Abstract
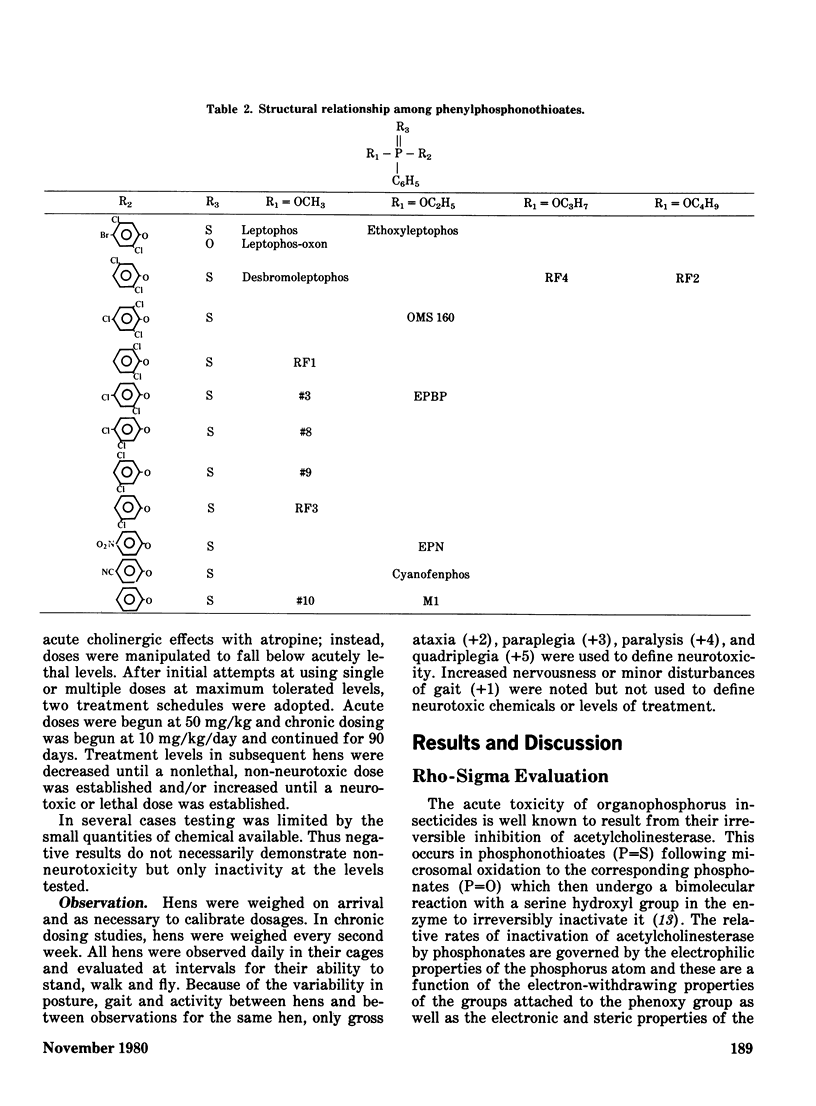
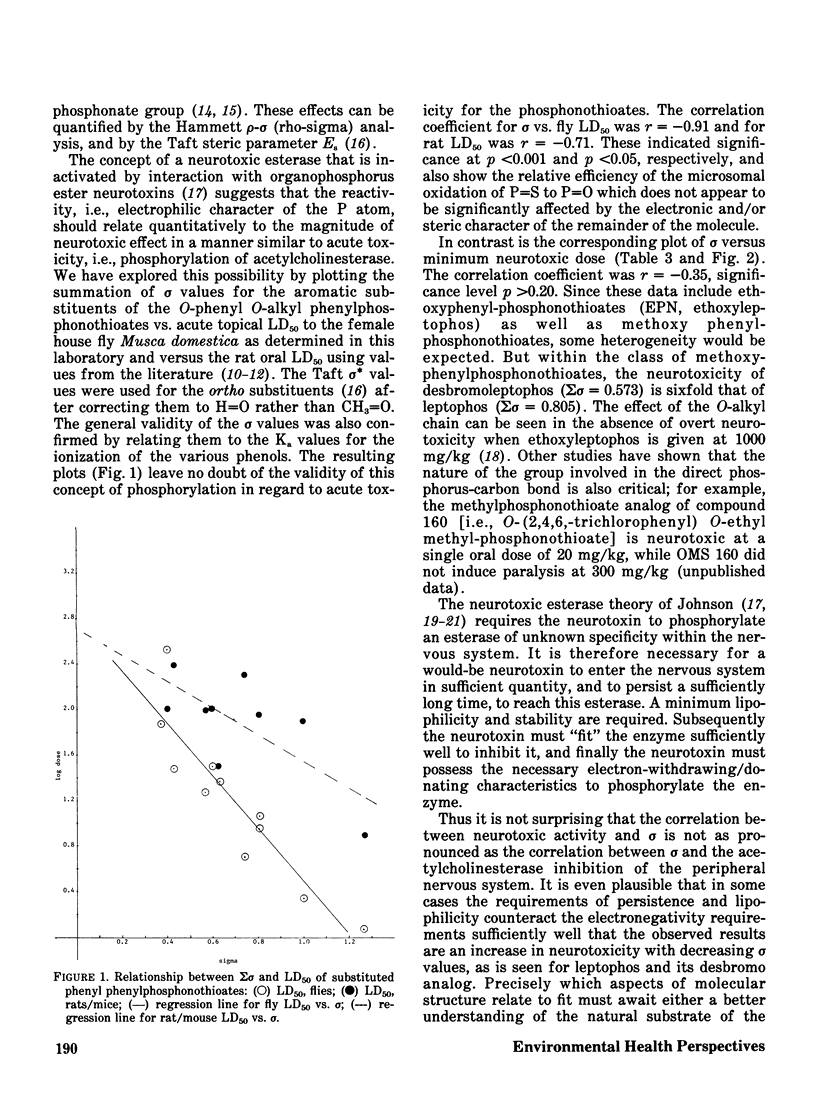
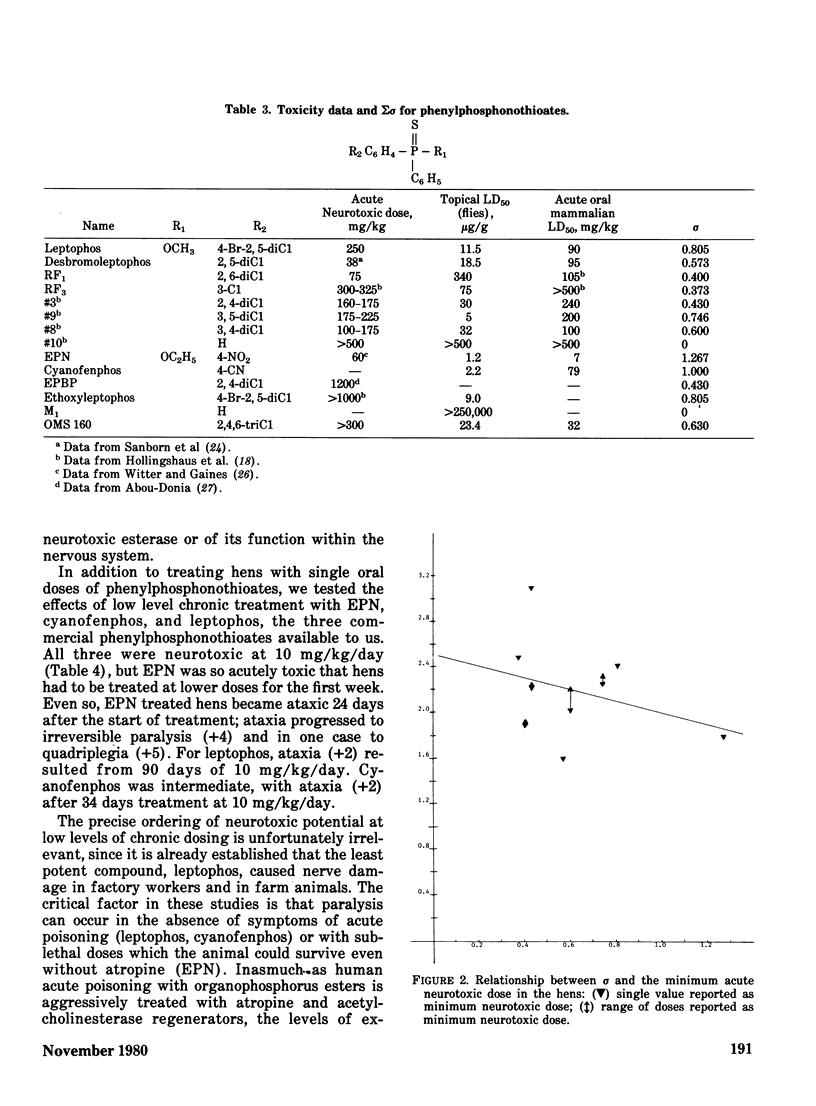
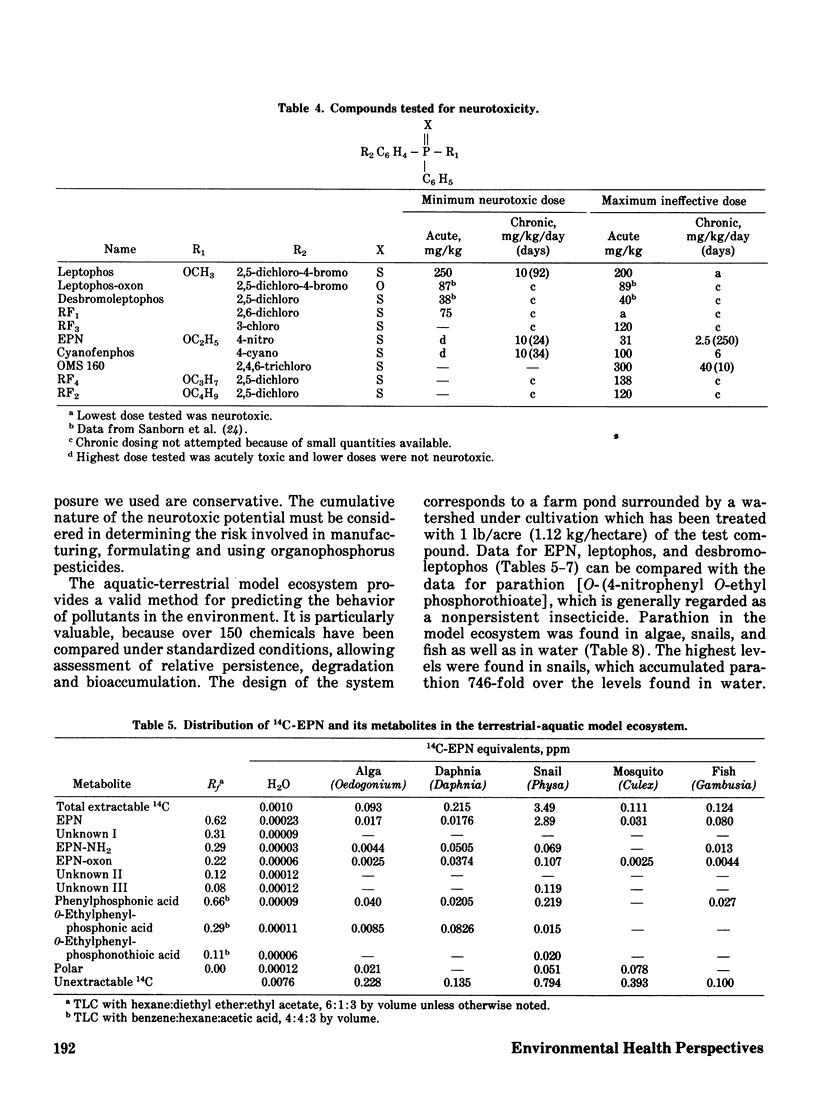
The phenylphosphonothioate insecticides EPN and leptophos, and several analogs, were evaluated with respect to their delayed neurotoxic effects in hens and their environmental behavior in a terrestrial-aquatic model ecosystem. Acute toxicity to insects was highly correlated with sigma sigma of the substituted phenyl group (regression coefficient r = -0.91) while acute toxicity to mammals was slightly less well correlated (regression coefficient r = -0.71), and neurotoxicity was poorly correlated with sigma sigma (regression coefficient r = -0.35). Both EPN and leptophos were markedly more persistent and bioaccumulative in the model ecosystem than parathion. Desbromoleptophos, a contaminant and metabolite of leptophos, was seen to be a highly stable and persistent terminal residue of leptophos.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DURHAM W. F., GAINES T. B., HAYES W. J., Jr Paralytic and related effects of certain organic phosphorus compounds. AMA Arch Ind Health. 1956 Apr;13(4):326–330. [PubMed] [Google Scholar]
- Fukuto T. R. Relationships between the structure of organophosphorus compounds and their activity as acetylcholinesterase inhibitors. Bull World Health Organ. 1971;44(1-3):31–42. [PMC free article] [PubMed] [Google Scholar]
- Gunther F. A., Iwata Y., Carman G. E., Smith C. A. The citrus reentry problem: research on its causes and effects, and approaches to its minimization. Residue Rev. 1977;67:1–132. doi: 10.1007/978-1-4684-7062-8_1. [DOI] [PubMed] [Google Scholar]
- Johnson M. K. A phosphorylation site in brain and the delayed neurotoxic effect of some organophosphorus compounds. Biochem J. 1969 Feb;111(4):487–495. doi: 10.1042/bj1110487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Mechanism of protection against the delayed neurotoxic effects of organophosphorus esters. Fed Proc. 1976 Jan;35(1):73–74. [PubMed] [Google Scholar]
- Johnson M. K. Organophosphorus and other inhibitors of brain 'neurotoxic esterase' and the development of delayed neurotoxicity in hens. Biochem J. 1970 Dec;120(3):523–531. doi: 10.1042/bj1200523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. The delayed neuropathy caused by some organophosphorus esters: mechanism and challenge. CRC Crit Rev Toxicol. 1975 Jun;3(3):289–316. doi: 10.3109/10408447509079861. [DOI] [PubMed] [Google Scholar]
- KELLY R. G., PEETS E. A., GORDON S., BUYSKE D. A. Determination of C-14 and H3 in biological samples by Schoeniger combustion and liquid scintillation techniques. Anal Biochem. 1961 Jun;2:267–273. doi: 10.1016/s0003-2697(61)80010-9. [DOI] [PubMed] [Google Scholar]
- Lu P. Y., Metcalf R. L., Carlson E. M. Environmental fate of five radio-labeled coal conversion by-products evaluated in a laboratory model ecosystem. Environ Health Perspect. 1978 Jun;24:201–208. doi: 10.1289/ehp.7824201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITTER R. F., GAINES T. B. RELATIONSHIP BETWEEN DEPRESSION OF BRAIN OR PLASMA CHOLINESTERASE AND PARALYSIS IN CHICKENS CAUSED BY CERTAIN ORGANIC PHOSPHORUS COMPOUNDS. Biochem Pharmacol. 1963 Dec;12:1377–1386. doi: 10.1016/0006-2952(63)90207-7. [DOI] [PubMed] [Google Scholar]


