1973 May-Jun;21(3):416–424. doi: 10.1021/jf60187a008. [DOI] [PubMed] [Google Scholar]
- McBain J. B., Menn J. J. S-methylation, oxidation, hydroxylation and conjugation of thiophenol in the rat. Biochem Pharmacol. 1969 Sep;18(9):2282–2285. doi: 10.1016/0006-2952(69)90340-2. [DOI] [PubMed] [Google Scholar]
- McBain J. B., Yamamoto I., Casida J. E. Mechanism of activation and deactivation of Dyfonate (o-ethyl S-phenyl ethylphosphonodithioate) by rat liver microsomes. Life Sci II. 1971 Aug 22;10(16):947–954. doi: 10.1016/0024-3205(71)90097-x. [DOI] [PubMed] [Google Scholar]
- Menn J. J., DeBaun J. R., McBain J. B. Recent advances in the metabolism of organophosphorus insecticides. Fed Proc. 1976 Dec;35(14):2598–2602. [PubMed] [Google Scholar]
- Menn J. J., Pallow F. M. Development of morphogenetic agents in insect control. Environ Lett. 1975;8(1):71–88. doi: 10.1080/00139307509435837. [DOI] [PubMed] [Google Scholar]
- Menn J. J., Still G. G. Metabolism of insecticides and herbicides in higher plants. CRC Crit Rev Toxicol. 1977 May;5(1):1–21. doi: 10.3109/10408447709101340. [DOI] [PubMed] [Google Scholar]
- Miaullis J. B., Hoffman L. J., DeBaun J. R., Menn J. J. Metabolism of O-ethyl S-4-chlorophenyl ethanephosphonodithioate (N-2596) in the rat. J Agric Food Chem. 1977 May-Jun;25(3):501–506. doi: 10.1021/jf60211a013. [DOI] [PubMed] [Google Scholar]
- Motoyama N., Dauterman W. C. The role of nonoxidative metabolism in organophosphorus resistance. J Agric Food Chem. 1974 May-Jun;22(3):350–356. doi: 10.1021/jf60193a055. [DOI] [PubMed] [Google Scholar]
- Pallos F. M., Gray R. A., Arneklev D. R., Brokke M. E. Antidotes protect corn from thiocarbamate herbicide injury. J Agric Food Chem. 1975 Jul-Aug;23(4):821–822. doi: 10.1021/jf60200a037. [DOI] [PubMed] [Google Scholar]
- Ptashne K. A., Wolcott R. M., Neal R. A. Oxygen-18 studies on the chemical mechanisms of the mixed function oxidase catalyzed desulfuration and dearylation reactions of parathion. J Pharmacol Exp Ther. 1971 Nov;179(2):380–385. [PubMed] [Google Scholar]
- Shimabukuro R. H. Herbicide metabolism by glutathione conjugation in plants. Environ Qual Saf. 1975;4:140–148. [PubMed] [Google Scholar]
- Suzuki T., Uchiyama M. Pathway of nitro reduction of parathion by spinach homogenate. J Agric Food Chem. 1975 Mar-Apr;23(2):281–286. doi: 10.1021/jf60198a057. [DOI] [PubMed] [Google Scholar]
- Wargo J. P., Honeycutt R. C., Adler I. L. Characterization of bound residues of nitrofen in cereal grains. J Agric Food Chem. 1975 Nov-Dec;23(6):1095–1097. doi: 10.1021/jf60202a027. [DOI] [PubMed] [Google Scholar]


