Abstract
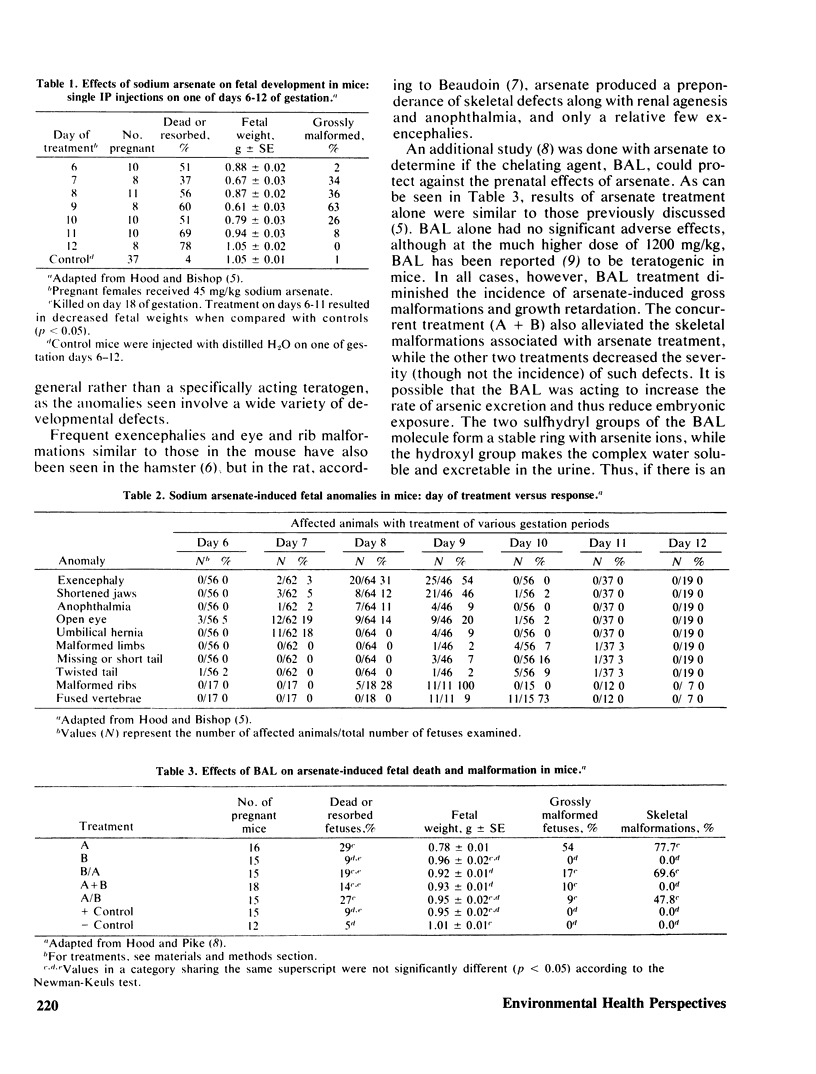
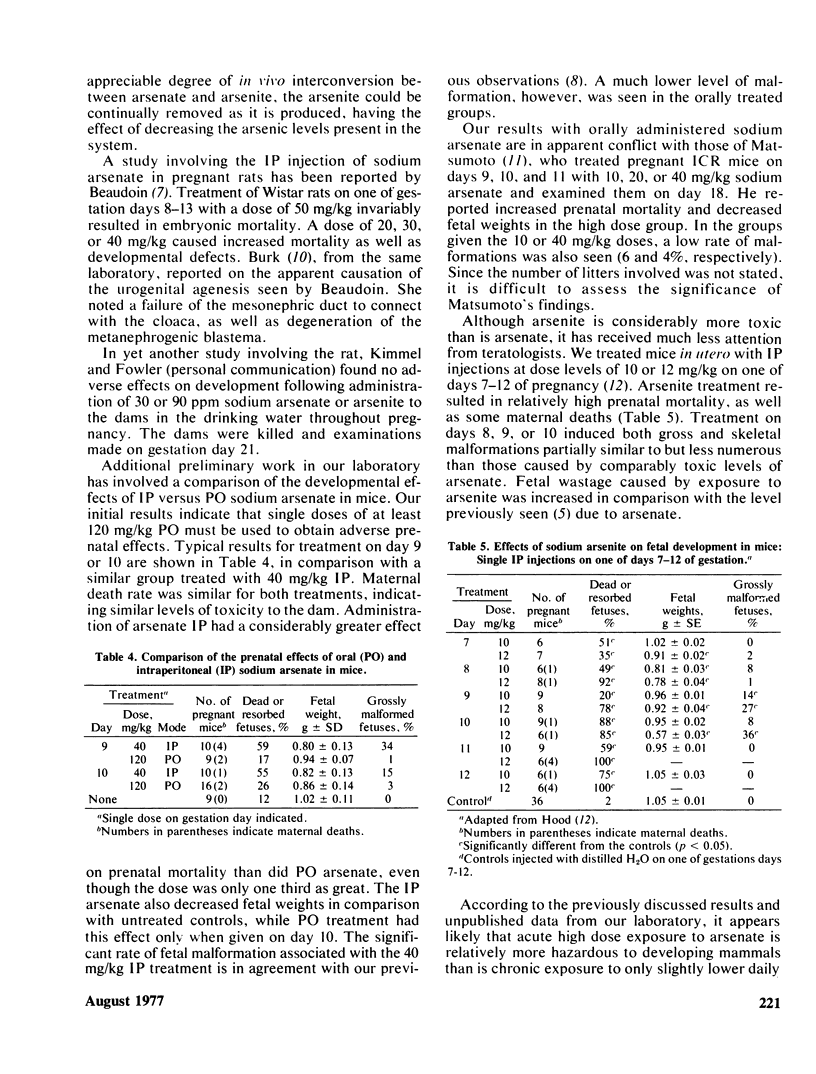
Initial experiments involving mouse development employed single IP injections of 45 mg/kg sodium arsenate on one of days 6-12 of gestation and produced a spectrum of developmental defects. Embryotoxicity was indicated by high prenatal mortality and decreased fetal weights. A chelating agent, 2,3-dimercaptopropanol (BAL), was then employed in an attempt to alleviate the adverse effects of prenatal arsenate. BAL was administered 4 hr before, concurrently with, or 4 hr after arsenate. All BAL treatments diminished arsenate-induced gross malformations and growth retardation; the concurrent treatment alleviated skeletal malformation. Injection of rats IP with arsenate has also been reported to result in teratogenicity, including renal agenesis. Further reports indicated that 40 mg/kg arsenate administered to mice by gavage on days 9–11 increased prenatal mortality, reduced fetal weights, and was associated with minor malformations. According to our recent work, however, single oral doses of arsenate must be around 120 mg/kg to cause prenatal toxicity. Multiple doses of 60 mg/kg on 3 days had little effect. Sodium arsenite has also been found to be fetotoxic and teratogenic. Such effects were seen at IP doses of 10–12 mg/kg.
Full text
PDF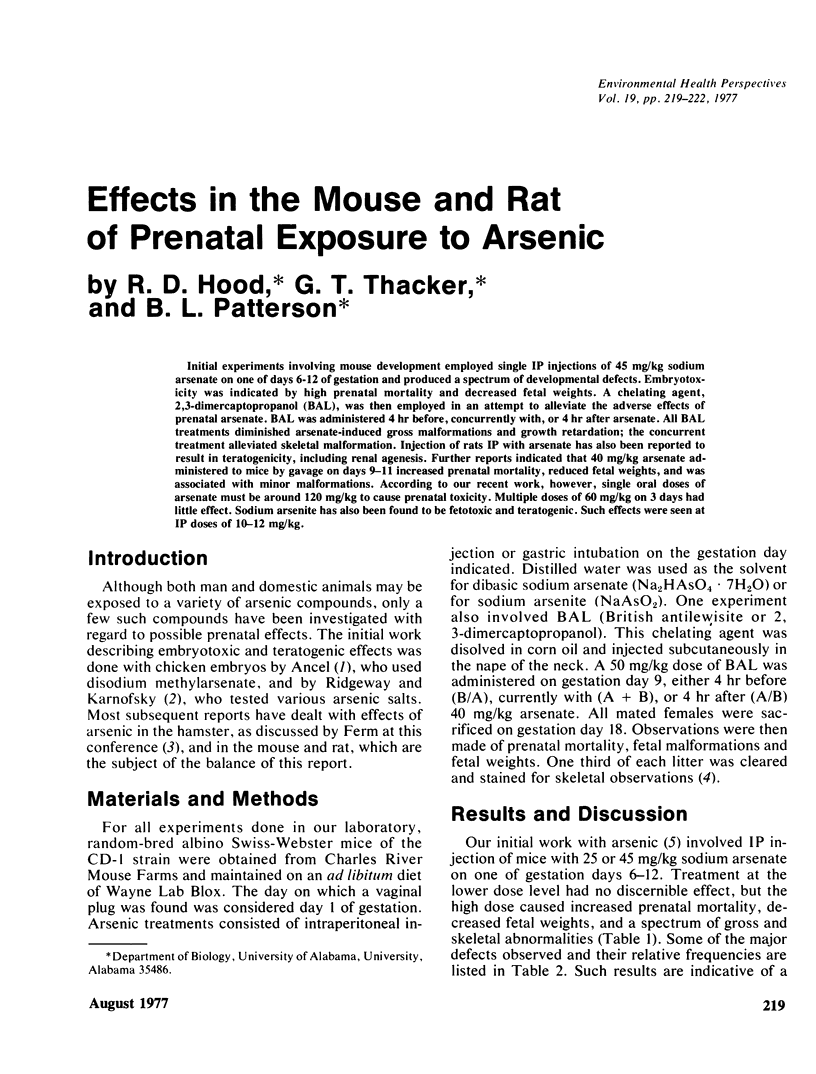

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudoin A. R. Teratogenicity of sodium arsenate in rats. Teratology. 1974 Oct;10(2):153–157. doi: 10.1002/tera.1420100211. [DOI] [PubMed] [Google Scholar]
- CRARY D. D. Modified benzyl alcohol clearing of alizarinstained specimens without loss of flexibility. Stain Technol. 1962 Mar;37:124–125. doi: 10.3109/10520296209114589. [DOI] [PubMed] [Google Scholar]
- Ferm V. H. Arsenic as a teratogenic agent. Environ Health Perspect. 1977 Aug;19:215–217. doi: 10.1289/ehp.7719215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferm V. H., Saxon A., Smith B. M. The teratogenic profile of sodium arsenate in the golden hamster. Arch Environ Health. 1971 May;22(5):557–560. doi: 10.1080/00039896.1971.10665901. [DOI] [PubMed] [Google Scholar]
- Hood R. D., Bishop S. L. Teratogenic effects of sodium arsenate in mice. Arch Environ Health. 1972 Jan;24(1):62–65. doi: 10.1080/00039896.1972.10666051. [DOI] [PubMed] [Google Scholar]
- Hood R. D. Effects of sodium arsenite on fetal development. Bull Environ Contam Toxicol. 1972 Apr;7(4):216–222. doi: 10.1007/BF01684401. [DOI] [PubMed] [Google Scholar]
- Hood R. D., Pike C. T. BAL alleviation of arsenate-induced teratogenesis in mice. Teratology. 1972 Oct;6(2):235–237. doi: 10.1002/tera.1420060216. [DOI] [PubMed] [Google Scholar]
- NISHIMURA H., TAKAGAKI S. Developmental anomalies in mice induced by 2,3-dimercaptopropanol (BAL). Anat Rec. 1959 Dec;135:261–267. doi: 10.1002/ar.1091350404. [DOI] [PubMed] [Google Scholar]
- RIDGWAY L. P., KARNOFSKY D. A. The effects of metals on the chick embryo: toxicity and production of abnormalities in development. Ann N Y Acad Sci. 1952 Aug 8;55(2):203–215. doi: 10.1111/j.1749-6632.1952.tb26536.x. [DOI] [PubMed] [Google Scholar]


