Abstract
The G protein-coupled receptor kinases (GRKs) and β-arrestins, families of molecules essential to the desensitization of G protein-dependent signaling via seven-transmembrane receptors (7TMRs), have been recently shown to also transduce G protein-independent signals from receptors. However, the physiologic consequences of this G protein-independent, GRK/β-arrestin-dependent signaling are largely unknown. Here, we establish that GRK/β-arrestin-mediated signal transduction via the angiotensin II (ANG) type 1A receptor (AT1AR) results in positive inotropic and lusitropic effects in isolated adult mouse cardiomyocytes. We used the “biased” AT1AR agonist [Sar1, Ile4, Ile8]-angiotensin II (SII), which is unable to stimulate Gαq-mediated signaling, but which has previously been shown to promote β-arrestin interaction with the AT1AR. Cardiomyocytes from WT, but not AT1AR-deficient knockout (KO) mice, exhibited positive inotropic and lusitropic responses to both ANG and SII. Responses of WT cardiomyocytes to ANG were dramatically reduced by protein kinase C (PKC) inhibition, whereas those to SII were unaffected. In contrast, cardiomyocytes from β-arrestin2 KO and GRK6 KO mice failed to respond to SII, but displayed preserved responses to ANG. Cardiomyocytes from GRK2 heterozygous knockout mice (GRK2+/−) exhibited augmented responses to SII in comparison to ANG, whereas those from GRK5 KO mice did not differ from those from WT mice. These findings indicate the existence of independent Gαq/PKC- and GRK6/β-arrestin2-dependent mechanisms by which stimulation of the AT1AR can modulate cardiomyocyte function, and which can be differentially activated by selective receptor ligands. Such ligands may have potential as a novel class of therapeutic agents.
Keywords: seven-transmembrane receptors, G protein-coupled receptor kinase, mice
Virtually all physiologic processes in higher organisms are critically regulated by signal transduction through seven-transmembrane receptors (7TMRs), the largest and most diverse family of cell surface receptors (1). The biological effects of 7TMR signal transduction have conventionally been attributed to activation of 7TMR-associated heterotrimeric G proteins, and consequent activation of second messenger-generating enzymes, production of second messengers, and activation of second messenger-dependent effector molecules (2). This classical understanding has been supported by numerous physiologic studies, and forms the basis for the utilization of drugs (agonists and antagonists) targeting 7TMRs, as therapeutic strategies for a vast array of diseases (1).
However, this simple paradigm has been altered by an increasing appreciation of the biochemical importance of both negative regulation of G protein-dependent signaling, and G protein-independent signal transduction by 7TMRs (3, 4). G protein activation and dependent downstream processes are predominantly antagonized by the sequential actions of two families of molecules, the G protein-coupled receptor kinases (GRKs) and the β-arrestins. Agonist binding to the 7TMR induces conformational changes, which result in GRK-catalyzed phosphorylation of 7TMR intracellular serine and threonine residues, which then promotes recruitment of β-arrestins to the receptor (4). β-Arrestin interaction with the receptor interdicts coupling of the receptor to G proteins (desensitization) (4), and facilitates receptor removal from the cell surface (internalization) (4). As with the biochemical functions of G proteins and downstream effectors, these roles of GRKs and β-arrestins in desensitization have been corroborated with respect to the regulation of numerous physiologic processes (4, 5).
Comparatively less is known regarding the physiologic and/or pathophysiologic roles of 7TMR-mediated G protein-independent signal transduction. However, recent evidence suggests that G protein-independent signal transduction via 7TMRs may carry out important physiologic functions in the cardiovascular system. For example, transgenic myocardium-specific overexpression of an angiotensin II (ANG) type 1A receptor (AT1AR) uncoupled from Gαq activation has distinct effects on myocardial signaling and ventricular function, in comparison to overexpression of the wild-type AT1AR (6). Yet, the proximal biochemical mediators of the observed phenotype are unknown.
The existence of G protein-independent, GRK/β-arrestin-mediated signal transduction has been established for several 7TMRs (7–13) as well as non-7TMRs (14, 15), and represents a potentially important mechanism by which 7TMRs modulate physiologic processes. For example, β-arrestins have been shown to couple 7TMRs, including the AT1AR (7, 8, 10), β2 adrenergic receptor (β2AR) (12, 16), V2 vasopressin receptor (V2R) (11), protease-activated receptor 2 (PAR2) (17), and parathyroid hormone receptor (9), to activation of the mitogen activated protein kinase ERK; furthermore, for the AT1AR and V2R, this process requires the activities of GRKs 5 and 6. To date, the physiologic consequences of β-arrestin-mediated signal transduction have not been extensively characterized. However, a recent report implicates β-arrestin2 in D2 dopamine receptor-dependent neurotransmission and behaviors (18).
We have previously shown that stimulation of the AT1AR in adult mouse cardiomyocytes results in positive inotropic effects (19), which have also been demonstrated in cells from other species (20) and in vivo (21). To assess the physiologic roles of β-arrestin-mediated signaling in the cardiovascular system, we measured systolic and diastolic functional responses of adult mouse cardiomyocytes to ANG and to a “biased agonist” of the AT1AR ([Sar1, Ile4, Ile8])-ANG (SII). In studies with HEK293 cells, we have shown that this ligand is unable to promote Gαq activation, but mediates β-arrestin2-dependent ERK activation, i.e., it is “biased” in favor of β-arrestin-mediated versus G protein-mediated signaling. We also tested the dependence of these responses on β-arrestin2 and specific GRK isoforms by using cardiomyocytes from gene-targeted mice deficient in each of these proteins (22–26). The results provide strong evidence for signal transduction via the GRK/β-arrestin system resulting in cardiovascular physiologic responses.
Results
Intracellular Calcium Mobilization in Cardiomyocytes Stimulated with ANG or SII.
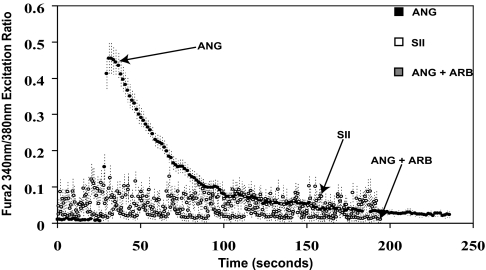
To determine whether SII activates Gαq-dependent pathways in cells expressing endogenous AT1ARs, calcium mobilization in neonatal rat atrial cardiomyocytes (these cells can be obtained at high yield and maintained in culture; ref. 27) in response to exposure to ANG or SII was assessed. As shown in Fig. 1, cells treated with ANG displayed robust calcium mobilization, whereas cells treated with SII showed no calcium mobilization, which was identical to cells treated with ANG in the presence of an angiotensin receptor blocker (ARB).
Fig. 1.
Mobilization of calcium in response to ANG and SII. Neonatal rat atrial cardiomyocytes were loaded with the calcium-binding dye Fura-2, and stimulated either with ANG (100 nM), SII (10 μM), or ANG in the presence of pretreatment with the AT1R antagonist (ARB) valsartan (50 μM). Calcium fluorimetric traces are shown, with the 340/380 nm excitation ratio (y axis) plotted as a function of time (x axis). Results displayed are mean ± SEM of three independent experiments.
Control of β-Arrestin Recruitment to the AT1AR by Specific GRKs.
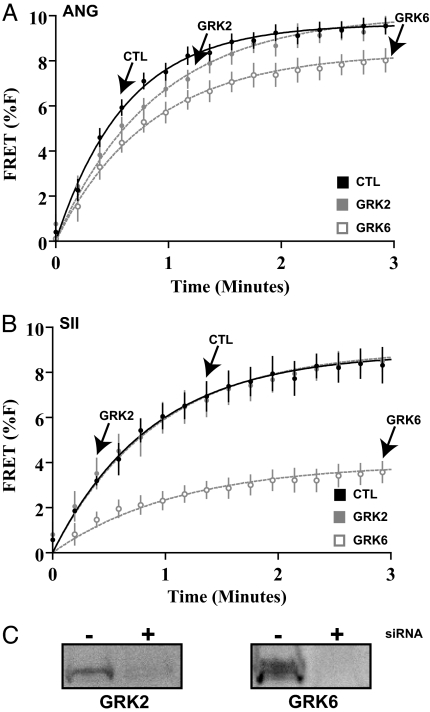
We have shown that SII stimulates β-arrestin2-dependent ERK activation through the AT1AR (7, 10, 13), and that the activities of GRK5 and GRK6 are required for this PKC-independent ERK activation (10). Before carrying out studies of the functional effects of β-arrestin-mediated signaling in cardiomyocytes, further characterization of β-arrestin2 recruitment to the AT1AR by ANG and SII, and its control by specific GRK isoforms was carried out. HEK293 cells were used for these studies (Materials and Methods). Using fluorescence resonance energy transfer (FRET), we found that both ANG and SII led to β-arrestin2 recruitment to the AT1AR with comparable kinetics and magnitude (Fig. 2). Recruitment in response to ANG was slightly diminished in the presence of siRNA targeted against GRK6, and to a lesser extent, GRK2 (Fig. 2A). However, in the case of SII-induced β-arrestin2 recruitment, siRNA against GRK6 led to substantial impairment (Fig. 2B). No effects of GRK5 siRNA on β-arrestin2 recruitment were observed for either ANG or SII (data not shown). These findings suggest that SII-induced signaling via β-arrestin2 depends on the upstream activity of GRK6.
Fig. 2.
Contribution of specific GRK isoforms to agonist-induced recruitment of β-arrestin2 to the AT1AR. HEK293 cells stably expressing AT1AR-mCFP and β-arrestin2-mYFP were stimulated with ANG (100 nM) or SII (10 μM), in the setting of exposure to the indicated siRNA. See Materials and Methods for details. (A) Modest effects of GRK2 or GRK6 deficiency on ANG-stimulated (ANG) recruitment of β-arrestin2 to the AT1AR. (B) Substantial effects of GRK6 deficiency, but not GRK2 deficiency, on SII-stimulated (SII) recruitment of β-arrestin2 to the AT1AR. (C) Representative immunoblot demonstrating efficacy of inhibition of GRK2 or GRK6 expression (n = 3 for all experiments, data displayed in A and B are mean ± SEM).
Effects of SII on Cardiomyocyte Function Via Signaling Through the AT1AR.
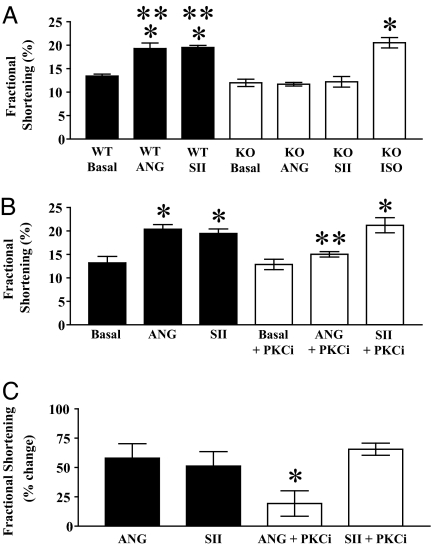
Having demonstrated that SII fails to activate Gαq-dependent signals in cardiomyocytes, we sought to determine whether, like ANG (19), SII has effects on cardiomyocyte function. As shown in Fig. 3A, WT cardiomyocytes displayed augmented percent fractional shortening in response to treatment with either ANG or SII in comparison to conditions of electrical stimulation alone. Furthermore, both ANG- and SII-induced positive inotropic responses were absent in cardiomyocytes from AT1AR KO mice (Fig. 3A), which nevertheless responded robustly to the βAR agonist isoproterenol. Similar data were observed for −dL/dtmax and +dL/dtmax (Fig. 6, which is published as supporting information on the PNAS web site). These data demonstrate that the effects of both AT1AR ligands are mediated by signaling through the AT1AR.
Fig. 3.
AT1AR-dependent changes in cardiomyocyte systolic function mediated by the natural agonist ANG and the biased agonist SII; effects of antagonism of PKC on responses to ANG and SII. (A) Absence of effects of ANG and SII on fractional shortening of cardiomyocytes from AT1AR KO mice. Fractional shortening of cardiomyocytes from contemporaneous WT (n = 4 animals; black bars) and AT1AR-deficient (KO) (n = 7 animals; white bars) mice, under conditions of pacing alone (Basal), or additional exposure to 10 μM ANG or SII as indicated. In four experiments (i.e., four individual animals), KO cardiomyocytes were additionally stimulated with 1 μM isoproterenol (Iso). ∗, P < 0.05 by one-way ANOVA with post hoc Bonferroni test relative to pertinent basal; ∗∗, P < 0.05 by one-way ANOVA with post hoc Bonferroni test relative to pertinent AT1AR KO (identical stimulation condition). (B and C) Differential effects of PKC antagonism on positive inotropic responses to ANG and SII. Fractional shortening (B, absolute values for each variable under indicated stimulation conditions; C, percent change in each variable in response to ANG or SII, relative to pertinent basal) of cardiomyocytes from WT mice (n = 4 animals), without (filled bars) or with (open bars) pretreatment with the PKC inhibitor Ro-31–8425 (1 μM), under conditions of pacing alone (Basal), or additional exposure to 10 μM ANG or SII as indicated. ∗, P < 0.05 by one-way ANOVA with post hoc Bonferroni test relative to pertinent basal (B) or ANG (C); ∗∗, P < 0.05 by one-way ANOVA with post hoc Bonferroni test relative to identical stimulation condition (B only). Data displayed are mean ± SEM. See Materials and Methods for experimental details.
Dependence of ANG Versus SII Effects on Activation of PKC.
To determine whether the SII-induced effects on cardiomyocyte contractile function are mediated by PKC signaling, we stimulated cardiomyocytes in the presence or absence of the PKC inhibitor Ro-31–8425. As shown in Fig. 3 B and C, whereas ANG-induced positive inotropic responses were inhibited substantially (by 71%) by the PKC inhibitor Ro-31–8425, responses to SII were unaffected. Similar findings for −dL/dtmax and +dL/dtmax were observed (Fig. 7, which is published as supporting information on the PNAS web site). Thus, the functional effects of SII are independent of PKC activation, consistent with selective transmission of Gαq-independent signals. In contrast, in this experimental system, the functional effects of ANG predominantly depend on PKC activation, and presumably depend on upstream activation of Gαq.
Dependence of ANG Versus SII Effects on β-Arrestin2.
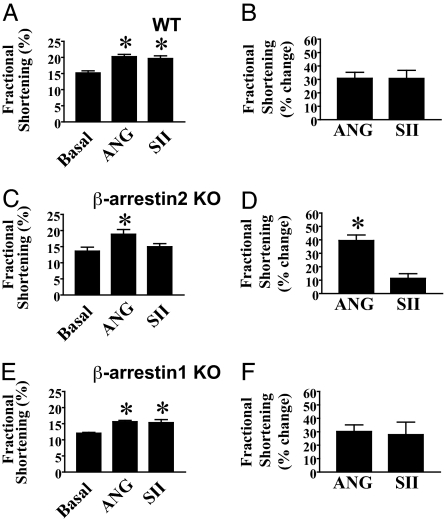
Accumulating biochemical evidence suggests that, in many cases, a component of G protein-independent signaling via 7TMRs is mediated by β-arrestins. To test whether the positive inotropic responses of cardiomyocytes to SII are, in fact, β-arrestin-dependent, cardiomyocytes from β-arrestin2 KO mice were assayed for responses to ANG and SII, along with cardiomyocytes from a contemporaneous set of WT mice. Although WT cardiomyocytes displayed similar increases in percent fractional shortening in response to ANG and SII (Fig. 4A and B), β-arrestin2 KO cardiomyocytes displayed minimal increases in percent fractional shortening in response to SII despite preserved responses to ANG [Fig. 4C, SII versus basal (P > 0.05), ANG versus SII (P < 0.05); Fig. 4D, ANG versus SII (P < 0.05)]. Similar data were observed for −dL/dtmax and +dL/dtmax (Fig. 8, which is published as supporting information on the PNAS web site). These data demonstrate that AT1AR signaling via β-arrestin2 is required for SII to exert positive inotropic and lusitropic effects.
Fig. 4.
Effects of deficiency in β-arrestin2 or β-arrestin1 on changes in systolic cardiomyocyte function in response to ANG and SII. (A and B) WT cardiomyocytes display positive inotropic responses to both ANG and SII. Fractional shortening of cardiomyocytes from a series of WT mice (n = 12 animals) analyzed contemporaneously with the experiments in C–F and Fig. 5, under conditions of pacing alone (Basal), or additional exposure to 10 μM ANG or SII as indicated. (A) Absolute values for each variable under indicated stimulation conditions. (B) Percentage change in each variable in response to ANG or SII, relative to basal. (C and D) β-arrestin2 KO cardiomyocytes display severely defective positive inotropic responses to SII, but unaffected responses to ANG. Fractional shortening of cardiomyocytes from β-arrestin2 KO mice (n = 5 animals) under conditions of pacing alone (Basal), or additional exposure to 10 μM ANG or SII as indicated. (C) Absolute values for each variable under indicated stimulation conditions. (D) Percent change in each variable in response to ANG or SII, relative to basal. (E and F) In contrast, β-arrestin1 KO cardiomyocytes exhibit equivalent positive inotropic responses to ANG and SII. Fractional shortening of cardiomyocytes from β-arrestin1 KO mice (n = 5 animals) under conditions of pacing alone (Basal), or additional exposure to 10 μM ANG or SII as indicated. (E) Absolute values for each variable under indicated stimulation conditions. (F) Percent change in each variable in response to ANG or SII, relative to basal. ∗, P < 0.05 by one-way ANOVA with post hoc Bonferroni test relative to basal, and between ANG and SII when relevant (A, C, and E; in C, the asterisk for ANG thus represents significance relative to both basal and SII, whereas in A, the asterisk for either ANG or SII represents significance relative to basal), and by Student's paired t test between ANG and SII (B, D, and F). Data displayed are mean ± SEM. See Materials and Methods for experimental details.
We have previously reported that β-arrestin1 negatively regulates SII-mediated ERK activation by the AT1AR in HEK293 cells (28). Additionally, β-arrestin1 KO mice have been shown to exhibit augmented cardiovascular system responses to β-AR stimulation with isoproterenol in vivo (24). Thus, we examined responses to ANG and SII in cardiomyocytes isolated from β-arrestin1 KO mice. ANG and SII were found to elicit equivalent increases in percentage of fractional shortening (Fig. 4 E and F), and −dL/dtmax and +dL/dtmax (Fig. 8), of β-arrestin1 KO cardiomyocytes.
Dependence of ANG Versus SII Effects on Specific GRKs.
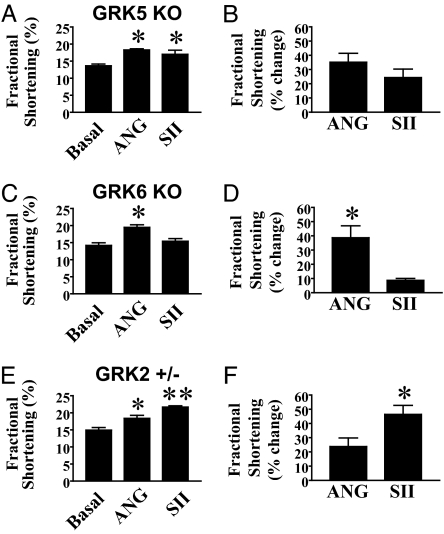
We next tested whether the ANG- and SII-mediated cardiomyocyte functional responses depended on specific GRK isoforms. Because we have previously shown that in HEK293 cells expressing the AT1AR, GRK5 and GRK6 are individually required for β-arrestin2-mediated ERK activation (10), we initially evaluated cardiomyocytes from GRK5 KO and GRK6 KO mice, and subsequently cardiomyocytes from GRK2 heterozygous KO mice (homozygous GRK2 KO mice suffer embryonic lethality as a consequence of heart failure; ref. 22). As shown in Fig. 5A and B, GRK5 KO cardiomyocytes exhibit equivalent increases in percentage of fractional shortening in response to ANG or SII; similar data were observed for −dL/dtmax and +dL/dtmax (Fig. 9, which is published as supporting information on the PNAS web site). In contrast, GRK6 KO cardiomyocytes, like β-arrestin2 KO cardiomyocytes, display augmentations in percent fractional shortening in response to ANG, but not to SII [Fig. 5C, SII versus basal (P > 0.05), ANG versus SII (P < 0.05); Fig. 5D, ANG versus SII (P < 0.05)]. Similar data were obtained for −dL/dtmax and +dL/dtmax (Fig. 9). In contrast to the effects of GRK6 deficiency, cardiomyocytes from GRK2+/− mice exhibited enhanced percent fractional shortening in response to both ANG and SII (Fig. 5 E and F), and surprisingly, increased responsiveness to SII in comparison to ANG [Fig. 5E, ANG versus SII (P < 0.05); Fig. 5F, ANG versus SII (P < 0.05)]. These data demonstrate that positive inotropic and lusitropic effects mediated by G protein-independent signaling via the AT1AR require GRK6, but not GRK5. Furthermore, these data are consistent with our findings in HEK293 cells that GRK6 is the predominant GRK isoform required for SII-induced β-arrestin2 recruitment to the AT1AR (see Fig. 2). Moreover, GRK2 appears to negatively regulate β-arrestin-dependent signaling via the AT1AR, thereby controlling the balance between G protein-dependent and -independent signaling.
Fig. 5.
Effects of deficiency of specific GRK isoforms on changes in systolic cardiomyocyte function in response to ANG and SII. (A and B) GRK5 KO cardiomyocytes display positive inotropic responses to both ANG and SII. Fractional shortening of cardiomyocytes from GRK5 KO mice (n = 5 animals) under conditions of pacing alone (Basal), or additional exposure to 10 μM ANG or SII as indicated. (A) Absolute values for each variable under indicated stimulation conditions. (B) Percent change in each variable in response to ANG or SII, relative to basal. (C and D) GRK6 KO cardiomyocytes display severely defective positive inotropic responses to SII, but unaffected responses to ANG. Fractional shortening of cardiomyocytes from GRK6 KO mice (n = 5 animals) under conditions of pacing alone (Basal), or additional exposure to 10 μM ANG or SII as indicated are shown. (C) Absolute values for each variable under indicated stimulation conditions. (D) Percent change in each variable in response to ANG or SII, relative to basal. (E and F) GRK2 heterozygous KO (+/−) cardiomyocytes display augmented positive inotropic responses to SII. Fractional shortening of cardiomyocytes from GRK2+/− mice (n = 5 animals) under conditions of pacing alone (Basal), or additional exposure to 10 μM ANG or SII as indicated. (E) Absolute values for each variable under indicated stimulation conditions. (F) Percent change in each variable in response to ANG or SII, relative to basal. ∗, P < 0.05 by one-way ANOVA with post hoc Bonferroni test relative to basal, and between ANG and SII when relevant (A, C, and E; in C, the asterisk for ANG thus represents significance relative to both basal and SII, whereas in A and E, the asterisk for either ANG or SII represents significance relative to basal), and by Student's paired t test between ANG and SII (B, D, and F); ∗∗, P < 0.05 by ANOVA with post hoc Bonferroni test for SII relative to both basal and ANG (E only). Data displayed are mean ± SEM. See Materials and Methods for experimental details.
Discussion
All of the physiologic functions of 7TMR signal transduction have traditionally been attributed to activation of heterotrimeric G proteins, historically the first identified effectors of 7TMRs (2). However, the recently appreciated existence of G protein-independent signal transduction by 7TMRs indicates that this conventional understanding must be reassessed. In our studies of adult mouse cardiomyocytes expressing endogenous cell surface receptors, we found that the AT1AR mediates the augmentation of contraction and relaxation in mouse cardiomyocytes by distinct pathways: one, G protein-dependent (presumably, Gαq) and involving PKC, and two, in which the signal is carried by β-arrestin2 and GRK6.
We observed that the effects of ANG on isolated cardiomyocyte function are predominantly mediated via the Gαq/PKC pathway. However, ≈30% of ANG-mediated cardiomyocyte functional responses were insensitive to PKC inhibition. This component of ANG-induced effects may be β-arrestin-mediated (13). One might expect that in the absence of PKC-mediated signaling, ANG would have been able to fully engage the β-arrestin mediated mechanism, to the same extent as SII. As shown in Fig. 3A, this is not the case. Although the explanation for this finding is not clear, this may reflect distinct conformational states of the AT1AR induced by the two ligands. The apparent lack of impairment in ANG-induced positive inotropic and lusitropic responses in β-arrestin2 KO (and GRK6 KO) mice may be due to robust activation of Gαq-dependent signaling pathways by the high concentrations of ANG used in these experiments, which compensates for the deficiency in activation of β-arrestin-dependent pathways. Finally, the positive inotropic and lusitropic effects of SII also indicate that β-arrestin-mediated signaling is sufficient for the AT1AR to modulate cardiomyocyte function. Thus, activation of either the Gαq-dependent or GRK/β-arrestin-dependent signaling pathways alone appears to be sufficient to mediate full effects on myocardial function in the setting of optimal AT1AR stimulation by either conventional or “biased” agonist ligands.
The molecular mechanisms by which β-arrestin2 promotes an AT1AR-mediated inotropic effect on cardiomyocytes are currently unknown. Broadly, two possibilities exist: (i) coupling of β-arrestin to the regulation of cytosolic [Ca2+], and/or (ii) coupling of β-arrestin to myofilament proteins. These two possibilities encompass the factors governing myocardial contractile function at the molecular level. The first mechanism suggests Ca2+ channel/pump proteins as possible β-arrestin-dependent effectors and/or interaction partners. These include the l-type Ca2+ channel, IP3 receptor, ryanodine receptor, phospholamban, and SERCA. Alternatively, myofilament proteins that are known to be involved in regulating sensitivity to Ca2+, such as troponin and myosin-binding protein C, may be effectors or interaction partners of β-arrestin. Further understanding of the relevant mechanisms will require studies examining β-arrestin interaction with such proteins, or the role of β-arrestin in regulating their functions.
Our studies were conducted on cardiomyocytes from healthy mice that had not undergone any cardiovascular manipulations. However, under pathophysiologic conditions, the roles of G protein-dependent and -independent signal transduction via the AT1AR and other 7TMRs may be considerably different. Numerous lines of evidence suggest that chronic activation of either Gαs or Gαq, the primary Gα proteins to which many myocardial 7TMRs couple, results in deleterious effects on ventricular function in several experimental systems, and even clinically (29). With respect to the AT1AR, overexpression of Gαq results in ventricular dysfunction (30), whereas inhibition of Gαq by transgenic overexpression of an inhibitory peptide prevents high afterload-induced left ventricular dysfunction (31) and hypertrophy (32). In contrast, G protein-independent, GRK/β-arrestin-dependent signaling has been shown to be cytoprotective in several cellular systems (33–35), and thus might be beneficial to myocardial function, especially if sustained chronically. Supporting this notion, in the setting of overexpression of a Gαq-uncoupled mutant AT1AR in the myocardium of mice, cardiomyocytes display reduced cell death in comparison to cardiomyocytes from mice that overexpress the WT AT1AR (6).
Isolated cardiomyocyte function and ventricular function in vivo have been shown to correlate in a variety of experimental studies (19, 36). Thus, our work raises the possibility of developing drugs for 7TMRs that selectively signal in a β-arrestin-dependent fashion. For example, in either the acute or chronic setting, agents such as SII, which are null with respect to activation of Gαq, will competitively antagonize ANG-mediated Gαq activation; they are thus ARBs. However, they still retain the ability to carry β-arrestin-dependent signals from the AT1AR to mediate effects on cardiomyocyte function; thus, they are simultaneously “biased agonists” with respect to β-arrestin-dependent signaling. These features distinguish such drugs from the conventional antagonists of many 7TMRs currently used in clinical practice (e.g., βAR blockers, ARBs, etc.), which nonselectively inhibit all signals from 7TMRs. Based on the biochemical evidence implicating β-arrestins in cytoprotective signaling through activation of effectors such as ERK (33), PI-3-Kinase (34), and Akt (34, 37), these ligands may function as an entirely unique class of drugs, “super receptor blockers,” agents that bifunctionally antagonize cytotoxic G protein-mediated signaling while actively inducing cytoprotective GRK/β-arrestin-dependent signaling.
Materials and Methods
Reagents.
ANG (Sigma, St. Louis, MO) and SII (Cleveland Clinic Core Synthesis Facility, Cleveland, OH) were dissolved in PBS without calcium and magnesium to concentrations of 1 mM before use in cardiomyocyte functional assays; final concentrations in these assays were 10 μM for each drug. The PKC inhibitor Ro-31–8425 (Calbiochem, San Diego, CA) was dissolved in DMSO, and the final concentration in assays was 1 μM. The ARB valsartan was dissolved in 100% ethanol to a concentration of 20 mM, and used at a final concentration of 50 μM.
Calcium Fluorimetry.
Neonatal rat atrial cardiomyocytes were isolated and cultured as described (27). Cells were loaded with the dye Fura-2 (as per manufacturers instructions, Invitrogen, Carlsbad, CA), and treated with either ANG (100 nM) or SII (10 μM), in the absence or presence of 50 μM valsartan pretreatment. The instantaneous 340/380 nm excitation ratio for Fura-2 was calculated and plotted as a function of time.
FRET Assays.
Plasmids.
β-arrestin2-mYFP has been described (38). Rat AT1AR was amplified by PCR to encode 5′ HindIII and 3′ XhoI restriction sites, with the termination codon replaced to encode a diglycine linker. The PCR product was cut, purified, and ligated into a pcDNA3.1-mCFP vector (39) to generate AT1AR-mCFP.
siRNA silencing of gene expression.
Chemically synthesized siRNA duplexes with 3′ dTdT overhangs were obtained from Dharmacon (Lafayette, CO) for GRK2 and GRK6, transfected into HEK293 cells by using Gene Silencer (Gene Therapy Systems, San Diego, CA), and efficiency of GRK silencing was validated by immunoblotting, as described (10, 11).
Imaging.
Details of imaging protocols have been described (38).
Real-time GRK activity assay.
HEK293 cells stably transfected with AT1AR-mCFP and β-arrestin2-mYFP were used to measure GRK functional activity by quantifying the rate and extent of either ANG (100 nM) or SII (10 μM)-stimulated β-arrestin association with the AT1AR as measured by FRET, as described for the β2-AR (38). See Supporting Text, which is published as supporting information on the PNAS web site, for more detail.
Animals.
All animals used in these studies were adult male mice of 8–20 weeks of age. All mouse strains were back-crossed to the C57BL/6 background ≥10 generations. Animals were handled according to approved protocols and animal welfare regulations of the Institutional Review Board at Duke University Medical Center. In addition to WT C57BL/6 mice, the following gene-targeted deficient [homozygous or heterozygous knockout (KO), as indicated] mouse strains were used, all of which have been described: AT1AR KO (40), β-arrestin1 KO (24), β-arrestin2 KO (23), GRK2 heterozygous KO (+/−) (22), GRK5 KO (26), and GRK6 KO (25).
Cardiomyocyte Isolation and Functional Assays.
Adult mouse cardiomyocyte isolation, experimentation, and analysis from the aforementioned strains were performed as described (19). See Supporting Text for more detail.
Statistical Analyses.
Statistical analyses performed were one-way ANOVA with post hoc Bonferroni tests, or Student's paired t tests. A P value of <0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Kristine Hesser Porter of the Rockman laboratory for technical expertise in conducting the myocyte experiments and Dr. Marc G. Caron (Duke University, Durham, NC) for providing GRK2 heterozygous knockout mice. This work was supported in part by National Institutes of Health Grants HL16037 and HL70631 (to R.J.L.) and HL075443 (to H.A.R.). R.J.L. is an Investigator with the Howard Hughes Medical Institute.
Abbreviations
- 7TMR
seven-transmembrane receptor
- GRK
G protein-coupled receptor kinase
- ANG
angiotensin II
- AT1AR
ANG type 1A receptor
- SII
([Sar1, Ile4, Ile8])-ANG
- ARB
angiotensin receptor blocker.
Footnotes
The authors declare no conflict of interest.
References
- 1.Rees S, Morrow D, Kenakin T. Receptors Channels. 2002;8:261–268. [PubMed] [Google Scholar]
- 2.Gilman AG. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ, Shenoy SK. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 4.Lefkowitz RJ, Whalen EJ. Curr Opin Cell Biol. 2004;16:162–168. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Rockman HA, Koch WJ, Lefkowitz RJ. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 6.Zhai P, Yamamoto M, Galeotti J, Liu J, Masurekar M, Thaisz J, Irie K, Holle E, Yu X, Kupershmidt S, et al. J Clin Invest. 2005;115:3045–3056. doi: 10.1172/JCI25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Proc Natl Acad Sci USA. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. J Biol Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 9.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 13.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. J Clin Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin FT, Daaka Y, Lefkowitz RJ. J Biol Chem. 1998;273:31640–31643. doi: 10.1074/jbc.273.48.31640. [DOI] [PubMed] [Google Scholar]
- 16.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeFea KA, Zalevsky J, Thoma MS, Dery O, Mullins RD, Bunnett NW. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Barki-Harrington L, Luttrell LM, Rockman HA. Circulation. 2003;108:1611–1618. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- 20.Lefroy DC, Crake T, Del Monte F, Vescovo G, Dalla Libera L, Harding S, Poole-Wilson PA. Am J Physiol. 1996;270:H2060–H2069. doi: 10.1152/ajpheart.1996.270.6.H2060. [DOI] [PubMed] [Google Scholar]
- 21.Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Proc Natl Acad Sci USA. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr., Lefkowitz RJ, Caron MG, Giros B. Proc Natl Acad Sci USA. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 24.Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. Circ Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 25.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Proc Natl Acad Sci USA. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT. Neuron. 1999;24:1029–1036. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- 27.Mohler PJ, Gramolini AO, Bennett V. J Biol Chem. 2002;277:10599–10607. doi: 10.1074/jbc.M110958200. [DOI] [PubMed] [Google Scholar]
- 28.Ahn S, Wei H, Garrison TR, Lefkowitz RJ. J Biol Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 29.Adams JW, Brown JH. Oncogene. 2001;20:1626–1634. doi: 10.1038/sj.onc.1204275. [DOI] [PubMed] [Google Scholar]
- 30.D'Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., Jr Proc Natl Acad Sci USA. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 32.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 33.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Povsic TJ, Kohout TA, Lefkowitz RJ. J Biol Chem. 2003;278:51334–51339. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- 35.Revankar CM, Vines CM, Cimino DF, Prossnitz ER. J Biol Chem. 2004;279:24578–24584. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- 36.Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. J Biol Chem. 1998;273:18180–18184. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- 37.Goel R, Phillips-Mason PJ, Raben DM, Baldassare JJ. J Biol Chem. 2002;277:18640–18648. doi: 10.1074/jbc.M108995200. [DOI] [PubMed] [Google Scholar]
- 38.Violin JD, Ren XR, Lefkowitz RJ. J Biol Chem. 2006;281:20577–20588. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- 39.Violin JD, Zhang J, Tsien RY, Newton AC. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.