Abstract
Synaptic vesicle (SV) exocytosis mediating neurotransmitter release occurs spontaneously at low intraterminal calcium concentrations and is stimulated by a rise in intracellular calcium. Exocytosis is compensated for by the reformation of vesicles at plasma membrane and endosomes. Although the adaptor complex AP-3 was proposed to be involved in the formation of SVs from endosomes, whether its function has an indirect effect on exocytosis remains unknown. Using mocha mice, which are deficient in functional AP-3, we identify an AP-3-dependent tetanus neurotoxin-resistant asynchronous release that can be evoked at hippocampal mossy fiber (MF) synapses. Presynaptic targeting of the tetanus neurotoxin-resistant vesicle soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) tetanus neurotoxin-insensitive vesicle-associated membrane protein (TI-VAMP) is lost in mocha hippocampal MF terminals, whereas the localization of synaptobrevin 2 is unaffected. In addition, quantal release in mocha cultures is more frequent and more sensitive to sucrose. We conclude that lack of AP-3 results in more constitutive secretion and loss of an asynchronous evoked release component, suggesting an important function of AP-3 in regulating SV exocytosis at MF terminals.
Keywords: hippocampus, neurotransmitter release, tetanus neurotoxin
The release of a neurotransmitter at the synapse requires the fusion and recycling of synaptic vesicles (SVs). The fusion of SVs with the plasma membrane depends on the formation of soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes between vesicle SNAREs and plasma membrane SNAREs, as demonstrated by the striking sensitivity of neurotransmitter release to clostridial neurotoxins, particularly tetanus neurotoxin (TeNT; for a review, see ref. 1). Several models of the recycling of SVs have been proposed: endosomal recycling, SV budding from the plasma membrane (2), and kiss-and-run and kiss-and-stay (for a review, see ref. 3). Endosomal recycling involves the molecular coat AP-3, as suggested from experiments in neuroendocrine cells (4), but the importance of AP-3 in neurotransmitter release is still unclear. AP-3 is composed of four subunits, and two different AP-3 complexes are expressed in brain, the ubiquitous AP-3A, composed of the δ, σ3, β3A, and μ3A subunits; and the neuronal-specific AP-3B, composed of the δ, σ3, β3B, and μ3B subunits (5, 6). mocha mice are deficient for the δ subunit and therefore lack both AP-3A and AP-3B complexes. These mice have neurological disorders, including hyperactivity and spontaneous seizures. In this paper, we set out to understand the importance of AP-3 function in neurotransmitter release by characterizing basal and evoked neurotransmitter release in mocha mice.
Results
Asynchronous Release Evoked at Mossy Fiber (MF) Terminals Is Lost in mocha Mice.
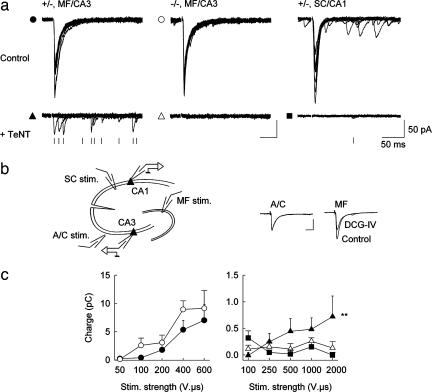
AP-3 is particularly concentrated in the hilus and CA3 region of the hippocampus in heterozygous control (+/−) mice (Fig. 5a, which is published as supporting information on the PNAS web site)]. Therefore, to assess the role of AP-3 in neurotransmitter release, we compared synaptic transmission at MF-CA3 synapses in organotypic hippocampal cultures from mocha (−/−) and heterozygous control (+/−) littermates (Fig. 1a). We first examined Ca2+-dependent release evoked by MF stimulation. A stimulating electrode placed at the hilar border of the granule cell layer reliably evoked large postsynaptic currents (PSCs) that were specifically suppressed by the group 2 metabotropic glutamate receptor agonist DCG-IV (Fig. 1b; ref. 7). The amplitude of the PSCs gradually increased with stimulation intensities ranging from 15 to 600 V·μs. The correlation between the average charge of the PSCs and the stimulation intensity was not significantly different in cultures prepared from control vs. mocha mice (Fig. 1c). Cleavage of the SV SNARE synaptobrevin 2 (Syb2) by preincubation of the cultures with TeNT for >72 h caused a dramatic reduction in transmitter release evoked by MF stimulation. In cultures prepared from control mice, stimuli up to 2,000 V·μs evoked small unreliable PSCs, reminiscent of evoked transmission in cultured hippocampal neurons from Syb2 knockout mice (8). Release at stimulated synapses was usually asynchronous and occurred within ≈200 ms after stimulation with an average probability of 0.26 ± 0.05 at the highest stimulation intensities (n = 7). In contrast, no PSC could be evoked by MF stimulation in cultures prepared from mocha littermate mice.
Fig. 1.
Evoked synaptic transmission in slice cultures prepared from control and mocha mice. (a) PSCs evoked in CA3 pyramidal cells by MF stimulation. Ten superimposed sample traces are shown for maximal stimulation intensity. (Upper) Control cultures; (Lower) cultures treated for at least 72 h with TeNT (500 ng/ml). After TeNT treatment, unreliable asynchronous responses (marked with a tick) can be detected in control (+/−) but not mocha (−/−) cultures. EPSCs evoked by Schaffer collateral stimulation in CA1 cells are also completely abolished by prior incubation with TeNT. (b) Stimulation arrangement. MF were stimulated with a patch pipette located at the hilar border of the granule cell layer (MF stim.). Associational/commissural fibers (A/C) were stimulated in stratum oriens. Responses to either stimulation were distinguishable by their sensitivity to DCG-IV (1 μM). (c) Comparison of responses (integrated over 200 ms of the stimulus artifact) evoked by increasing MF stimulations in control vs. mocha cultures or by SC stimulation in CA1 cells. (Left) in control cultures, no significant difference could be detected between control cells (filled circles) and mocha CA3 cells (open circles, n = 4 and 7 cells, respectively; two-way ANOVA; P > 0.05). (Right) After treatment with TeNT, responses were evoked in control CA3 cells (filled triangles) but not in mocha CA3 cells (open triangles) or control CA1 cells (filled squares, n = 7, 11, and 5 cells, respectively; two-way ANOVA; P < 0.02 for control CA3 vs. mocha CA3 or control CA1 and P > 0.05 for mocha CA3 vs. control CA1).
Loss of Presynaptic TI-VAMP in MF Terminals.
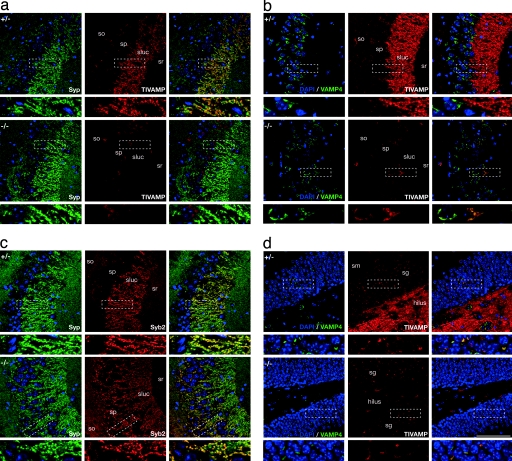
Our observations may reflect the contribution of a TeNT-insensitive AP-3-dependent pathway of transmitter release at MF terminals. We have shown that tetanus neurotoxin-insensitive vesicle-associated membrane protein (TI-VAMP) (i) is present at a high level in SVs at MF terminals (9), (ii) interacts with the δ subunit of the AP-3 complex, and (iii) is mistargeted in mocha fibroblasts (10). TI-VAMP is therefore the best candidate v-SNARE to support TeNT-resistant vesicle exocytosis at MF terminals. Because TI-VAMP is not expressed at Schaffer collateral terminals onto rat CA1 pyramidal cells (ref. 9; Fig. 5a), we anticipated this form of exocytosis may not be observed at these synapses. Consistent with this prediction, evoked release was entirely impaired by TeNT at Schaffer collateral synapses onto CA1 pyramidal cells in control mice (Fig. 1 a and b). We therefore compared TI-VAMP localization in hippocampal sections from control and mocha mice. In the CA3 and dentate gyrus areas of control mice, TI-VAMP was localized in the MF terminals, as demonstrated by its colocalization with the presynaptic marker synaptophysin (Syp, Figs. 2a and 5a). This was further confirmed in cultured granule cells, because we found that 67.20 ± 6.65% of TI-VAMP positive punctae were also Syp-positive (Fig. 6a, which is published as supporting information on the PNAS web site). However, in mocha sections, TI-VAMP labeling in MFs was completely lost (Figs. 2a and 5b). Thus, AP-3 is required for the presynaptic targeting of TI-VAMP to MF terminals. In contrast, Syb2 localization was unchanged in brain sections from mocha mice as compared with control (Fig. 2b), suggesting that the presynaptic targeting of Syb2 is independent of AP-3. Other SV proteins, including Rab3a, synaptotagmin 1, showed a presynaptic targeting similar in control and mocha mice (Fig. 5b and L.D. and T.G., unpublished observations). Neurite outgrowth and TI-VAMP localization to the growth cone were unaffected in differentiating mocha neurons (see Supporting Text and Fig. 7, which are published as supporting information on the PNAS web site). Together, these results suggest that a form of TeNT-insensitive AP-3-dependent evoked release exists at MF-CA3 synapses, which likely involves TI-VAMP as a v-SNARE.
Fig. 2.
TI-VAMP is absent from MF terminals and accumulates in the cell body of mocha neurons. Fifty-micrometer vibratome sections from adult control (+/−) and mocha (−/−) mice were labeled with DAPI (blue) and monoclonal antibodies against TI-VAMP or Syb2, polyclonal antibodies against Syp or VAMP4. (a) TI-VAMP is lost from the terminals of granule cells (mossy synapses) innervating CA3 pyramidal cells in mocha brain. A dense signal for Syp (green) is detected throughout the CA3 region in stratum oriens (so), stratum pyramidale (sp), and stratum radiatum (sr) in control (+/−) hippocampus. The strong immunofluorescence presented in the stratum lucidum (sluc) corresponds to MF terminals innervating CA3 pyramidal dendrites. Lower images are magnifications of the boxed windows. In control (+/−) mice, confocal images show that TI-VAMP (red) immunoreactivity colocalizes in mossy synapses with Syp puncta in sluc. However, in mocha (−/−) mice, TI-VAMP is not detected in presynaptic terminals (sluc) of granule cells, whereas a faint immunofluorescence is detected in pyramidal cell soma (boxed window in a and c). (b) Syb2 localization is unaffected in the mocha hippocampus. As for Syp immunoreactivity, Syb2 is detected in control and mocha mice throughout so, sp, sluc, and sr. (c and d) TI-VAMP is mislocalized in the granule cells of mocha mice. In control mice, TI-VAMP is present in MF in both the lucidum (c) and the hilus of the dentate gyrus (d). In mocha mice, the strong labeling of the lucidum and hilus in the dentate gyrus is not present. Double labeling of TI-VAMP and VAMP4 shows that the remaining TI-VAMP colocalizes with VAMP4 in the cell bodies of pyramidal (sp) and granule cells [stratum granulosum (sg)]. (Scale bar, 100 μm.)
TI-VAMP Is Blocked in the Cell Body of mocha Neurons.
We then examined the subcellular localization of TI-VAMP in mocha hippocampal granule cells from which MFs originate. We found that TI-VAMP accumulated in the cell bodies of granule cells, as shown by colocalization with VAMP4, a vesicular SNARE located in early endosomes (11, 12) and the trans-Golgi network (ref. 13; Fig. 2d). However, the AP-3-dependent sorting of TI-VAMP in the perinuclear VAMP4-positive compartment may not be specific to granule cells, because a strong colocalization of TI-VAMP and VAMP4 was also found in the mocha CA3 pyramidal cells (Fig. 2c). The accumulation of TI-VAMP in cell bodies in mocha neurons was also observed in cultured hippocampal pyramidal neurons by immunolabeling (Fig. 6b), as well as immunogold labeling in ultrathin cryosections analyzed by electron microscopy (Fig. 6c). TI-VAMP labeling in mocha neurons was found restricted to the cytosolic side of Golgi cisternea, whereas in control pyramidal neurons, TI-VAMP labeled vesicles and the plasma membrane, consistent with its function as a secretory v-SNARE in control but not mocha neurons. Therefore, an AP-3-dependent sorting of TI-VAMP at the level of a VAMP4-positive perinuclear compartment is required for the proper targeting of TI-VAMP.
Increased Basal Release in Mocha and Brefeldin A (BFA)-Treated MF Terminals.
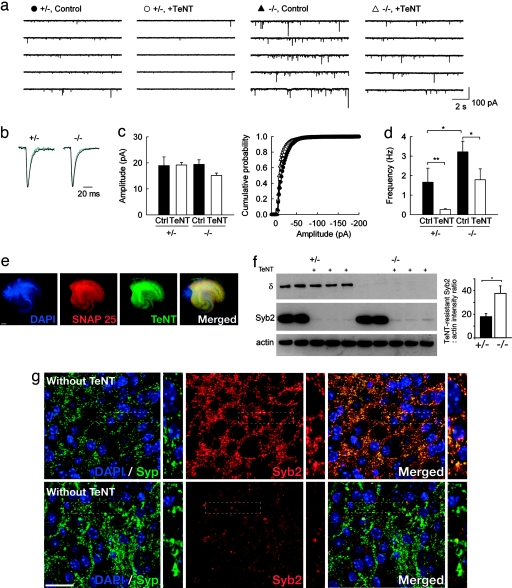
We then asked whether the lack of AP-3 and TI-VAMP may impact Ca2+-independent constitutive release at MF terminals from mocha mice. Miniature excitatory PSCs (mEPSCs) were recorded from CA3 pyramidal cells in slice cultures prepared from control and mocha littermates. In cultures from mocha mice, the frequency of mEPSCs was ≈2-fold higher than in control cultures (3.22 ± 0.55 vs. 1.66 ± 0.72 Hz, P < 0.03; Fig. 3a). However, their mean amplitude was unchanged (19.44 ± 1.73 vs. 18.97 ± 3.32 pA, P = 0.91), as were their rise time (10–90% of peak, 1.59 ± 0.09 vs. 1.45 ± 0.19 ms, P = 0.32) and decay time constant (3.86 ± 0.12 vs. 3.37 ± 0.24 ms, P = 0.14; Fig. 3 b and c). These results suggest the lack of AP-3 did not alter the rate of fusion pore opening or the number of postsynaptic receptors at excitatory synapses onto CA3 pyramidal cells. In addition, the higher mEPSC frequency in mocha cultures was not affected by the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (A.S. and J.-C.P., unpublished observations), suggesting it did not reflect a greater activation of presynaptic NMDA receptors (14, 15) because of the lack of Zn release from MF terminals in mocha mice (16, 17). After 72-h incubation with TeNT, mEPSC frequency in control cultures was reduced by ≈84% (to 0.27 ± 0.04 Hz, n = 9; Fig. 3d), with no apparent change in their mean amplitude (19.18 ± 0.99 vs. 18.97 ± 3.32 pA, P = 0.89), again consistent with observations in cultured hippocampal neurons from Syb2 knockout mice (8). In contrast, quantal release in mocha cultures was more resistant to TeNT and was reduced in frequency by only ≈44% (to 1.79 ± 0.56 Hz, n = 8). These observations suggest the absence of AP-3 not only increases Ca-independent quantal release but also reduces the effect of TeNT. Apart from TI-VAMP and Syb2, no other v-SNARE protein is known to be present at excitatory synapses on CA3 pyramidal cells. Therefore, we analyzed the penetration and cleavage efficiency of TeNT in the slice cultures. We labeled TeNT-treated slice cultures with DAPI and antibodies against TeNT and SNAP-25. We found that TeNT penetrated throughout mocha (Fig. 3e) as well as control explants (L.D. and T.G., unpublished observations). Furthermore, although the vast majority of Syb2 was cleaved in control and mocha explants after a 72-h incubation with TeNT, a small fraction of Syb2 was still detected by Western blotting. Quantification of the blots revealed twice as much TeNT-resistant Syb2 protein in mocha cultures as compared with control (Fig. 3f). Treatment with TeNT drastically reduced Syb2 labeling, but the remaining signal corresponded largely to Syb2 present at synapses, as revealed by colocalization with Syp (Fig. 3g); thus the numerous TeNT-resistant mEPSCs observed in mocha are likely to be mediated by a presynaptic pool of Syb2 that resisted the treatment with TeNT.
Fig. 3.
Ca-independent quantal release at excitatory synapses on CA3 cells in control and mocha cultured slices. (a) Representative traces of mEPSCs recorded in CA3 pyramidal cells from control (+/−) and mocha (−/−) cultures, treated or not with TeNT. (b) Averaged mEPSCs (≈100) detected from the above recordings. Black traces, control; blue traces, after TeNT treatment. No difference in either their rate of onset or decay was apparent. (c) (Left) Average amplitude of mEPSCs recorded in all four conditions. No significant difference was observed (n = 7, 9, 11, and 8 cells, respectively; P > 0.05). (Right) Cumulative amplitude histograms from the same four data sets. The distributions were not significantly different (Kolmogorov–Smirnov test, P > 0.05). (d) Mean frequencies of mEPSCs were significantly different between control and TeNT-treated cultures in both control and mocha cultures (P < 0.005 and P < 0.05, respectively). mEPSC frequency was also different in control vs. mocha culture in the absence of TeNT (P < 0.05). (e) mocha culture slices treated with TeNT for 72 h were fixed and labeled with antibodies against SNAP25 (red), TeNT (green), and DAPI (blue). The whole surface of the explant can be visualized by either DAPI (nucleus) or SNAP25 (neuronal plasma membrane). Note that TeNT staining is uniformly distributed, confirming the extended penetration of the toxin. (Scale bar, 200 μm.) (f) Culture slices used in electrophysiological recordings were lysed and analyzed by Western blotting with antibodies against AP-3δ, Syb2, and actin (as a loading control). A 72-h treatment with TeNT resulted in efficient cleavage of Syb2, although quantification of the remaining Syb2 revealed a 2-fold increase in TeNT-resistant Syb2 in mocha slices [mean ± SEM, control 17.83 ± 2.35, n = 6; mocha 37.58 ± 6.31, n = 6, P < 0.015 (Mann–Whitney rank sum test)]. (g) mocha-cultured slices treated with or without TeNT for 72 h were fixed and labeled with antibodies against Syp (green), Syb2 (red), and DAPI (blue). Note that the remaining Syb2 labeling after TeNT treatment is very faint. The rare remaining Syb2 puncta are mainly synaptic. (Scale bar, 50 μm.)
Newly formed vesicles derived from endosomes disappear with a half life of ≈36 min after treatment with the fungal drug BFA, which specifically targets the AP-3-dependent pathway in PC12 cells (4, 18–20). To test the effect of acute inactivation of the AP-3 pathway, we therefore incubated control hippocampal slices with BFA for ≈2–4 h. In BFA-treated slices, mEPSC frequency was increased by ≈127%, as compared with control (2.59 ± 0.33 vs. 1.14 ± 0.25 Hz, n = 14 and 12 cells, respectively; P < 0.005; Fig. 8 a–c, which is published as supporting information on the PNAS web site). The ratio of synaptic TI-VAMP/synaptic Syp measured by immunolabeling was not significantly altered by BFA neither in the hilus nor in the stratum lucidum (Fig. 8d). This is consistent with the fact that longer times may be required to clear TI-VAMP from MF terminals and demonstrate that the mocha phenotype can be reproduced by acute pharmacological inactivation of AP-3-dependent SV formation.
Increased Sensitivity of Release to Osmotic Stimulation in mocha MF Terminals.
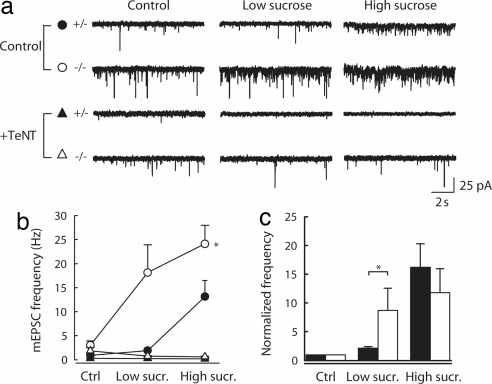
Syb2 is resistant to TeNT (21) in SNARE complexes (21) that may be clamped by complexin and synaptotagmin before calcium rise (22). TeNT-resistant Syb2 was associated with release-competent SVs (23, 24). Our previous results could suggest that the lack of AP-3 and TI-VAMP in MF SVs may thus increase the capacity of Syb2 to form TeNT-resistant clamped SNARE complexes, thereby enhancing the probability of calcium-independent fusion at mocha MF terminals. To test this hypothesis, we examined the rate of release induced by focal application of a hypertonic solution, because previous studies showed that hypertonic solution specifically recruits readily releasable quanta at hippocampal synapses (25) and stimulates secretion in a calcium independent manner. We thus compared the effects of focal applications of a 0.5 M sucrose solution through a patch pipette positioned in stratum lucidum ≈25 μm away from the somata of the recorded pyramidal cells. Because effective sucrose concentration at release sites may be difficult to control in slice cultures, we used varying injection pressures to compare the sucrose sensitivity of release in mocha vs. control cultures. In cells recorded from control cultures, application of sucrose with low pressure (0.25 psi) caused a ≈2-fold increase in mEPSC frequency, whereas at a higher pressure (1.5 psi), a further ≈7-fold increase was observed (Fig. 4). The sensitivity of the release rate to sucrose was significantly increased in recordings from mocha cultures; even low-pressure application of sucrose caused a ≈9-fold increase in mEPSC frequency, which was further enhanced by another ≈35% at high pressure (Fig. 4). Interestingly, however, recruitment of readily releasable vesicles by sucrose was disrupted in both control and mocha cultures by prior incubation with TeNT, and application of hypertonic solution failed to produce an increase in mEPSC frequency even at high pressure (Fig. 4 a and b). Taken together, these results suggest that an AP-3-dependent mechanism decreases the sucrose sensitivity of constitutive secretion.
Fig. 4.
Differential recruitment of readily releasable vesicles by hypertonic sucrose applications in control vs. mocha cultured slices. (a) Sample traces from individual recordings of mEPSCs recorded in the presence of tetrodotoxin and an external Ca/Mg ratio of 0.1 before and during application of a 500 mOsm sucrose solution in the presence of a 2.5 ml/min normal artificial cerebrospinal fluid perfusion. High sucrose was applied by pressure injection through a patch pipette to the proximal dendrites of the recorded cell in stratum lucidum. Low sucrose, ≈0.25 psi; high sucrose, 1.5 psi. (b) Average frequency of mEPSCs recorded in all conditions. The sensitivity of mEPSC frequency to sucrose was significantly different in control vs. mocha cultures (n = 4 and 6 cells, respectively; two-way ANOVA, P < 0.01). After treatment with TeNT, sucrose application failed to increase mEPSC frequency (n = 9 and 7 cells, respectively). (c) Frequencies were normalized to control values, showing a greater relative increase in mocha (open bars) compared with control slices (filled bars) upon low-pressure (Mann–Whitney, P < 0.05) but not high-pressure application of sucrose (P = 0.5).
Discussion
mocha mice are deficient for AP-3δ subunit and therefore lack both ubiquitous AP-3A and neuronal AP-3B complexes. Here, we have shown that presynaptic TI-VAMP, a well established AP-3δ cargo (10, 26), is lost in mocha CA3 MFs. Other AP-3 cargoes, including the zinc transporter ZnT-3 (16, 17), the chloride channel ClC-3 (27), and the phosphatidylinositol-4-kinase type IIα (28), are mislocalized in mocha CA3 MFs. A previous study in ZnT3 knockout mice showed the lack of vesicular zinc in MFs does not significantly affect the MF-associated excitability of CA3 pyramidal cells (29). In addition, we observed that the higher mEPSC frequency in mocha cultures was not affected by blocking NMDA receptors, further suggesting that the lack of Zn release from MF terminals in mocha mice does not explain the phenotype we observed. Similarly, the lack of MF ClC-3 is unlikely to explain the increased quantal release from mocha MF terminals mice. The loss of CIC-3 rather affects acidification of SVs resulting in a slight reduction of quantal size (30). Finally, recent data suggested AP-3 may regulate the volume of large dense-core vesicles in chromaffin cells (31). This, however, is unlikely to apply to MF terminals, because (i) we did not observe any change in quantal size in mocha cultures, and (ii) the pathway of SV reformation largely differs from that of secretory granules, the latter maturing by removal of material from immature secretory granules. Treatment of hippocampal slice cultures with TeNT revealed an asynchronous component of secretion evoked in CA3 pyramidal cells by single stimulation of MFs. This asynchronous release is unlikely to be mediated by the low amount of TeNT-resistant Syb2, because it was not observed in mocha cultures, which showed more TeNT-resistant Syb2. Furthermore, this asynchronous component was observed at MF-CA3 but not CA1–CA3 synapses, where TI-VAMP is not expressed. Because no other TeNT-resistant v-SNARE was ever detected at MF terminals, we suggest TI-VAMP likely mediates the asynchronous evoked release unraveled in our experiments. In conclusion, although we cannot exclude that other AP-3 cargoes lost in mocha MF terminals may participate to the mocha phenotype, the loss of TI-VAMP seems most likely to explain the perturbation of evoked SV release described in the present study.
Asynchronous release was not observed in TeNT-untreated explants, suggesting inactivation of Syb2 may be required for the expression of this asynchronous evoked release. This observation strongly suggests TI-VAMP and Syb2 may both be present on the same rather than distinct SVs. Consistent with this scenario, Syb2 was shown to have a higher rate of SNARE complex assembly than TI-VAMP both in vitro and in vivo (10), predicting that evoked release mediated by TI-VAMP would be detected only after cleavage of Syb2. In addition, cleavage of Syb2 by TeNT was shown to modify the coupling of intracellular calcium and release-competent vesicles (32), suggesting that removal of TeNT-sensitive v-SNAREs allows for the expression of a secretory machinery that may be hard to observe otherwise. Interestingly, Sr+ preferentially stimulates asynchronous release (33), and different synaptotagmin isoforms show different sensitivities to Ca2+ and Sr+ (34). For instance, Synaptotagmin 1 and 7 have different sensitivities to calcium (35), the latter interacting with TI-VAMP in fibroblasts (36) and showing biochemical properties suitable for a Ca2+ sensor for asynchronous release (35, 37, 38). Therefore, we speculate that MF SVs may be equipped with two distinct fusion machineries for exocytosis, one depending on Syb2 and the other on TI-VAMP as a v-SNARE, which may be recruited in different conditions.
We have shown a higher basal calcium-independent release in mocha cultures, with no apparent change in quantal size or current kinetics. This observation strongly argues for a presynaptic difference between control and mocha MF-CA3 synapses. This is not the result of a greater density of MF terminals onto mocha CA3 pyramidal cells because of axonal sprouting induced in organotypic cultures (39) or a greater contribution of recurrent CA3-CA3 synapses to miniature EPSCs in mocha cultures. Indeed, we found an equal Syp staining in control and mocha brain sections and the increased quantal release was also observed upon pharmacological disruption of AP-3 by BFA in acute hippocampal slices. In addition, quantal release evoked by hyperosmotic solution directly applied onto MF terminals was also increased in mocha cultures. Therefore, the most likely explanation is that more vesicles are ready to fuse because of the absence of AP-3 and possibly TI-VAMP in mocha vs. control MF terminals. How AP-3-dependent sorting may decrease basal release remains to be explored.
In conclusion, our data suggest that the molecular mechanism of transmitter release at MF terminals reaches a high degree of complexity with at least two exocytic v-SNAREs, Syb2 and TI-VAMP. AP-3 function is important for both constitutive as well as evoked release, raising the possibility that specific forms of synaptic plasticity might occur at terminals expressing AP-3 cargoes like TI-VAMP (9).
Materials and Methods
Animals.
Heterozygous mocha mice were originally obtained from M. Robinson (Cambridge Institute for Medical Research, Cambridge, U.K.) and then bred in-house. The experiments were carried out in accordance with the European Community Council Directive of November 24, 1986 (86/609/EEC). All efforts were made to minimize the number of animals used and their suffering.
Immunofluorescence.
mocha and control (heterozygous littermates) 1- to 2-month-old mice were anesthetized with 35% chloral hydrate or pentobarbital and perfused through the heart with 4% paraformaldehyde. The dissected brains were fixed overnight in 4% paraformaldehyde in PBS, cut on a vibratome into 30-μm-thick sections, and processed for immunofluorescence as described (9). Confocal laser-scanning microscopy was performed by using an SP2 confocal microscope (Leica Microsystems, Mannheim, Germany). Images were assembled by using Adobe Photoshop (Adobe Systems, San Jose, CA).
Western Blotting.
SDS/PAGE analysis was performed by using 4–12% NuPAGE (Invitrogen, Cergy-Pontoise, France) gradient gels and the manufacturer's buffers and then processed for Western blotting. Blots were quantified by using ImageJ (National Institutes of Health, Bethesda, MD), and statistical significance was estimated by using Mann–Whitney rank sum tests performed under SigmaStat (SPSS, Chicago, IL)
Electrophysiological Recordings.
Organotypic hippocampal slices from 6-day-old mice were maintained in culture as described (40, 41). After 2–3 days, cultures were preincubated 24 h in serum-free medium and then grown another 3–4 days in fresh serum-free medium with or without TeNT (500 ng/ml) as indicated. Electrophysiological recordings were carried out as described in Supporting Text, which is published as supporting information on the PNAS web site. For MF stimulation, the stimulating electrode was positioned at the hilar border of the granule cell layer. For Schaffer collateral stimulation, a cut was made between areas CA3 and CA1, and the electrode was positioned in stratum radiatum ≈50–100 μm apart from the recorded cell.
Values are expressed as mean ± SEM. Statistical significance was estimated by using Mann–Whitney or Wilcoxon rank sum tests or two-way ANOVA performed under SigmaStat (SPSS). Additional details are available in Supporting Text.
Supplementary Material
Acknowledgments
We are grateful to Lucien Cabanié (Unité Mixte de Recherche 144, Institut Curie, Paris, France) for the production of Cl158.2, Mathilde Chaineau and Agathe Van der Linden for help with mocha mice, and Richard Miles for support and critical reading of the manuscript. This work was supported in part by grants from Institut National de la Santé et de la Recherche Médicale (Avenir Program), the European Commission (“Signaling and Traffic” STREP 503229), the Association Française contre les Myopathies, the Ministère de la Recherche (ACI-BDP), the Fondation pour la Recherche Médicale, the Human Frontier Science Program (RGY0027/2001-B101), and the Fondation pour la Recherche sur le Cerveau (to T.G.) R.R. was supported by a postdoctoral fellowship from the Fondation pour la Recherche Médicale, A.S. was supported by a fellowship from the Swiss National Science Foundation, and L.D. was supported by a postdoctoral fellowship from the Association pour la Recherche sur le Cancer.
Abbreviations
- SV
synaptic vesicle
- TeNT
tetanus neurotoxin
- MF
mossy fiber
- PSC
postsynaptic current
- Syb2
synaptobrevin 2
- BFA
brefeldin A
- mEPSC
miniature excitatory PSC
- Syp
synaptophysin
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- TI-VAMP
tetanus neurotoxin-insensitive vesicle-associated membrane protein.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Galli T, Haucke V. Sci STKE 2004. 2004 doi: 10.1126/stke.2642004re19. re19. [DOI] [PubMed] [Google Scholar]
- 2.Takei K, Mundigl O, Daniell L, De Camilli P. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudhof TC. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 4.Faundez V, Horng JT, Kelly RB. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- 5.Simpson F, Peden AA, Christopoulou L, Robinson MS. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamiya H, Shinozaki H, Yamamoto C. J Physiol (London) 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 9.Muzerelle A, Alberts P, Martinez-Arca S, Jeannequin O, Lafaye P, Mazie J-C, Galli T, Gaspar P. Neuroscience. 2003;122:59–75. doi: 10.1016/s0306-4522(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Arca S, Rudge R, Vacca M, Raposo G, Camonis J, Proux-Gillardeaux V, Daviet L, Formstecher E, Hamburger A, Filippini F, et al. Proc Natl Acad Sci USA. 2003;100:9011–9016. doi: 10.1073/pnas.1431910100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. J Cell Biol. 2002;156:653–654. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzoli SO, Bethani I, Zwilling D, Wenzel D, Siddiqui TJ, Brandhorst D, Jahn R. Traffic. 2006;7:1163–1176. doi: 10.1111/j.1600-0854.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 13.Zeng Q, Tran TT, Tan HX, Hong W. J Biol Chem. 2003;278:23046–23054. doi: 10.1074/jbc.M303214200. [DOI] [PubMed] [Google Scholar]
- 14.Sjostrom PJ, Turrigiano GG, Nelson SB. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- 15.Woodhall G, Evans DI, Cunningham MO, Jones RS. J Neurophysiol. 2001;86:1644–1651. doi: 10.1152/jn.2001.86.4.1644. [DOI] [PubMed] [Google Scholar]
- 16.Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, et al. Neuron. 1998;21:111–122. doi: 10.1016/s0896-6273(00)80519-x. [DOI] [PubMed] [Google Scholar]
- 17.Vogt K, Mellor J, Tong G, Nicoll R. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- 18.Faundez V, Horng JT, Kelly RB. J Cell Biol. 1997;138:505–515. doi: 10.1083/jcb.138.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blagoveshchenskaya AD, Hewitt EW, Cutler DF. Mol Biol Cell. 1999;10:3979–3990. doi: 10.1091/mbc.10.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, Faundez V. Mol Biol Cell. 2004;15:575–587. doi: 10.1091/mbc.E03-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giraudo CG, Eng WS, Melia TJ, Rothman JE. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 23.Xu T, Binz T, Niemann H, Neher E. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- 24.Lonart G, Sudhof TC. J Biol Chem. 2000;275:27703–27707. [PubMed] [Google Scholar]
- 25.Rosenmund C, Stevens CF. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 26.Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, Falcon-Perez JM, Dell'angelica EC, Peden AA, Werner E, et al. Mol Biol Cell. 2006;17:4014–4026. doi: 10.1091/mbc.E06-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salazar G, Love R, Styers ML, Werner E, Peden A, Rodriguez S, Gearing M, Wainer BH, Faundez V. J Biol Chem. 2004;279:25430–25439. doi: 10.1074/jbc.M402331200. [DOI] [PubMed] [Google Scholar]
- 28.Salazar G, Craige B, Wainer BH, Guo J, De Camilli P, Faundez V. Mol Biol Cell. 2005;16:3692–3704. doi: 10.1091/mbc.E05-01-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopantsev V, Wenzel HJ, Cole TB, Palmiter RD, Schwartzkroin PA. Neuroscience. 2003;116:237–248. doi: 10.1016/s0306-4522(02)00570-5. [DOI] [PubMed] [Google Scholar]
- 30.Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, Bosl MR, Ruether K, Jahn H, Draguhn A, et al. Neuron. 2001;29:185–196. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 31.Grabner CP, Price SD, Lysakowski A, Cahill AL, Fox AP. Proc Natl Acad Sci USA. 2006;103:10035–10040. doi: 10.1073/pnas.0509844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaba T, Stein A, Jahn R, Neher E. Science. 2005;309:491–494. doi: 10.1126/science.1112645. [DOI] [PubMed] [Google Scholar]
- 33.Goda Y, Stevens CF. Proc Natl Acad Sci USA. 1994;91:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Sudhof TC. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 35.Sugita S, Shin OH, Han WP, Lao Y, Sudhof TC. EMBO J. 2002;21:270–280. doi: 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW. J Biol Chem. 2004;279:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 37.Sugita S, Han WP, Butz S, Liu XR, FernandezChacon R, Lao Y, Sudhof TC. Neuron. 2001;30:459–473. doi: 10.1016/s0896-6273(01)00290-2. [DOI] [PubMed] [Google Scholar]
- 38.Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER. Proc Natl Acad Sci USA. 2005;102:5210–5214. doi: 10.1073/pnas.0500941102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutierrez R, Heinemann U. Brain Res. 1999;815:304–316. doi: 10.1016/s0006-8993(98)01101-9. [DOI] [PubMed] [Google Scholar]
- 40.Stoppini L, Buchs PA, Muller D. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 41.Musleh W, Yaghoubi S, Baudry M. Brain Res. 1997;770:298–301. doi: 10.1016/s0006-8993(97)00851-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.