Abstract
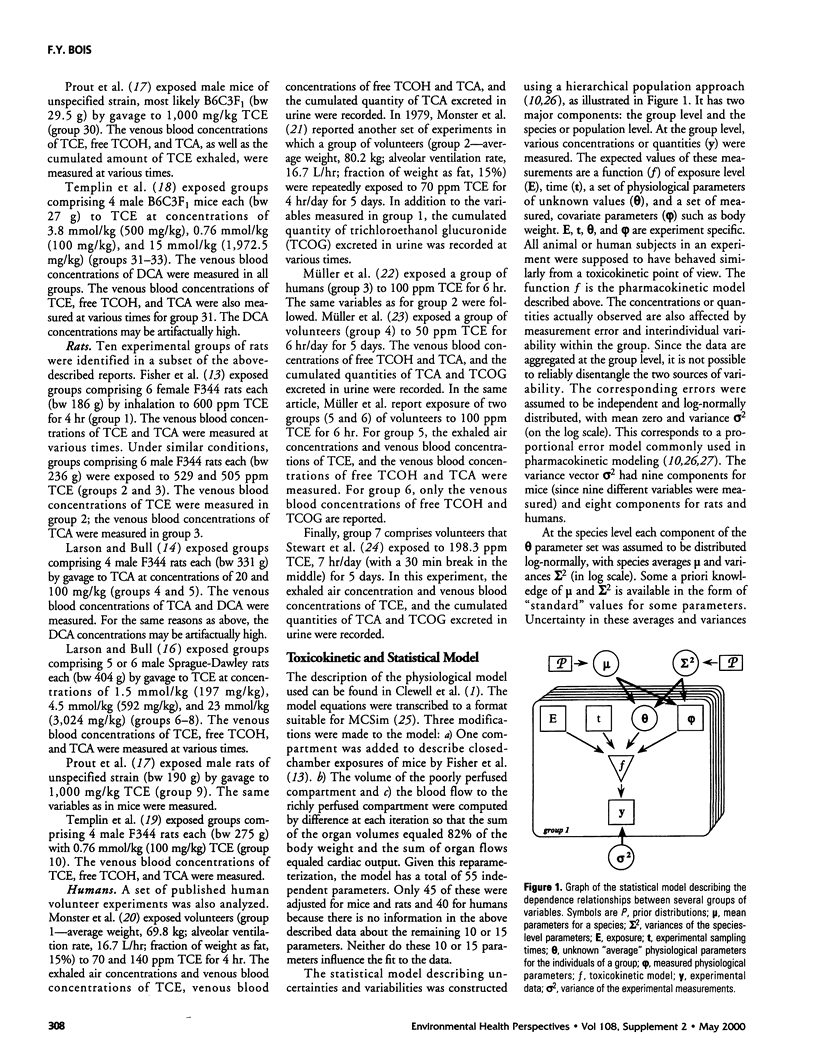
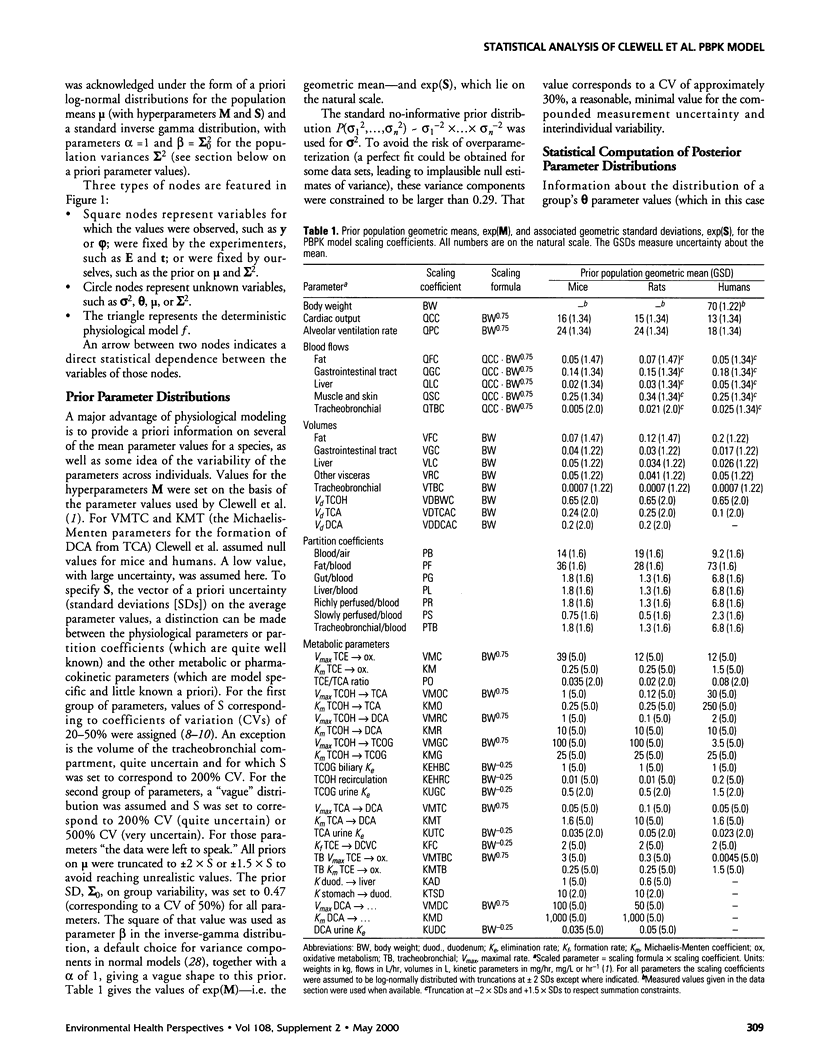
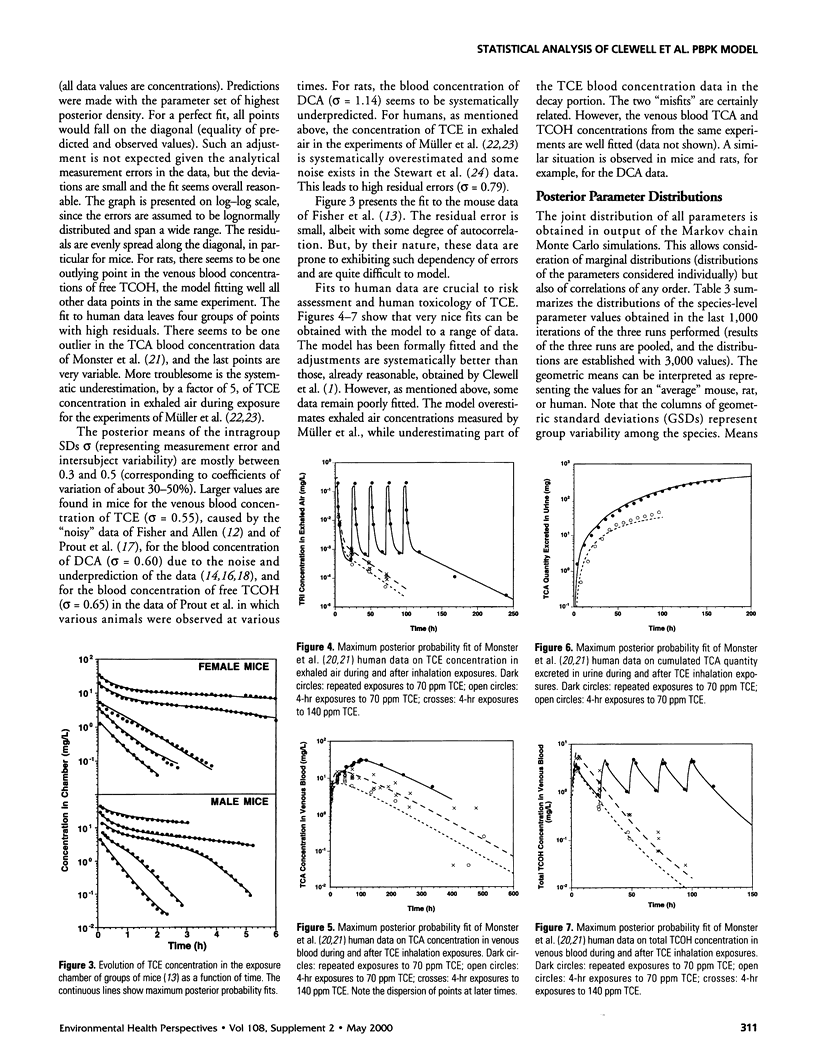
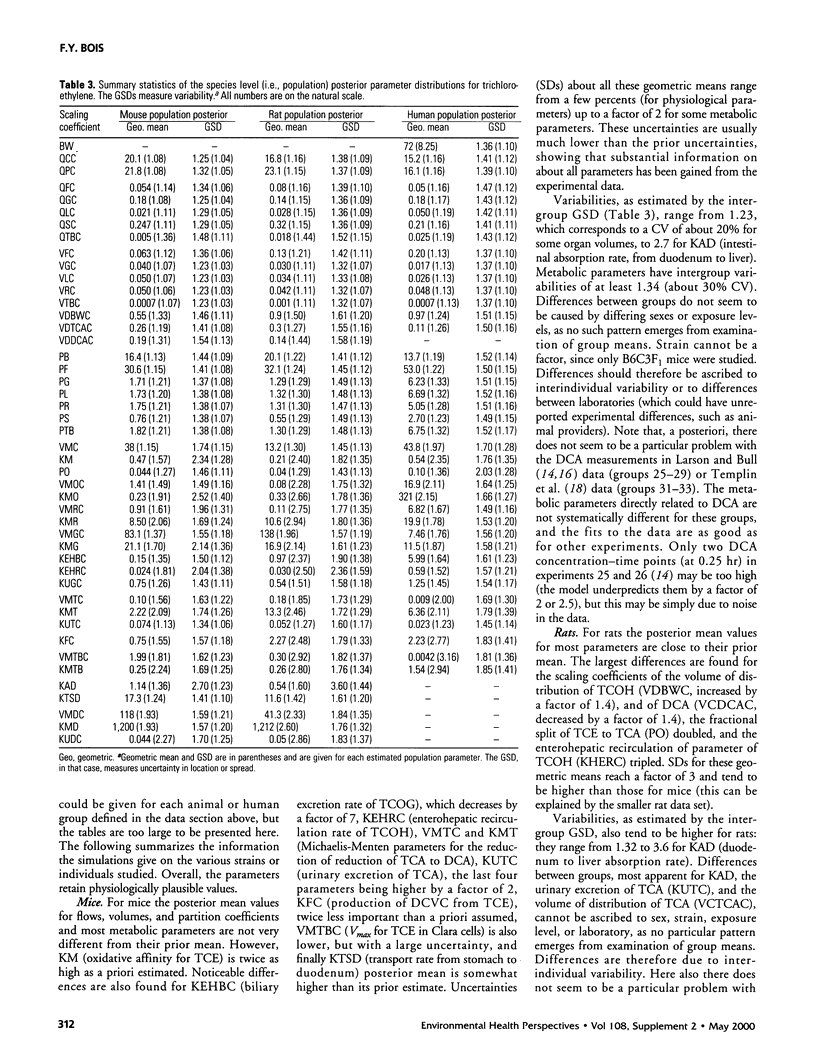
A physiologically based pharmacokinetic model for trichloroethylene (TCE) in rodents and humans was calibrated with published toxicokinetic data sets. A Bayesian statistical framework was used to combine previous information about the model parameters with the data likelihood, to yield posterior parameter distributions. The use of the hierarchical statistical model yielded estimates of both variability between experimental groups and uncertainty in TCE toxicokinetics. After adjustment of the model by Markov chain Monte Carlo sampling, estimates of variability for the animal or human metabolic parameters ranged from a factor of 1.5-2 (geometric standard deviation [GSD]). Uncertainty was of the same order as variability for animals and higher than variability for humans. The model was used to make posterior predictions for several measures of cancer risk. These predictions were affected by both uncertainties and variability and exhibited GSDs ranging from 2 to 6 in mice and rats and from 2 to 10 for humans.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrand I., Ovrum P. Exposure to trichloroethylene I. Uptake and distribution in man. Scand J Work Environ Health. 1976 Dec;2(4):199–211. [PubMed] [Google Scholar]
- Bois F. Y., Gelman A., Jiang J., Maszle D. R., Zeise L., Alexeef G. Population toxicokinetics of tetrachloroethylene. Arch Toxicol. 1996;70(6):347–355. doi: 10.1007/s002040050284. [DOI] [PubMed] [Google Scholar]
- Bois F. Y. Statistical analysis of Fisher et al. PBPK model of trichloroethylene kinetics. Environ Health Perspect. 2000 May;108 (Suppl 2):275–282. doi: 10.1289/ehp.00108s2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnick S. B., Kawai R., Nedelman J. R., Lemaire M., Niederberger W., Sato H. Perspectives in pharmacokinetics. Physiologically based pharmacokinetic modeling as a tool for drug development. J Pharmacokinet Biopharm. 1995 Apr;23(2):217–229. doi: 10.1007/BF02354273. [DOI] [PubMed] [Google Scholar]
- Clewell H. J., 3rd, Gentry P. R., Covington T. R., Gearhart J. M. Development of a physiologically based pharmacokinetic model of trichloroethylene and its metabolites for use in risk assessment. Environ Health Perspect. 2000 May;108 (Suppl 2):283–305. doi: 10.1289/ehp.00108s2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández J. G., Droz P. O., Humbert B. E., Caperos J. R. Trichloroethylene exposure. Simulation of uptake, excretion, and metabolism using a mathematical model. Br J Ind Med. 1977 Feb;34(1):43–55. doi: 10.1136/oem.34.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. W., Allen B. C. Evaluating the risk of liver cancer in humans exposed to trichloroethylene using physiological models. Risk Anal. 1993 Feb;13(1):87–95. doi: 10.1111/j.1539-6924.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Gargas M. L., Allen B. C., Andersen M. E. Physiologically based pharmacokinetic modeling with trichloroethylene and its metabolite, trichloroacetic acid, in the rat and mouse. Toxicol Appl Pharmacol. 1991 Jun 15;109(2):183–195. doi: 10.1016/0041-008x(91)90167-d. [DOI] [PubMed] [Google Scholar]
- Guberan E., Fernandez J. Control of industrial exposure to tetrachloroethylene by measuring alveolar concentrations: theoretical approach using a mathematical model. Br J Ind Med. 1974 Apr;31(2):159–167. doi: 10.1136/oem.31.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Otsuji H., Imamura T., Komoike Y. Urinary excretion of total trichloro-compounds, trichloroethanol, and trichloroacetic acid as a measure of exposure to trichloroethylene and tetrachloroethylene. Br J Ind Med. 1972 Jul;29(3):328–333. doi: 10.1136/oem.29.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson G., Jonsson F., Bois F. Development of new technique for risk assessment using physiologically based toxicokinetic models. Am J Ind Med. 1999 Sep;Suppl 1:101–103. doi: 10.1002/(sici)1097-0274(199909)36:1+<101::aid-ajim36>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kohn M. C. Achieving credibility in risk assessment models. Toxicol Lett. 1995 Sep;79(1-3):107–114. doi: 10.1016/0378-4274(95)03362-o. [DOI] [PubMed] [Google Scholar]
- Laparé S., Tardif R., Brodeur J. Effect of various exposure scenarios on the biological monitoring of organic solvents in alveolar air. II. 1,1,1-Trichloroethane and trichloroethylene. Int Arch Occup Environ Health. 1995;67(6):375–394. doi: 10.1007/BF00381051. [DOI] [PubMed] [Google Scholar]
- Larson J. L., Bull R. J. Metabolism and lipoperoxidative activity of trichloroacetate and dichloroacetate in rats and mice. Toxicol Appl Pharmacol. 1992 Aug;115(2):268–277. doi: 10.1016/0041-008x(92)90332-m. [DOI] [PubMed] [Google Scholar]
- Larson J. L., Bull R. J. Species differences in the metabolism of trichloroethylene to the carcinogenic metabolites trichloroacetate and dichloroacetate. Toxicol Appl Pharmacol. 1992 Aug;115(2):278–285. doi: 10.1016/0041-008x(92)90333-n. [DOI] [PubMed] [Google Scholar]
- Louis T. A. Assessing, accommodating, and interpreting the influences of heterogeneity. Environ Health Perspect. 1991 Jan;90:215–222. doi: 10.1289/ehp.90-1519510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster A. C., Boersma G., Duba W. C. Kinetics of trichloroethylene in repeated exposure of volunteers. Int Arch Occup Environ Health. 1979 Jan 15;42(3-4):283–292. doi: 10.1007/BF00377782. [DOI] [PubMed] [Google Scholar]
- Monster A. C., Boersma G., Duba W. C. Pharmacokinetics of trichloroethylene in volunteers, influence of workload and exposure concentration. Int Arch Occup Environ Health. 1976 Dec 15;38(2):87–102. doi: 10.1007/BF00378619. [DOI] [PubMed] [Google Scholar]
- Müller G., Spassovski M., Henschler D. Metabolism of trichloroethylene in man. II. Pharmacokinetics of metabolites. Arch Toxicol. 1974;32(4):283–295. doi: 10.1007/BF00330110. [DOI] [PubMed] [Google Scholar]
- Müller G., Spassowski M., Henschler D. Metabolism of trichloroethylene in man. III. Interaction of trichloroethylene and ethanol. Arch Toxicol. 1975;33(3):173–189. doi: 10.1007/BF00311271. [DOI] [PubMed] [Google Scholar]
- Opdam J. J. Intra and interindividual variability in the kinetics of a poorly and highly metabolising solvent. Br J Ind Med. 1989 Dec;46(12):831–845. doi: 10.1136/oem.46.12.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prout M. S., Provan W. M., Green T. Species differences in response to trichloroethylene. I. Pharmacokinetics in rats and mice. Toxicol Appl Pharmacol. 1985 Jul;79(3):389–400. doi: 10.1016/0041-008x(85)90137-1. [DOI] [PubMed] [Google Scholar]
- Sato A., Nakajima T., Fujiwara Y., Murayama N. A pharmacokinetic model to study the excretion of trichloroethylene and its metabolites after an inhalation exposure. Br J Ind Med. 1977 Feb;34(1):56–63. doi: 10.1136/oem.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear R. C., Bois F. Y. Parameter variability and the interpretation of physiologically based pharmacokinetic modeling results. Environ Health Perspect. 1994 Dec;102 (Suppl 11):61–66. doi: 10.1289/ehp.94102s1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R. D., Dodd H. C., Gay H. H., Erley D. S. Experimental human exposure to trichloroethylene. Arch Environ Health. 1970 Jan;20(1):64–71. doi: 10.1080/00039896.1970.10665543. [DOI] [PubMed] [Google Scholar]
- Templin M. V., Parker J. C., Bull R. J. Relative formation of dichloroacetate and trichloroacetate from trichloroethylene in male B6C3F1 mice. Toxicol Appl Pharmacol. 1993 Nov;123(1):1–8. doi: 10.1006/taap.1993.1214. [DOI] [PubMed] [Google Scholar]
- Templin M. V., Stevens D. K., Stenner R. D., Bonate P. L., Tuman D., Bull R. J. Factors affecting species differences in the kinetics of metabolites of trichloroethylene. J Toxicol Environ Health. 1995 Apr;44(4):435–447. doi: 10.1080/15287399509531972. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Astrand I. Exposure to trichloroethylene monitored by analysis of metabolites in blood and urine. J Occup Med. 1976 Apr;18(4):224–226. [PubMed] [Google Scholar]
- Vozeh S., Steimer J. L., Rowland M., Morselli P., Mentre F., Balant L. P., Aarons L. The use of population pharmacokinetics in drug development. Clin Pharmacokinet. 1996 Feb;30(2):81–93. doi: 10.2165/00003088-199630020-00001. [DOI] [PubMed] [Google Scholar]
- Woodruff T. J., Bois F. Y. Optimization issues in physiological toxicokinetic modeling: a case study with benzene. Toxicol Lett. 1993 Aug;69(2):181–196. doi: 10.1016/0378-4274(93)90103-5. [DOI] [PubMed] [Google Scholar]


