Abstract
Trichloroethylene (TCE) pharmacokinetics have been studied in experimental animals and humans for over 30 years. Compartmental and physiologically based pharmacokinetic (PBPK) models have been developed for the uptake, distribution, and metabolism of TCE and the production, distribution, metabolism, and elimination of P450-mediated metabolites of TCE. TCE is readily taken up into systemic circulation by oral and inhalation routes of exposure and is rapidly metabolized by the hepatic P450 system and to a much lesser degree, by direct conjugation with glutathione. Recent PBPK models for TCE and its metabolites have focused on the major metabolic pathway for metabolism of TCE (P450-mediated metabolic pathway). This article briefly reviews selected published compartmental and PBPK models for TCE. Trichloroacetic acid (TCA) is considered a principle metabolite responsible for TCE-induced liver cancer in mice. Liver cancer in mice was considered a critical effect by the U.S. Environmental Protection Agency for deriving the current maximum contaminant level for TCE in water. In the literature both whole blood and plasma measurements of TCA are reported in mice and humans. To reduce confusion about disparately measured and model-predicted levels of TCA in plasma and whole blood, model-predicted outcomes are compared for first-generation (plasma) and second-generation (whole blood) PBPK models published by Fisher and colleagues. Qualitatively, animals and humans metabolize TCE in a similar fashion, producing the same metabolites. Quantitatively, PBPK models for TCE and its metabolites are important tools for providing dosimetry comparisons between experimental animals and humans. TCE PBPK models can be used today to aid in crafting scientifically sound public health decisions for TCE.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas R. R., Seckel C. S., Kidney J. K., Fisher J. W. Pharmacokinetic analysis of chloral hydrate and its metabolism in B6C3F1 mice. Drug Metab Dispos. 1996 Dec;24(12):1340–1346. [PubMed] [Google Scholar]
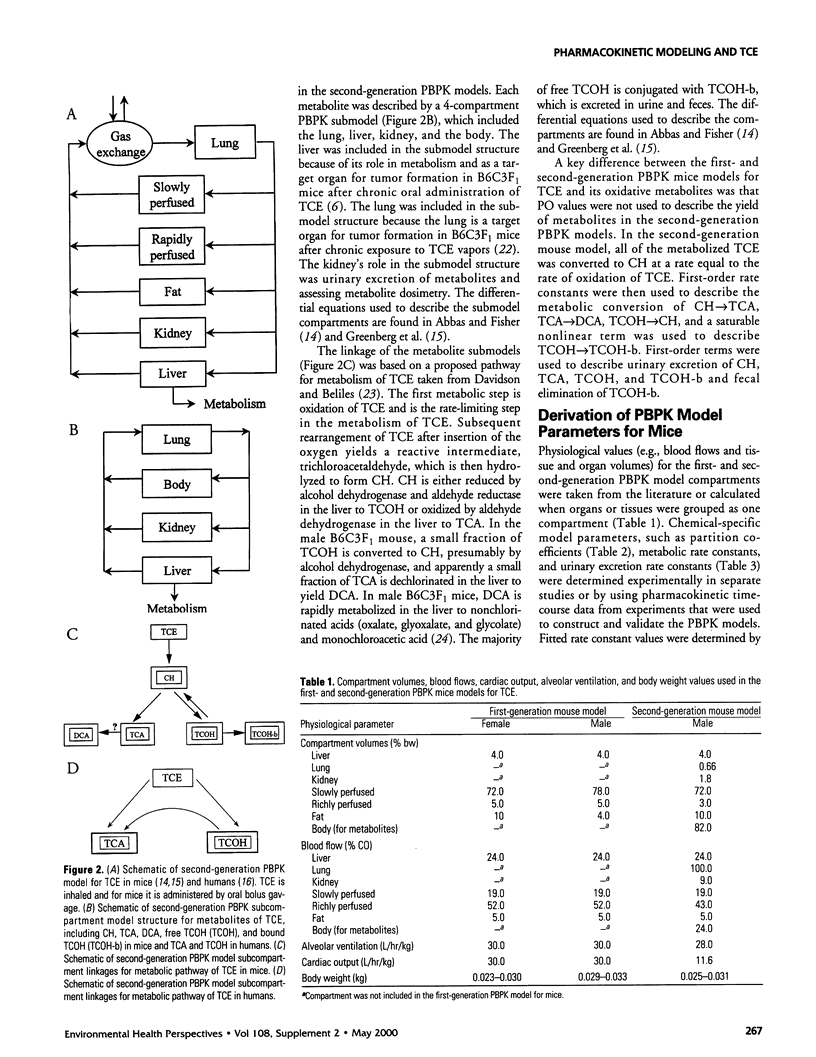
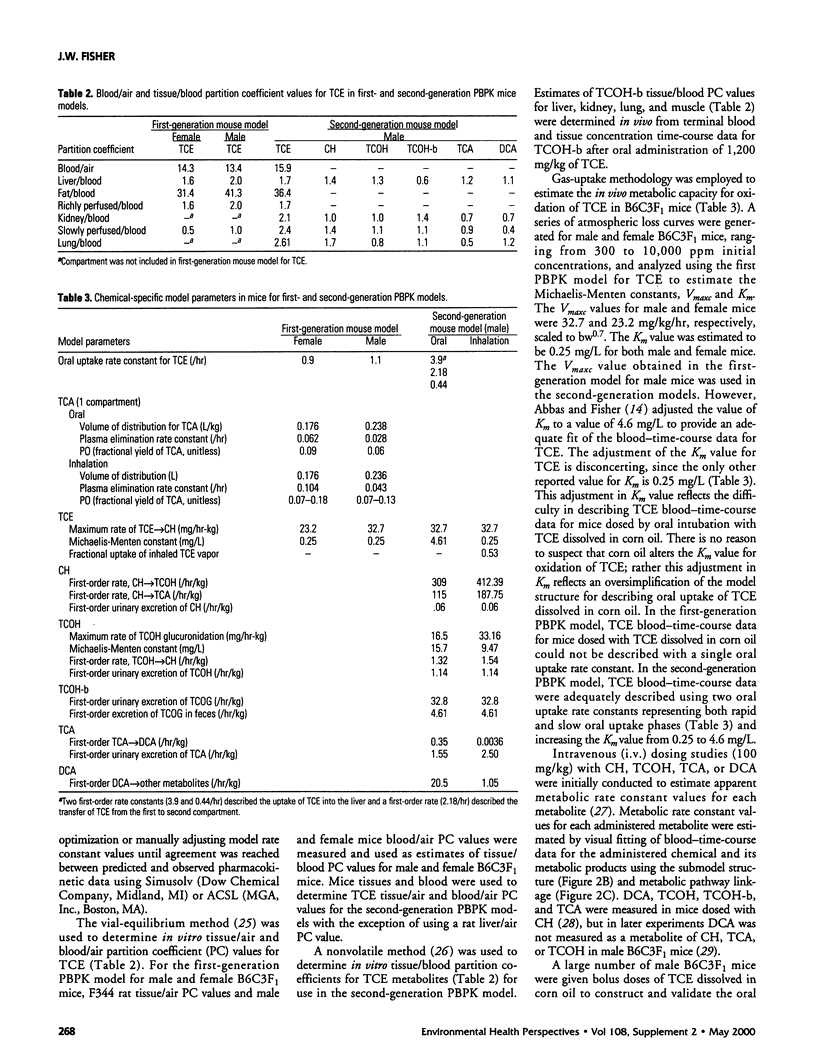
- Abbas R., Fisher J. W. A physiologically based pharmacokinetic model for trichloroethylene and its metabolites, chloral hydrate, trichloroacetate, dichloroacetate, trichloroethanol, and trichloroethanol glucuronide in B6C3F1 mice. Toxicol Appl Pharmacol. 1997 Nov;147(1):15–30. doi: 10.1006/taap.1997.8190. [DOI] [PubMed] [Google Scholar]
- Allen B. C., Fisher J. W. Pharmacokinetic modeling of trichloroethylene and trichloroacetic acid in humans. Risk Anal. 1993 Feb;13(1):71–86. doi: 10.1111/j.1539-6924.1993.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Andersen M. E., Gargas M. L., Clewell H. J., 3rd, Severyn K. M. Quantitative evaluation of the metabolic interactions between trichloroethylene and 1,1-dichloroethylene in vivo using gas uptake methods. Toxicol Appl Pharmacol. 1987 Jun 30;89(2):149–157. doi: 10.1016/0041-008x(87)90035-4. [DOI] [PubMed] [Google Scholar]
- Beland F. A., Schmitt T. C., Fullerton N. F., Young J. F. Metabolism of chloral hydrate in mice and rats after single and multiple doses. J Toxicol Environ Health A. 1998 Jun 12;54(3):209–226. doi: 10.1080/009841098158917. [DOI] [PubMed] [Google Scholar]
- Bogen K. T., Gold L. S. Trichloroethylene cancer risk: simplified calculation of PBPK-based MCLs for cytotoxic end points. Regul Toxicol Pharmacol. 1997 Feb;25(1):26–42. doi: 10.1006/rtph.1996.1070. [DOI] [PubMed] [Google Scholar]
- Bogen K. T. Pharmacokinetics for regulatory risk analysis: the case of trichloroethylene. Regul Toxicol Pharmacol. 1988 Dec;8(4):447–466. doi: 10.1016/0273-2300(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Clewell H. J., Gentry P. R., Gearhart J. M., Allen B. C., Andersen M. E. Considering pharmacokinetic and mechanistic information in cancer risk assessments for environmental contaminants: examples with vinyl chloride and trichloroethylene. Chemosphere. 1995 Jul;31(1):2561–2578. doi: 10.1016/0045-6535(95)00124-q. [DOI] [PubMed] [Google Scholar]
- Cronin W. J., 4th, Oswald E. J., Shelley M. L., Fisher J. W., Flemming C. D. A trichloroethylene risk assessment using a Monte Carlo analysis of parameter uncertainty in conjunction with physiologically-based pharmacokinetic modeling. Risk Anal. 1995 Oct;15(5):555–565. doi: 10.1111/j.1539-6924.1995.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Dallas C. E., Gallo J. M., Ramanathan R., Muralidhara S., Bruckner J. V. Physiological pharmacokinetic modeling of inhaled trichloroethylene in rats. Toxicol Appl Pharmacol. 1991 Sep 1;110(2):303–314. doi: 10.1016/s0041-008x(05)80013-4. [DOI] [PubMed] [Google Scholar]
- Davidson I. W., Beliles R. P. Consideration of the target organ toxicity of trichloroethylene in terms of metabolite toxicity and pharmacokinetics. Drug Metab Rev. 1991;23(5-6):493–599. doi: 10.3109/03602539109029772. [DOI] [PubMed] [Google Scholar]
- Fernández J. G., Droz P. O., Humbert B. E., Caperos J. R. Trichloroethylene exposure. Simulation of uptake, excretion, and metabolism using a mathematical model. Br J Ind Med. 1977 Feb;34(1):43–55. doi: 10.1136/oem.34.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. W., Allen B. C. Evaluating the risk of liver cancer in humans exposed to trichloroethylene using physiological models. Risk Anal. 1993 Feb;13(1):87–95. doi: 10.1111/j.1539-6924.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Gargas M. L., Allen B. C., Andersen M. E. Physiologically based pharmacokinetic modeling with trichloroethylene and its metabolite, trichloroacetic acid, in the rat and mouse. Toxicol Appl Pharmacol. 1991 Jun 15;109(2):183–195. doi: 10.1016/0041-008x(91)90167-d. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Mahle D., Abbas R. A human physiologically based pharmacokinetic model for trichloroethylene and its metabolites, trichloroacetic acid and free trichloroethanol. Toxicol Appl Pharmacol. 1998 Oct;152(2):339–359. doi: 10.1006/taap.1998.8486. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Whittaker T. A., Taylor D. H., Clewell H. J., 3rd, Andersen M. E. Physiologically based pharmacokinetic modeling of the lactating rat and nursing pup: a multiroute exposure model for trichloroethylene and its metabolite, trichloroacetic acid. Toxicol Appl Pharmacol. 1990 Mar 1;102(3):497–513. doi: 10.1016/0041-008x(90)90045-v. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Whittaker T. A., Taylor D. H., Clewell H. J., 3rd, Andersen M. E. Physiologically based pharmacokinetic modeling of the pregnant rat: a multiroute exposure model for trichloroethylene and its metabolite, trichloroacetic acid. Toxicol Appl Pharmacol. 1989 Jul;99(3):395–414. doi: 10.1016/0041-008x(89)90149-x. [DOI] [PubMed] [Google Scholar]
- Gearhart J. M., Mahle D. A., Greene R. J., Seckel C. S., Flemming C. D., Fisher J. W., Clewell H. J., 3rd Variability of physiologically based pharmacokinetic (PBPK) model parameters and their effects on PBPK model predictions in a risk assessment for perchloroethylene (PCE). Toxicol Lett. 1993 May;68(1-2):131–144. doi: 10.1016/0378-4274(93)90126-i. [DOI] [PubMed] [Google Scholar]
- Herren-Freund S. L., Pereira M. A., Khoury M. D., Olson G. The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicol Appl Pharmacol. 1987 Sep 15;90(2):183–189. doi: 10.1016/0041-008x(87)90325-5. [DOI] [PubMed] [Google Scholar]
- Jepson G. W., Hoover D. K., Black R. K., McCafferty J. D., Mahle D. A., Gearhart J. M. A partition coefficient determination method for nonvolatile chemicals in biological tissues. Fundam Appl Toxicol. 1994 May;22(4):519–524. doi: 10.1006/faat.1994.1059. [DOI] [PubMed] [Google Scholar]
- Johanson G. Modelling of respiratory exchange of polar solvents. Ann Occup Hyg. 1991 Jun;35(3):323–339. doi: 10.1093/annhyg/35.3.323. [DOI] [PubMed] [Google Scholar]
- Ketcha M. M., Stevens D. K., Warren D. A., Bishop C. T., Brashear W. T. Conversion of trichloroacetic acid to dichloroacetic acid in biological samples. J Anal Toxicol. 1996 Jul-Aug;20(4):236–241. doi: 10.1093/jat/20.4.236. [DOI] [PubMed] [Google Scholar]
- Koizumi A. Potential of physiologically based pharmacokinetics to amalgamate kinetic data of trichloroethylene and tetrachloroethylene obtained in rats and man. Br J Ind Med. 1989 Apr;46(4):239–249. doi: 10.1136/oem.46.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J. L., Bull R. J. Metabolism and lipoperoxidative activity of trichloroacetate and dichloroacetate in rats and mice. Toxicol Appl Pharmacol. 1992 Aug;115(2):268–277. doi: 10.1016/0041-008x(92)90332-m. [DOI] [PubMed] [Google Scholar]
- MARSHALL E. K., Jr, OWENS A. H., Jr Absorption, excretion and metabolic fate of chloral hydrate and trichloroethanol. Bull Johns Hopkins Hosp. 1954 Jul;95(1):1–18. [PubMed] [Google Scholar]
- Muralidhara S., Bruckner J. V. Simple method for rapid measurement of trichloroethylene and its major metabolites in biological samples. J Chromatogr B Biomed Sci Appl. 1999 Sep 10;732(1):145–153. doi: 10.1016/s0378-4347(99)00282-0. [DOI] [PubMed] [Google Scholar]
- Pleil J. D., Lindstrom A. B. Collection of a single alveolar exhaled breath for volatile organic compounds analysis. Am J Ind Med. 1995 Jul;28(1):109–121. doi: 10.1002/ajim.4700280110. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Andersen M. E. A physiologically based description of the inhalation pharmacokinetics of styrene in rats and humans. Toxicol Appl Pharmacol. 1984 Mar 30;73(1):159–175. doi: 10.1016/0041-008x(84)90064-4. [DOI] [PubMed] [Google Scholar]
- Sato A., Nakajima T., Fujiwara Y., Murayama N. A pharmacokinetic model to study the excretion of trichloroethylene and its metabolites after an inhalation exposure. Br J Ind Med. 1977 Feb;34(1):56–63. doi: 10.1136/oem.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon T. W. Combining physiologically based pharmacokinetic modeling with Monte Carlo simulation to derive an acute inhalation guidance value for trichloroethylene. Regul Toxicol Pharmacol. 1997 Dec;26(3):257–270. doi: 10.1006/rtph.1997.1168. [DOI] [PubMed] [Google Scholar]


