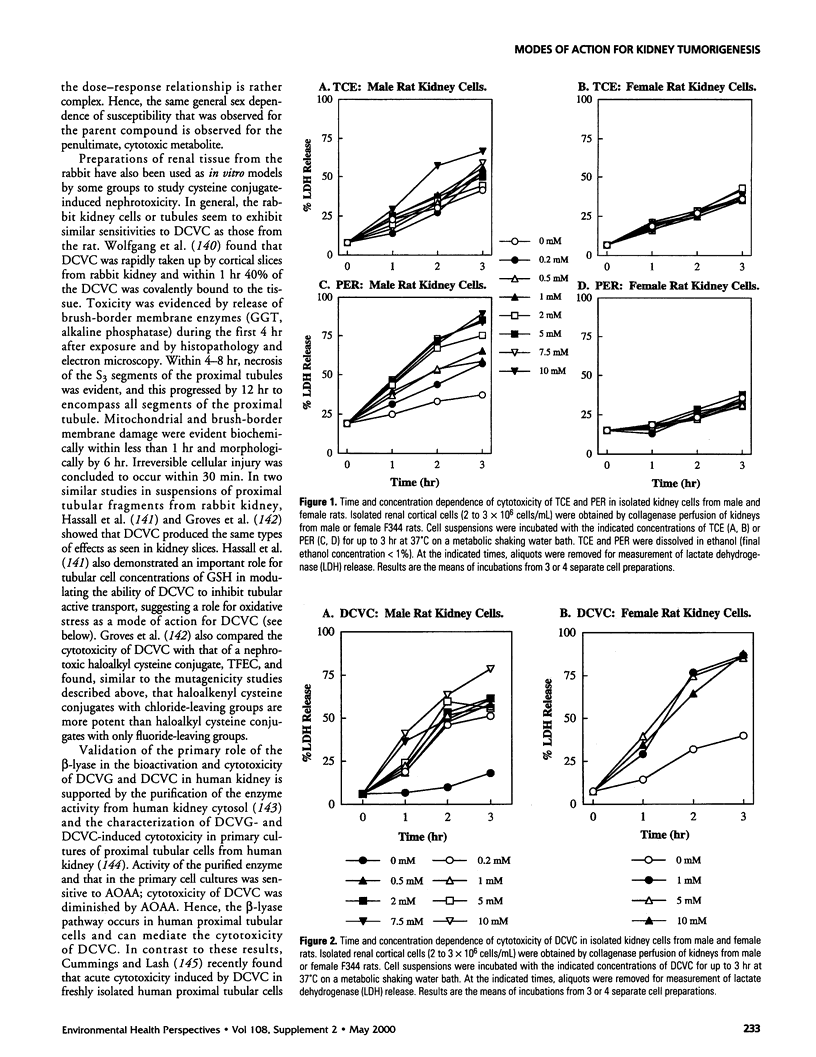
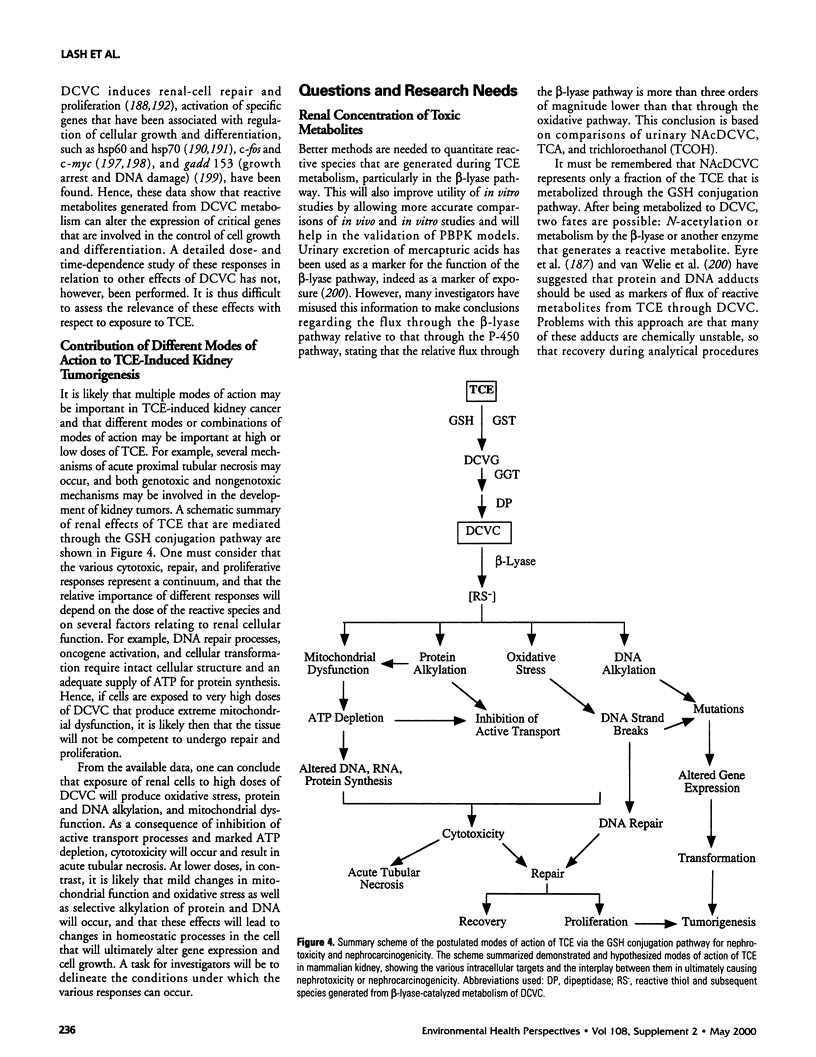
Abstract
This article focuses on the various models for kidney toxicity due to trichloroethylene (TCE) and its glutathione-dependent metabolites, in particular S-(1,2-dichlorovinyl)-l-cysteine. Areas of controversy regarding the relative importance of metabolic pathways, species differences in toxic responses, rates of generation of reactive metabolites, and dose-dependent phenomena are highlighted. The first section briefly reviews information on the incidence and risk factors of kidney cancer in the general U.S. population. Epidemiological data on incidence of kidney cancer in male workers exposed occupationally to TCE are also summarized. This is contrasted with cancer bioassay data from laboratory animals, that highlights sex and species differences and, consequently, the difficulties in making risk assessments for humans based on animal data. The major section of the article considers proposed modes of action for TCE or its metabolites in kidney, including peroxisome proliferation, alpha(2u)-globulin nephropathy, genotoxicity, and acute and chronic toxicity mechanisms. The latter comprise oxidative stress, alterations in calcium ion homeostasis, mitochondrial dysfunction, protein alkylation, cellular repair processes, and alterations in gene expression and cell proliferation. Finally, the status of risk assessment for TCE based on the kidneys as a target organ and remaining questions and research needs are discussed.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarsaether N., Aarsland A., Kryvi H., Nilsson A., Svardal A., Ueland P. M., Berge R. K. Changes in peroxisomes and mitochondria in liver of ethionine exposed rats: a biochemical and morphological investigation. Carcinogenesis. 1989 Jun;10(6):987–994. doi: 10.1093/carcin/10.6.987. [DOI] [PubMed] [Google Scholar]
- Anttila A., Pukkala E., Sallmén M., Hernberg S., Hemminki K. Cancer incidence among Finnish workers exposed to halogenated hydrocarbons. J Occup Environ Med. 1995 Jul;37(7):797–806. doi: 10.1097/00043764-199507000-00008. [DOI] [PubMed] [Google Scholar]
- Ashby J. Alpha 2 mu-globulin nephropathy in white ravens. Environ Health Perspect. 1996 Dec;104(12):1264–1267. doi: 10.1289/ehp.104-1469542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby J. The relevance of mechanistic data to the interpretation and extrapolation to humans of rodent carcinogenicity data. Environ Health Perspect. 1997 Sep;105(9):902–903. doi: 10.1289/ehp.105-1470344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson O., Seldén A., Andersson K., Hogstedt C. Updated and expanded Swedish cohort study on trichloroethylene and cancer risk. J Occup Med. 1994 May;36(5):556–562. [PubMed] [Google Scholar]
- Banki K., Anders M. W. Inhibition of rat kidney mitochondrial DNA, RNA and protein synthesis by halogenated cysteine S-conjugates. Carcinogenesis. 1989 Apr;10(4):767–772. doi: 10.1093/carcin/10.4.767. [DOI] [PubMed] [Google Scholar]
- Blair A., Hartge P., Stewart P. A., McAdams M., Lubin J. Mortality and cancer incidence of aircraft maintenance workers exposed to trichloroethylene and other organic solvents and chemicals: extended follow up. Occup Environ Med. 1998 Mar;55(3):161–171. doi: 10.1136/oem.55.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemen L. J., Tomenson J. Increased incidence of renal cell tumours in a cohort of cardboard workers exposed to trichloroethylene. Arch Toxicol. 1995;70(2):129–133. doi: 10.1007/BF02733675. [DOI] [PubMed] [Google Scholar]
- Borghoff S. J., Lehman-McKeeman L. D., Short B. G., Hard G. C., Swenberg J. A. Critique of R. Melnick's "An alternative hypothesis on the role of chemically induced protein droplet (alpha 2u-globulin) nephropathy in renal carcinogenesis". Regul Toxicol Pharmacol. 1993 Oct;18(2):357–364. doi: 10.1006/rtph.1993.1061. [DOI] [PubMed] [Google Scholar]
- Bruschi S. A., Bull R. J. In vitro cytotoxicity of mono-, di-, and trichloroacetate and its modulation by hepatic peroxisome proliferation. Fundam Appl Toxicol. 1993 Oct;21(3):366–375. doi: 10.1006/faat.1993.1109. [DOI] [PubMed] [Google Scholar]
- Bruschi S. A., West K. A., Crabb J. W., Gupta R. S., Stevens J. L. Mitochondrial HSP60 (P1 protein) and a HSP70-like protein (mortalin) are major targets for modification during S-(1,1,2,2-tetrafluoroethyl)-L-cysteine-induced nephrotoxicity. J Biol Chem. 1993 Nov 5;268(31):23157–23161. [PubMed] [Google Scholar]
- Brüning T., Golka K., Makropoulos V., Bolt H. M. Preexistence of chronic tubular damage in cases of renal cell cancer after long and high exposure to trichloroethylene. Arch Toxicol. 1996;70(3-4):259–260. doi: 10.1007/s002040050271. [DOI] [PubMed] [Google Scholar]
- Brüning T., Lammert M., Kempkes M., Thier R., Golka K., Bolt H. M. Influence of polymorphisms of GSTM1 and GSTT1 for risk of renal cell cancer in workers with long-term high occupational exposure to trichloroethene. Arch Toxicol. 1997;71(9):596–599. doi: 10.1007/s002040050432. [DOI] [PubMed] [Google Scholar]
- Brüning T., Sundberg A. G., Birner G., Lammert M., Bolt H. M., Appelkvist E. L., Nilsson R., Dallner G. Glutathione transferase alpha as a marker for tubular damage after trichloroethylene exposure. Arch Toxicol. 1999 Jun-Jul;73(4-5):246–254. doi: 10.1007/s002040050613. [DOI] [PubMed] [Google Scholar]
- Brüning T., Vamvakas S., Makropoulos V., Birner G. Acute intoxication with trichloroethene: clinical symptoms, toxicokinetics, metabolism, and development of biochemical parameters for renal damage. Toxicol Sci. 1998 Feb;41(2):157–165. doi: 10.1006/toxs.1997.2401. [DOI] [PubMed] [Google Scholar]
- Brüning T., Weirich G., Hornauer M. A., Höfler H., Brauch H. Renal cell carcinomas in trichloroethene (TRI) exposed persons are associated with somatic mutations in the von Hippel-Lindau (VHL) tumour suppressor gene. Arch Toxicol. 1997;71(5):332–335. doi: 10.1007/s002040050394. [DOI] [PubMed] [Google Scholar]
- Bull R. J. Mode of action of liver tumor induction by trichloroethylene and its metabolites, trichloroacetate and dichloroacetate. Environ Health Perspect. 2000 May;108 (Suppl 2):241–259. doi: 10.1289/ehp.00108s2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R. J., Templin M., Larson J. L., Stevens D. K. The role of dichloroacetate in the hepatocarcinogenicity of trichloroethylene. Toxicol Lett. 1993 May;68(1-2):203–211. doi: 10.1016/0378-4274(93)90131-g. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S. K., Tuchweber B. Studies of acute nephrotoxic potential of trichloroethylene in Fischer 344 rats. J Toxicol Environ Health. 1988;23(2):147–158. doi: 10.1080/15287398809531102. [DOI] [PubMed] [Google Scholar]
- Chauveau D., Duvic C., Chrétien Y., Paraf F., Droz D., Melki P., Hélénon O., Richard S., Grünfeld J. P. Renal involvement in von Hippel-Lindau disease. Kidney Int. 1996 Sep;50(3):944–951. doi: 10.1038/ki.1996.395. [DOI] [PubMed] [Google Scholar]
- Chen J. C., Stevens J. L., Trifillis A. L., Jones T. W. Renal cysteine conjugate beta-lyase-mediated toxicity studied with primary cultures of human proximal tubular cells. Toxicol Appl Pharmacol. 1990 May;103(3):463–473. doi: 10.1016/0041-008x(90)90319-p. [DOI] [PubMed] [Google Scholar]
- Chen Q., Jones T. W., Brown P. C., Stevens J. L. The mechanism of cysteine conjugate cytotoxicity in renal epithelial cells. Covalent binding leads to thiol depletion and lipid peroxidation. J Biol Chem. 1990 Dec 15;265(35):21603–21611. [PubMed] [Google Scholar]
- Chen Q., Jones T. W., Stevens J. L. Early cellular events couple covalent binding of reactive metabolites to cell killing by nephrotoxic cysteine conjugates. J Cell Physiol. 1994 Nov;161(2):293–302. doi: 10.1002/jcp.1041610214. [DOI] [PubMed] [Google Scholar]
- Chen Q., Yu K., Holbrook N. J., Stevens J. L. Activation of the growth arrest and DNA damage-inducible gene gadd 153 by nephrotoxic cysteine conjugates and dithiothreitol. J Biol Chem. 1992 Apr 25;267(12):8207–8212. [PubMed] [Google Scholar]
- Chen Q., Yu K., Stevens J. L. Regulation of the cellular stress response by reactive electrophiles. The role of covalent binding and cellular thiols in transcriptional activation of the 70-kilodalton heat shock protein gene by nephrotoxic cysteine conjugates. J Biol Chem. 1992 Dec 5;267(34):24322–24327. [PubMed] [Google Scholar]
- Chow W. H., Gridley G., McLaughlin J. K., Mandel J. S., Wacholder S., Blot W. J., Niwa S., Fraumeni J. F., Jr Protein intake and risk of renal cell cancer. J Natl Cancer Inst. 1994 Aug 3;86(15):1131–1139. doi: 10.1093/jnci/86.15.1131. [DOI] [PubMed] [Google Scholar]
- Chow W. H., McLaughlin J. K., Linet M. S., Niwa S., Mandel J. S. Use of analgesics and risk of renal cell cancer. Int J Cancer. 1994 Nov 15;59(4):467–470. doi: 10.1002/ijc.2910590406. [DOI] [PubMed] [Google Scholar]
- Chow W. H., McLaughlin J. K., Mandel J. S., Blot W. J., Niwa S., Fraumeni J. F., Jr Reproductive factors and the risk of renal cell cancer among women. Int J Cancer. 1995 Jan 27;60(3):321–324. doi: 10.1002/ijc.2910600307. [DOI] [PubMed] [Google Scholar]
- Chow W. H., McLaughlin J. K., Mandel J. S., Wacholder S., Niwa S., Fraumeni J. F., Jr Risk of renal cell cancer in relation to diuretics, antihypertensive drugs, and hypertension. Cancer Epidemiol Biomarkers Prev. 1995 Jun;4(4):327–331. [PubMed] [Google Scholar]
- Cimini A. M., Sulli A., Stefanini S., Serafini B., Moreno S., Rossi L., Giorgi M., Ceru M. P. Effects of Di-(2-ethylhexyl)phthalate on peroxisomes of liver, kidney and brain of lactating rats and their pups. Cell Mol Biol (Noisy-le-grand) 1994 Dec;40(8):1063–1076. [PubMed] [Google Scholar]
- Commandeur J. N., Boogaard P. J., Mulder G. J., Vermeulen N. P. Mutagenicity and cytotoxicity of two regioisomeric mercapturic acids and cysteine S-conjugates of trichloroethylene. Arch Toxicol. 1991;65(5):373–380. doi: 10.1007/BF02284259. [DOI] [PubMed] [Google Scholar]
- Counts R. S., Nowak G., Wyatt R. D., Schnellmann R. G. Nephrotoxicant inhibition of renal proximal tubule cell regeneration. Am J Physiol. 1995 Aug;269(2 Pt 2):F274–F281. doi: 10.1152/ajprenal.1995.269.2.F274. [DOI] [PubMed] [Google Scholar]
- Crebelli R., Bignami M., Conti L., Carere A. Mutagenicity of trichloroethylene in Salmonella typhimurium TA100. Ann Ist Super Sanita. 1982;18(1):117–121. [PubMed] [Google Scholar]
- Crebelli R., Conti G., Conti L., Carere A. Mutagenicity of trichloroethylene, trichloroethanol and chloral hydrate in Aspergillus nidulans. Mutat Res. 1985 Mar;155(3):105–111. doi: 10.1016/0165-1218(85)90126-0. [DOI] [PubMed] [Google Scholar]
- Cummings B. S., Lash L. H. Metabolism and toxicity of trichloroethylene and S-(1,2-dichlorovinyl)-L-cysteine in freshly isolated human proximal tubular cells. Toxicol Sci. 2000 Feb;53(2):458–466. doi: 10.1093/toxsci/53.2.458. [DOI] [PubMed] [Google Scholar]
- Darnerud P. O., Brandt I., Feil V. J., Bakke J. E. Dichlorovinyl cysteine (DCVC) in the mouse kidney: tissue-binding and toxicity after glutathione depletion and probenecid treatment. Arch Toxicol. 1989;63(5):345–350. doi: 10.1007/BF00303121. [DOI] [PubMed] [Google Scholar]
- David N. J., Wolman R., Milne F. J., van Niekerk I. Acute renal failure due to trichloroethylene poisoning. Br J Ind Med. 1989 May;46(5):347–349. doi: 10.1136/oem.46.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. J. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- De Craemer D., Vamecq J., Roels F., Vallée L., Pauwels M., Van den Branden C. Peroxisomes in liver, heart, and kidney of mice fed a commercial fish oil preparation: original data and review on peroxisomal changes induced by high-fat diets. J Lipid Res. 1994 Jul;35(7):1241–1250. [PubMed] [Google Scholar]
- Dekant W., Berthold K., Vamvakas S., Henschler D., Anders M. W. Thioacylating intermediates as metabolites of S-(1,2-dichlorovinyl)-L-cysteine and S-(1,2,2-trichlorovinyl)-L-cysteine formed by cysteine conjugate beta-lyase. Chem Res Toxicol. 1988 May-Jun;1(3):175–178. doi: 10.1021/tx00003a008. [DOI] [PubMed] [Google Scholar]
- Dekant W., Martens G., Vamvakas S., Metzler M., Henschler D. Bioactivation of tetrachloroethylene. Role of glutathione S-transferase-catalyzed conjugation versus cytochrome P-450-dependent phospholipid alkylation. Drug Metab Dispos. 1987 Sep-Oct;15(5):702–709. [PubMed] [Google Scholar]
- Dekant W., Vamvakas S., Berthold K., Schmidt S., Wild D., Henschler D. Bacterial beta-lyase mediated cleavage and mutagenicity of cysteine conjugates derived from the nephrocarcinogenic alkenes trichloroethylene, tetrachloroethylene and hexachlorobutadiene. Chem Biol Interact. 1986 Oct 15;60(1):31–45. doi: 10.1016/0009-2797(86)90015-3. [DOI] [PubMed] [Google Scholar]
- Dietrich D. R. Doubting nongenotoxic mechanisms of renal cancer: comparing apples and oranges in the alpha2u-globulin hypothesis. Environ Health Perspect. 1997 Sep;105(9):898–902. doi: 10.1289/ehp.105-1470358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfarra A. A., Jakobson I., Anders M. W. Mechanism of S-(1,2-dichlorovinyl)glutathione-induced nephrotoxicity. Biochem Pharmacol. 1986 Jan 15;35(2):283–288. doi: 10.1016/0006-2952(86)90527-7. [DOI] [PubMed] [Google Scholar]
- Elfarra A. A., Lash L. H., Anders M. W. Alpha-ketoacids stimulate rat renal cysteine conjugate beta-lyase activity and potentiate the cytotoxicity of S-(1,2-dichlorovinyl)-L-cysteine. Mol Pharmacol. 1987 Feb;31(2):208–212. [PubMed] [Google Scholar]
- Eyre R. J., Stevens D. K., Parker J. C., Bull R. J. Acid-labile adducts to protein can be used as indicators of the cysteine S-conjugate pathway of trichloroethene metabolism. J Toxicol Environ Health. 1995 Dec;46(4):443–464. doi: 10.1080/15287399509532048. [DOI] [PubMed] [Google Scholar]
- Eyre R. J., Stevens D. K., Parker J. C., Bull R. J. Renal activation of trichloroethene and S-(1,2-dichlorovinyl)-L-cysteine and cell proliferative responses in the kidneys of F344 rats and B6C3F1 mice. J Toxicol Environ Health. 1995 Dec;46(4):465–481. doi: 10.1080/15287399509532049. [DOI] [PubMed] [Google Scholar]
- Finkle W. D., McLaughlin J. K., Rasgon S. A., Yeoh H. H., Low J. E. Increased risk of renal cell cancer among women using diuretics in the United States. Cancer Causes Control. 1993 Nov;4(6):555–558. doi: 10.1007/BF00052431. [DOI] [PubMed] [Google Scholar]
- Flamm W. G., Lehman-McKeeman L. D. The human relevance of the renal tumor-inducing potential of d-limonene in male rats: implications for risk assessment. Regul Toxicol Pharmacol. 1991 Feb;13(1):70–86. doi: 10.1016/0273-2300(91)90042-t. [DOI] [PubMed] [Google Scholar]
- Gamble J. F., Pearlman E. D., Nicolich M. J. A nested case-control study of kidney cancer among refinery/petrochemical workers. Environ Health Perspect. 1996 Jun;104(6):642–650. doi: 10.1289/ehp.96104642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeptar A. R., Commandeur J. N., van Ommen B., van Bladeren P. J., Vermeulen N. P. Metabolism and kinetics of trichloroethylene in relation to toxicity and carcinogenicity. Relevance of the mercapturic acid pathway. Chem Res Toxicol. 1995 Jan-Feb;8(1):3–21. doi: 10.1021/tx00043a001. [DOI] [PubMed] [Google Scholar]
- Goldsworthy T. L., Lyght O., Burnett V. L., Popp J. A. Potential role of alpha-2 mu-globulin, protein droplet accumulation, and cell replication in the renal carcinogenicity of rats exposed to trichloroethylene, perchloroethylene, and pentachloroethane. Toxicol Appl Pharmacol. 1988 Nov;96(2):367–379. doi: 10.1016/0041-008x(88)90095-6. [DOI] [PubMed] [Google Scholar]
- Goldsworthy T. L., Popp J. A. Chlorinated hydrocarbon-induced peroxisomal enzyme activity in relation to species and organ carcinogenicity. Toxicol Appl Pharmacol. 1987 Apr;88(2):225–233. doi: 10.1016/0041-008x(87)90008-1. [DOI] [PubMed] [Google Scholar]
- Gonsowski C. T., Laster M. J., Eger E. I., 2nd, Ferrell L. D., Kerschmann R. L. Toxicity of compound A in rats. Effect of a 3-hour administration. Anesthesiology. 1994 Mar;80(3):556–565. doi: 10.1097/00000542-199403000-00012. [DOI] [PubMed] [Google Scholar]
- Gonsowski C. T., Laster M. J., Eger E. I., 2nd, Ferrell L. D., Kerschmann R. L. Toxicity of compound A in rats. Effect of increasing duration of administration. Anesthesiology. 1994 Mar;80(3):566–573. doi: 10.1097/00000542-199403000-00013. [DOI] [PubMed] [Google Scholar]
- Green T., Dow J., Ellis M. K., Foster J. R., Odum J. The role of glutathione conjugation in the development of kidney tumours in rats exposed to trichloroethylene. Chem Biol Interact. 1997 Jul 11;105(2):99–117. doi: 10.1016/s0009-2797(97)00040-9. [DOI] [PubMed] [Google Scholar]
- Green T., Dow J., Foster J. R., Hext P. M. Formic acid excretion in rats exposed to trichloroethylene: a possible explanation for renal toxicity in long-term studies. Toxicology. 1998 May 15;127(1-3):39–47. doi: 10.1016/s0300-483x(98)00020-1. [DOI] [PubMed] [Google Scholar]
- Green T., Odum J. Structure/activity studies of the nephrotoxic and mutagenic action of cysteine conjugates of chloro- and fluoroalkenes. Chem Biol Interact. 1985 Jun;54(1):15–31. doi: 10.1016/s0009-2797(85)80149-6. [DOI] [PubMed] [Google Scholar]
- Groves C. E., Hayden P. J., Lock E. A., Schnellmann R. G. Differential cellular effects in the toxicity of haloalkene and haloalkane cysteine conjugates to rabbit renal proximal tubules. J Biochem Toxicol. 1993 Mar;8(1):49–56. doi: 10.1002/jbt.2570080108. [DOI] [PubMed] [Google Scholar]
- Groves C. E., Lock E. A., Schnellmann R. G. Role of lipid peroxidation in renal proximal tubule cell death induced by haloalkene cysteine conjugates. Toxicol Appl Pharmacol. 1991 Jan;107(1):54–62. doi: 10.1016/0041-008x(91)90330-h. [DOI] [PubMed] [Google Scholar]
- Groves C. E., Lock E. A., Schnellmann R. G. The effects of haloalkene cysteine conjugates on cytosolic free calcium levels in suspensions of rat renal proximal tubules. J Biochem Toxicol. 1990 Fall;5(3):187–192. doi: 10.1002/jbt.2570050309. [DOI] [PubMed] [Google Scholar]
- Hashmi M., Vamvakas S., Anders M. W. Bioactivation mechanism of S-(3-oxopropyl)-N-acetyl-L-cysteine, the mercapturic acid of acrolein. Chem Res Toxicol. 1992 May-Jun;5(3):360–365. doi: 10.1021/tx00027a007. [DOI] [PubMed] [Google Scholar]
- Hassall C. D., Brendel K., Gandolfi A. J. Regulation of a S(trans-1,2-dichlorovinyl)-L-cysteine-induced renal tubular toxicity by glutathione. J Appl Toxicol. 1983 Dec;3(6):321–325. doi: 10.1002/jat.2550030610. [DOI] [PubMed] [Google Scholar]
- Hayden P. J., Ichimura T., McCann D. J., Pohl L. R., Stevens J. L. Detection of cysteine conjugate metabolite adduct formation with specific mitochondrial proteins using antibodies raised against halothane metabolite adducts. J Biol Chem. 1991 Oct 5;266(28):18415–18418. [PubMed] [Google Scholar]
- Hayden P. J., Stevens J. L. Cysteine conjugate toxicity, metabolism, and binding to macromolecules in isolated rat kidney mitochondria. Mol Pharmacol. 1990 Mar;37(3):468–476. [PubMed] [Google Scholar]
- Hayden P. J., Welsh C. J., Yang Y., Schaefer W. H., Ward A. J., Stevens J. L. Formation of mitochondrial phospholipid adducts by nephrotoxic cysteine conjugate metabolites. Chem Res Toxicol. 1992 Mar-Apr;5(2):232–237. doi: 10.1021/tx00026a013. [DOI] [PubMed] [Google Scholar]
- Huff J. Mechanisms, chemical carcinogenesis, and risk assessment: cell proliferation and cancer. Am J Ind Med. 1995 Feb;27(2):293–300. doi: 10.1002/ajim.4700270213. [DOI] [PubMed] [Google Scholar]
- Huff J. Response: alpha-2-mu-Globulin Nephropathy, Posed Mechanisms, and White Ravens. Environ Health Perspect. 1996 Dec;104(12):1264–1267. doi: 10.1289/ehp.104-1469546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilinskaja O., Vamvakas S. Alterations of the renal function in the isolated perfused rat kidney system after in vivo and in vitro application of S-(1,2-dichlorovinyl)-L-cysteine and S-(2,2-dichlorovinyl)-L-cysteine. Arch Toxicol. 1996;70(3-4):224–229. doi: 10.1007/s002040050264. [DOI] [PubMed] [Google Scholar]
- Iyer R. A., Frink E. J., Jr, Ebert T. J., Anders M. W. Cysteine conjugate beta-lyase-dependent metabolism of compound A (2-[fluoromethoxy]-1,1,3,3,3-pentafluoro-1-propene) in human subjects anesthetized with sevoflurane and in rats given compound A. Anesthesiology. 1998 Mar;88(3):611–618. doi: 10.1097/00000542-199803000-00009. [DOI] [PubMed] [Google Scholar]
- Jaffe D. R., Gandolfi A. J., Nagle R. B. Chronic toxicity of S-(trans-1,2-dichlorovinyl)-L-cysteine in mice. J Appl Toxicol. 1984 Dec;4(6):315–319. doi: 10.1002/jat.2550040607. [DOI] [PubMed] [Google Scholar]
- Jin L., Davis M. R., Kharasch E. D., Doss G. A., Baillie T. A. Identification in rat bile of glutathione conjugates of fluoromethyl 2,2-difluoro-1-(trifluoromethyl)vinyl ether, a nephrotoxic degradate of the anesthetic agent sevoflurane. Chem Res Toxicol. 1996 Mar;9(2):555–561. doi: 10.1021/tx950162m. [DOI] [PubMed] [Google Scholar]
- Jones T. W., Qin C., Schaeffer V. H., Stevens J. L. Immunohistochemical localization of glutamine transaminase K, a rat kidney cysteine conjugate beta-lyase, and the relationship to the segment specificity of cysteine conjugate nephrotoxicity. Mol Pharmacol. 1988 Nov;34(5):621–627. [PubMed] [Google Scholar]
- Kandel L., Laster M. J., Eger E. I., 2nd, Kerschmann R. L., Martin J. Nephrotoxicity in rats undergoing a one-hour exposure to compound A. Anesth Analg. 1995 Sep;81(3):559–563. doi: 10.1097/00000539-199509000-00024. [DOI] [PubMed] [Google Scholar]
- Kays S. E., Berdanier C. D., Swagler A. R., Lock E. A., Schnellmann R. G. An in vitro model of renal proximal tubule cell regeneration. J Pharmacol Toxicol Methods. 1993 Aug;29(4):211–215. doi: 10.1016/1056-8719(93)90027-c. [DOI] [PubMed] [Google Scholar]
- Kays S. E., Schnellmann R. G. Regeneration of renal proximal tubule cells in primary culture following toxicant injury: response to growth factors. Toxicol Appl Pharmacol. 1995 Jun;132(2):273–280. doi: 10.1006/taap.1995.1108. [DOI] [PubMed] [Google Scholar]
- Keller K. A., Callan C., Prokocimer P., Delgado-Herrera L., Friedman M. B., Hoffman G. M., Wooding W. L., Cusick P. K., Krasula R. W. Inhalation toxicity study of a haloalkene degradant of sevoflurane, Compound A (PIFE), in Sprague-Dawley rats. Anesthesiology. 1995 Dec;83(6):1220–1232. doi: 10.1097/00000542-199512000-00013. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Jubert C. Compound A uptake and metabolism to mercapturic acids and 3,3,3-trifluoro-2-fluoromethoxypropanoic acid during low-flow sevoflurane anesthesia: biomarkers for exposure, risk assessment, and interspecies comparison. Anesthesiology. 1999 Nov;91(5):1267–1278. doi: 10.1097/00000542-199911000-00017. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Jubert C., Spracklin D. K., Hoffman G. M. Dose-dependent metabolism of fluoromethyl-2,2-difluoro-1-(trifluoromethyl)vinyl ether (compound A), an anesthetic degradation product, to mercapturic acids and 3,3,3-trifluoro-2-(fluoromethoxy)propanoic acid in rats. Toxicol Appl Pharmacol. 1999 Oct 1;160(1):49–59. doi: 10.1006/taap.1999.8751. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Thorning D., Garton K., Hankins D. C., Kilty C. G. Role of renal cysteine conjugate beta-lyase in the mechanism of compound A nephrotoxicity in rats. Anesthesiology. 1997 Jan;86(1):160–171. doi: 10.1097/00000542-199701000-00020. [DOI] [PubMed] [Google Scholar]
- Kim H., Kim S. G., Lee M. Y., Novak R. F. Evidence for elevation of cytochrome P4502E1 (alcohol-inducible form) mRNA levels in rat kidney following pyridine administration. Biochem Biophys Res Commun. 1992 Jul 31;186(2):846–853. doi: 10.1016/0006-291x(92)90823-4. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature. 1997 Apr 24;386(6627):761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- Kluwe W. M. The relevance of hepatic peroxisome proliferation in rats to assessment of human carcinogenic risk for pharmaceuticals. Regul Toxicol Pharmacol. 1994 Oct;20(2):170–186. doi: 10.1006/rtph.1994.1068. [DOI] [PubMed] [Google Scholar]
- Krejci M. E., Ridgewell R. E., Koechel D. A. Acute effects of the D-isomer of S-(1,2-dichlorovinyl)cysteine on renal function and ultrastructure in the pentobarbital-anesthetized dog: site-specific toxicity involving the S1 and S2 cells of the proximal tubule. Toxicology. 1991;69(2):151–164. doi: 10.1016/0300-483x(91)90227-r. [DOI] [PubMed] [Google Scholar]
- Lake B. G. Peroxisome proliferation: current mechanisms relating to nongenotoxic carcinogenesis. Toxicol Lett. 1995 Dec;82-83:673–681. doi: 10.1016/0378-4274(95)03513-3. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Anders M. W. Cytotoxicity of S-(1,2-dichlorovinyl)glutathione and S-(1,2-dichlorovinyl)-L-cysteine in isolated rat kidney cells. J Biol Chem. 1986 Oct 5;261(28):13076–13081. [PubMed] [Google Scholar]
- Lash L. H., Anders M. W. Mechanism of S-(1,2-dichlorovinyl)-L-cysteine- and S-(1,2-dichlorovinyl)-L-homocysteine-induced renal mitochondrial toxicity. Mol Pharmacol. 1987 Oct;32(4):549–556. [PubMed] [Google Scholar]
- Lash L. H., Elfarra A. A., Anders M. W. Renal cysteine conjugate beta-lyase. Bioactivation of nephrotoxic cysteine S-conjugates in mitochondrial outer membrane. J Biol Chem. 1986 May 5;261(13):5930–5935. [PubMed] [Google Scholar]
- Lash L. H., Elfarra A. A., Rakiewicz-Nemeth D., Anders M. W. Bioactivation mechanism of cytotoxic homocysteine S-conjugates. Arch Biochem Biophys. 1990 Feb 1;276(2):322–330. doi: 10.1016/0003-9861(90)90727-g. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Fisher J. W., Lipscomb J. C., Parker J. C. Metabolism of trichloroethylene. Environ Health Perspect. 2000 May;108 (Suppl 2):177–200. doi: 10.1289/ehp.00108s2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L. H., Lipscomb J. C., Putt D. A., Parker J. C. Glutathione conjugation of trichloroethylene in human liver and kidney: kinetics and individual variation. Drug Metab Dispos. 1999 Mar;27(3):351–359. [PubMed] [Google Scholar]
- Lash L. H., Nelson R. M., Van Dyke R. A., Anders M. W. Purification and characterization of human kidney cytosolic cysteine conjugate beta-lyase activity. Drug Metab Dispos. 1990 Jan-Feb;18(1):50–54. [PubMed] [Google Scholar]
- Lash L. H., Putt D. A., Brashear W. T., Abbas R., Parker J. C., Fisher J. W. Identification of S-(1,2-dichlorovinyl)glutathione in the blood of human volunteers exposed to trichloroethylene. J Toxicol Environ Health A. 1999 Jan 8;56(1):1–21. doi: 10.1080/009841099158204. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Qian W., Putt D. A., Jacobs K., Elfarra A. A., Krause R. J., Parker J. C. Glutathione conjugation of trichloroethylene in rats and mice: sex-, species-, and tissue-dependent differences. Drug Metab Dispos. 1998 Jan;26(1):12–19. [PubMed] [Google Scholar]
- Lash L. H. Role of renal metabolism in risk to toxic chemicals. Environ Health Perspect. 1994 Dec;102 (Suppl 11):75–79. doi: 10.1289/ehp.94102s1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L. H., Sausen P. J., Duescher R. J., Cooley A. J., Elfarra A. A. Roles of cysteine conjugate beta-lyase and S-oxidase in nephrotoxicity: studies with S-(1,2-dichlorovinyl)-L-cysteine and S-(1,2-dichlorovinyl)-L-cysteine sulfoxide. J Pharmacol Exp Ther. 1994 Apr;269(1):374–383. [PubMed] [Google Scholar]
- Lash L. H., Xu Y., Elfarra A. A., Duescher R. J., Parker J. C. Glutathione-dependent metabolism of trichloroethylene in isolated liver and kidney cells of rats and its role in mitochondrial and cellular toxicity. Drug Metab Dispos. 1995 Aug;23(8):846–853. [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Lehman-McKeeman L. D., Rivera-Torres M. I., Caudill D. Lysosomal degradation of alpha 2u-globulin and alpha 2u-globulin-xenobiotic conjugates. Toxicol Appl Pharmacol. 1990 May;103(3):539–548. doi: 10.1016/0041-008x(90)90326-p. [DOI] [PubMed] [Google Scholar]
- Lindblad P., McLaughlin J. K., Mellemgaard A., Adami H. O. Risk of kidney cancer among patients using analgesics and diuretics: a population-based cohort study. Int J Cancer. 1993 Aug 19;55(1):5–9. doi: 10.1002/ijc.2910550103. [DOI] [PubMed] [Google Scholar]
- Lindblad P., Mellemgaard A., Schlehofer B., Adami H. O., McCredie M., McLaughlin J. K., Mandel J. S. International renal-cell cancer study. V. Reproductive factors, gynecologic operations and exogenous hormones. Int J Cancer. 1995 Apr 10;61(2):192–198. doi: 10.1002/ijc.2910610209. [DOI] [PubMed] [Google Scholar]
- Linet M. S., Chow W. H., McLaughlin J. K., Wacholder S., Yu M. C., Schoenberg J. B., Lynch C., Fraumeni J. F., Jr Analgesics and cancers of the renal pelvis and ureter. Int J Cancer. 1995 Jul 4;62(1):15–18. doi: 10.1002/ijc.2910620105. [DOI] [PubMed] [Google Scholar]
- Lock E. A., Schnellmann R. G. The effect of haloalkene cysteine conjugates on rat renal glutathione reductase and lipoyl dehydrogenase activities. Toxicol Appl Pharmacol. 1990 Jun 1;104(1):180–190. doi: 10.1016/0041-008x(90)90293-4. [DOI] [PubMed] [Google Scholar]
- Lynge E., Andersen A., Nilsson R., Barlow L., Pukkala E., Nordlinder R., Boffetta P., Grandjean P., Heikkilä P., Hörte L. G. Risk of cancer and exposure to gasoline vapors. Am J Epidemiol. 1997 Mar 1;145(5):449–458. doi: 10.1093/oxfordjournals.aje.a009127. [DOI] [PubMed] [Google Scholar]
- MacFarlane M., Foster J. R., Gibson G. G., King L. J., Lock E. A. Cysteine conjugate beta-lyase of rat kidney cytosol: characterization, immunocytochemical localization, and correlation with hexachlorobutadiene nephrotoxicity. Toxicol Appl Pharmacol. 1989 Apr;98(2):185–197. doi: 10.1016/0041-008x(89)90224-x. [DOI] [PubMed] [Google Scholar]
- Maher E. R., Kaelin W. G., Jr von Hippel-Lindau disease. Medicine (Baltimore) 1997 Nov;76(6):381–391. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- Maltoni C., Lefemine G., Cotti G., Perino G. Long-term carcinogenicity bioassays on trichloroethylene administered by inhalation to Sprague-Dawley rats and Swiss and B6C3F1 mice. Ann N Y Acad Sci. 1988;534:316–342. doi: 10.1111/j.1749-6632.1988.tb30120.x. [DOI] [PubMed] [Google Scholar]
- Mandel J. S., McLaughlin J. K., Schlehofer B., Mellemgaard A., Helmert U., Lindblad P., McCredie M., Adami H. O. International renal-cell cancer study. IV. Occupation. Int J Cancer. 1995 May 29;61(5):601–605. doi: 10.1002/ijc.2910610503. [DOI] [PubMed] [Google Scholar]
- Marshall F. F., Stewart A. K., Menck H. R. The National Cancer Data Base: report on kidney cancers. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1997 Dec 1;80(11):2167–2174. [PubMed] [Google Scholar]
- McCredie M., Pommer W., McLaughlin J. K., Stewart J. H., Lindblad P., Mandel J. S., Mellemgaard A., Schlehofer B., Niwa S. International renal-cell cancer study. II. Analgesics. Int J Cancer. 1995 Jan 27;60(3):345–349. doi: 10.1002/ijc.2910600312. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. K., Blot W. J. A critical review of epidemiology studies of trichloroethylene and perchloroethylene and risk of renal-cell cancer. Int Arch Occup Environ Health. 1997;70(4):222–231. doi: 10.1007/s004200050211. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. K., Chow W. H., Mandel J. S., Mellemgaard A., McCredie M., Lindblad P., Schlehofer B., Pommer W., Niwa S., Adami H. O. International renal-cell cancer study. VIII. Role of diuretics, other anti-hypertensive medications and hypertension. Int J Cancer. 1995 Oct 9;63(2):216–221. doi: 10.1002/ijc.2910630212. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. K., Gao Y. T., Gao R. N., Zheng W., Ji B. T., Blot W. J., Fraumeni J. F., Jr Risk factors for renal-cell cancer in Shanghai, China. Int J Cancer. 1992 Oct 21;52(4):562–565. doi: 10.1002/ijc.2910520411. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. K., Hrubec Z., Heineman E. F., Blot W. J., Fraumeni J. F., Jr Renal cancer and cigarette smoking in a 26-year followup of U.S. veterans. Public Health Rep. 1990 Sep-Oct;105(5):535–537. [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. K., Lindblad P., Mellemgaard A., McCredie M., Mandel J. S., Schlehofer B., Pommer W., Adami H. O. International renal-cell cancer study. I. Tobacco use. Int J Cancer. 1995 Jan 17;60(2):194–198. doi: 10.1002/ijc.2910600211. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. K. Renal cell cancer and exposure to gasoline: a review. Environ Health Perspect. 1993 Dec;101 (Suppl 6):111–114. doi: 10.1289/ehp.93101s6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. K., Silverman D. T., Hsing A. W., Ross R. K., Schoenberg J. B., Yu M. C., Stemhagen A., Lynch C. F., Blot W. J., Fraumeni J. F., Jr Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992 Jan 15;52(2):254–257. [PubMed] [Google Scholar]
- Mellemgaard A., Engholm G., McLaughlin J. K., Olsen J. H. Occupational risk factors for renal-cell carcinoma in Denmark. Scand J Work Environ Health. 1994 Jun;20(3):160–165. doi: 10.5271/sjweh.1413. [DOI] [PubMed] [Google Scholar]
- Mellemgaard A., Engholm G., McLaughlin J. K., Olsen J. H. Risk factors for renal cell carcinoma in Denmark. I. Role of socioeconomic status, tobacco use, beverages, and family history. Cancer Causes Control. 1994 Mar;5(2):105–113. doi: 10.1007/BF01830256. [DOI] [PubMed] [Google Scholar]
- Mellemgaard A., Engholm G., McLaughlin J. K., Olsen J. H. Risk factors for renal-cell carcinoma in Denmark. III. Role of weight, physical activity and reproductive factors. Int J Cancer. 1994 Jan 2;56(1):66–71. doi: 10.1002/ijc.2910560113. [DOI] [PubMed] [Google Scholar]
- Mellemgaard A., Lindblad P., Schlehofer B., Bergström R., Mandel J. S., McCredie M., McLaughlin J. K., Niwa S., Odaka N., Pommer W. International renal-cell cancer study. III. Role of weight, height, physical activity, and use of amphetamines. Int J Cancer. 1995 Jan 27;60(3):350–354. doi: 10.1002/ijc.2910600313. [DOI] [PubMed] [Google Scholar]
- Mellemgaard A., Niwa S., Mehl E. S., Engholm G., McLaughlin J. K., Olsen J. H. Risk factors for renal cell carcinoma in Denmark: role of medication and medical history. Int J Epidemiol. 1994 Oct;23(5):923–930. doi: 10.1093/ije/23.5.923. [DOI] [PubMed] [Google Scholar]
- Melnick R. L. An alternative hypothesis on the role of chemically induced protein droplet (alpha 2u-globulin) nephropathy in renal carcinogenesis. Regul Toxicol Pharmacol. 1992 Oct;16(2):111–125. doi: 10.1016/0273-2300(92)90052-b. [DOI] [PubMed] [Google Scholar]
- Melnick R. L. Critique does not validate assumptions in the model on alpha 2u-globulin and renal carcinogenesis. Regul Toxicol Pharmacol. 1993 Oct;18(2):365–368. doi: 10.1006/rtph.1993.1062. [DOI] [PubMed] [Google Scholar]
- Melnick R. L., Kohn M. C., Huff J. Weight of evidence versus weight of speculation to evaluate the alpha2u-globulin hypothesis. Environ Health Perspect. 1997 Sep;105(9):904–906. doi: 10.1289/ehp.105-1470356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. W., Kelsh M. A., Zhao K., Heringer S. Mortality of aerospace workers exposed to trichloroethylene. Epidemiology. 1998 Jul;9(4):424–431. [PubMed] [Google Scholar]
- Morio M., Fujii K., Satoh N., Imai M., Kawakami U., Mizuno T., Kawai Y., Ogasawara Y., Tamura T., Negishi A. Reaction of sevoflurane and its degradation products with soda lime. Toxicity of the byproducts. Anesthesiology. 1992 Dec;77(6):1155–1164. doi: 10.1097/00000542-199212000-00017. [DOI] [PubMed] [Google Scholar]
- Nagaya T., Ishikawa N., Hata H. Sister-chromatid exchanges in lymphocytes of workers exposed to trichloroethylene. Mutat Res. 1989 Mar;222(3):279–282. doi: 10.1016/0165-1218(89)90144-4. [DOI] [PubMed] [Google Scholar]
- Nagaya T., Ishikawa N., Hata H. Urinary total protein and beta-2-microglobulin in workers exposed to trichloroethylene. Environ Res. 1989 Oct;50(1):86–92. doi: 10.1016/s0013-9351(89)80050-7. [DOI] [PubMed] [Google Scholar]
- Nemali M. R., Usuda N., Reddy M. K., Oyasu K., Hashimoto T., Osumi T., Rao M. S., Reddy J. K. Comparison of constitutive and inducible levels of expression of peroxisomal beta-oxidation and catalase genes in liver and extrahepatic tissues of rat. Cancer Res. 1988 Sep 15;48(18):5316–5324. [PubMed] [Google Scholar]
- Odum J., Green T., Foster J. R., Hext P. M. The role of trichloracetic acid and peroxisome proliferation in the differences in carcinogenicity of perchloroethylene in the mouse and rat. Toxicol Appl Pharmacol. 1988 Jan;92(1):103–112. doi: 10.1016/0041-008x(88)90232-3. [DOI] [PubMed] [Google Scholar]
- Park S. B., Osterloh J. D., Vamvakas S., Hashmi M., Anders M. W., Cashman J. R. Flavin-containing monooxygenase-dependent stereoselective S-oxygenation and cytotoxicity of cysteine S-conjugates and mercapturates. Chem Res Toxicol. 1992 Mar-Apr;5(2):193–201. doi: 10.1021/tx00026a008. [DOI] [PubMed] [Google Scholar]
- Partanen T., Heikkilä P., Hernberg S., Kauppinen T., Moneta G., Ojajärvi A. Renal cell cancer and occupational exposure to chemical agents. Scand J Work Environ Health. 1991 Aug;17(4):231–239. doi: 10.5271/sjweh.1708. [DOI] [PubMed] [Google Scholar]
- Ponchaut S., Draye J. P., Veitch K., Van Hoof F. Influence of chronic administration of valproate on ultrastructure and enzyme content of peroxisomes in rat liver and kidney. Oxidation of valproate by liver peroxisomes. Biochem Pharmacol. 1991 May 15;41(10):1419–1428. doi: 10.1016/0006-2952(91)90557-l. [DOI] [PubMed] [Google Scholar]
- Pähler A., Parker J., Dekant W. Dose-dependent protein adduct formation in kidney, liver, and blood of rats and in human blood after perchloroethene inhalation. Toxicol Sci. 1999 Mar;48(1):5–13. doi: 10.1093/toxsci/48.1.5. [DOI] [PubMed] [Google Scholar]
- Rasmussen K., Brogren C. H., Sabroe S. Subclinical affection of liver and kidney function and solvent exposure. Int Arch Occup Environ Health. 1993;64(6):445–448. doi: 10.1007/BF00517951. [DOI] [PubMed] [Google Scholar]
- Rushton L., Alderson M. R. Epidemiological survey of oil distribution centres in Britain. Br J Ind Med. 1983 Aug;40(3):330–339. doi: 10.1136/oem.40.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton L. Further follow up of mortality in a United Kingdom oil distribution centre cohort. Br J Ind Med. 1993 Jun;50(6):561–569. doi: 10.1136/oem.50.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausen P. J., Duescher R. J., Elfarra A. A. Further characterization and purification of the flavin-dependent S-benzyl-L-cysteine S-oxidase activities of rat liver and kidney microsomes. Mol Pharmacol. 1993 Mar;43(3):388–396. [PubMed] [Google Scholar]
- Sausen P. J., Elfarra A. A. Reactivity of cysteine S-conjugate sulfoxides: formation of S-[1-chloro-2-(S-glutathionyl)vinyl]-L-cysteine sulfoxide by the reaction of S-(1,2-dichlorovinyl)-L-cysteine sulfoxide with glutathione. Chem Res Toxicol. 1991 Nov-Dec;4(6):655–660. doi: 10.1021/tx00024a010. [DOI] [PubMed] [Google Scholar]
- Seldén A., Hultberg B., Ulander A., Ahlborg G., Jr Trichloroethylene exposure in vapour degreasing and the urinary excretion of N-acetyl-beta-D-glucosaminidase. Arch Toxicol. 1993;67(3):224–226. doi: 10.1007/BF01973312. [DOI] [PubMed] [Google Scholar]
- Shimada T., Swanson A. F., Leber P., Williams G. M. Activities of chlorinated ethane and ethylene compounds in the Salmonella/rat microsome mutagenesis and rat hepatocyte/DNA repair assays under vapor phase exposure conditions. Cell Biol Toxicol. 1985 Jun;1(3):159–179. doi: 10.1007/BF00120162. [DOI] [PubMed] [Google Scholar]
- Siemiatycki J., Dewar R., Nadon L., Gérin M., Richardson L., Wacholder S. Associations between several sites of cancer and twelve petroleum-derived liquids. Results from a case-referent study in Montreal. Scand J Work Environ Health. 1987 Dec;13(6):493–504. doi: 10.5271/sjweh.2008. [DOI] [PubMed] [Google Scholar]
- Stevens J. L., Hatzinger P. B., Hayden P. J. Quantitation of multiple pathways for the metabolism of nephrotoxic cysteine conjugates using selective inhibitors of L-alpha-hydroxy acid oxidase (L-amino acid oxidase) and cysteine conjugate beta-lyase. Drug Metab Dispos. 1989 May-Jun;17(3):297–303. [PubMed] [Google Scholar]
- Stevens J., Hayden P., Taylor G. The role of glutathione conjugate metabolism and cysteine conjugate beta-lyase in the mechanism of S-cysteine conjugate toxicity in LLC-PK1 cells. J Biol Chem. 1986 Mar 5;261(7):3325–3332. [PubMed] [Google Scholar]
- Stewart P. A., Lee J. S., Marano D. E., Spirtas R., Forbes C. D., Blair A. Retrospective cohort mortality study of workers at an aircraft maintenance facility. II. Exposures and their assessment. Br J Ind Med. 1991 Aug;48(8):531–537. doi: 10.1136/oem.48.8.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonard M. D., Parker V. H. 2-Oxoacid dehydrogenases of rat liver mitochondria as the site of action of S-(1,2 dichlorovinyl)-L-cysteine and S-(1,2-dichlorovinyl)-3-mercaptopropionic acid. Biochem Pharmacol. 1971 Sep;20(9):2417–2427. doi: 10.1016/0006-2952(71)90242-5. [DOI] [PubMed] [Google Scholar]
- Stonard M. D., Parker V. H. The metabolism of S-(1,2 dichlorovinyl)-L-cysteine by rat liver mitochondria. Biochem Pharmacol. 1971 Sep;20(9):2429–2437. doi: 10.1016/0006-2952(71)90243-7. [DOI] [PubMed] [Google Scholar]
- Sundseth S. S., Waxman D. J. Sex-dependent expression and clofibrate inducibility of cytochrome P450 4A fatty acid omega-hydroxylases. Male specificity of liver and kidney CYP4A2 mRNA and tissue-specific regulation by growth hormone and testosterone. J Biol Chem. 1992 Feb 25;267(6):3915–3921. [PubMed] [Google Scholar]
- Swaen G. M. Increased incidence of renal cell tumours in a cohort of cardboard workers exposed to trichloroethylene. Arch Toxicol. 1995;70(2):127-8, 131-3. doi: 10.1007/BF02733674. [DOI] [PubMed] [Google Scholar]
- TERRACINI B., PARKER V. H. A PATHOLOGICAL STUDY ON THE TOXICITY OF S-DICHLOROVINYL-L-CYSTEINE. Food Cosmet Toxicol. 1965 Jul;3:67–74. doi: 10.1016/s0015-6264(65)80010-4. [DOI] [PubMed] [Google Scholar]
- Vamvakas S., Bittner D., Dekant W., Anders M. W. Events that precede and that follow S-(1,2-dichlorovinyl)-L-cysteine-induced release of mitochondrial Ca2+ and their association with cytotoxicity to renal cells. Biochem Pharmacol. 1992 Sep 25;44(6):1131–1138. doi: 10.1016/0006-2952(92)90377-u. [DOI] [PubMed] [Google Scholar]
- Vamvakas S., Brüning T., Thomasson B., Lammert M., Baumüller A., Bolt H. M., Dekant W., Birner G., Henschler D., Ulm K. Renal cell cancer correlated with occupational exposure to trichloroethene. J Cancer Res Clin Oncol. 1998;124(7):374–382. doi: 10.1007/s004320050186. [DOI] [PubMed] [Google Scholar]
- Vamvakas S., Dekant W., Henschler D. Assessment of unscheduled DNA synthesis in a cultured line of renal epithelial cells exposed to cysteine S-conjugates of haloalkenes and haloalkanes. Mutat Res. 1989 Apr;222(4):329–335. doi: 10.1016/0165-1218(89)90108-0. [DOI] [PubMed] [Google Scholar]
- Vamvakas S., Dekant W., Schiffmann D., Henschler D. Induction of unscheduled DNA synthesis and micronucleus formation in Syrian hamster embryo fibroblasts treated with cysteine S-conjugates of chlorinated hydrocarbons. Cell Biol Toxicol. 1988 Dec;4(4):393–403. doi: 10.1007/BF00117768. [DOI] [PubMed] [Google Scholar]
- Vamvakas S., Elfarra A. A., Dekant W., Henschler D., Anders M. W. Mutagenicity of amino acid and glutathione S-conjugates in the Ames test. Mutat Res. 1988 Sep;206(1):83–90. doi: 10.1016/0165-1218(88)90144-9. [DOI] [PubMed] [Google Scholar]
- Vamvakas S., Köster U. The nephrotoxin dichlorovinylcysteine induces expression of the protooncogenes c-fos and c-myc in LLC-PK1 cells--a comparative investigation with growth factors and 12-O-tetradecanoylphorbolacetate. Cell Biol Toxicol. 1993 Jan-Mar;9(1):1–13. doi: 10.1007/BF00755136. [DOI] [PubMed] [Google Scholar]
- Vamvakas S., Sharma V. K., Sheu S. S., Anders M. W. Perturbations of intracellular calcium distribution in kidney cells by nephrotoxic haloalkenyl cysteine S-conjugates. Mol Pharmacol. 1990 Oct;38(4):455–461. [PubMed] [Google Scholar]
- Van den Branden C., De Craemer D., Pauwels M., Vamecq J. Peroxisomes in mice fed a diet supplemented with low doses of fish oil. Lipids. 1995 Aug;30(8):701–705. doi: 10.1007/BF02537795. [DOI] [PubMed] [Google Scholar]
- Völkel W., Friedewald M., Lederer E., Pähler A., Parker J., Dekant W. Biotransformation of perchloroethene: dose-dependent excretion of trichloroacetic acid, dichloroacetic acid, and N-acetyl-S-(trichlorovinyl)-L-cysteine in rats and humans after inhalation. Toxicol Appl Pharmacol. 1998 Nov;153(1):20–27. doi: 10.1006/taap.1998.8548. [DOI] [PubMed] [Google Scholar]
- Wallin A., Zhang G., Jones T. W., Jaken S., Stevens J. L. Mechanism of the nephrogenic repair response. Studies on proliferation and vimentin expression after 35S-1,2-dichlorovinyl-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest. 1992 Apr;66(4):474–484. [PubMed] [Google Scholar]
- Ward J. M., Stevens J. L., Konishi N., Kurata Y., Uno H., Diwan B. A., Ohmori T. Vimentin metaplasia in renal cortical tubules of preneoplastic, neoplastic, aging, and regenerative lesions of rats and humans. Am J Pathol. 1992 Oct;141(4):955–964. [PMC free article] [PubMed] [Google Scholar]
- Weiss N. S. Cancer in relation to occupational exposure to trichloroethylene. Occup Environ Med. 1996 Jan;53(1):1–5. doi: 10.1136/oem.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M., Birner G., Dekant W. Sulfoxidation of mercapturic acids derived from tri- and tetrachloroethene by cytochromes P450 3A: a bioactivation reaction in addition to deacetylation and cysteine conjugate beta-lyase mediated cleavage. Chem Res Toxicol. 1996 Jan-Feb;9(1):41–49. doi: 10.1021/tx950075u. [DOI] [PubMed] [Google Scholar]
- White A. E., Takehisa S., Eger E. I., 2nd, Wolff S., Stevens W. C. Sister chromatid exchanges induced by inhaled anesthetics. Anesthesiology. 1979 May;50(5):426–430. doi: 10.1097/00000542-197905000-00010. [DOI] [PubMed] [Google Scholar]
- Wolfgang G. H., Gandolfi A. J., Nagle R. B., Brendel K., Stevens J. L. Assessment of S-(1,2-dichlorovinyl)-L-cysteine induced toxic events in rabbit renal cortical slices. Biochemical and histological evaluation of uptake, covalent binding, and toxicity. Chem Biol Interact. 1990;75(2):153–170. doi: 10.1016/0009-2797(90)90115-4. [DOI] [PubMed] [Google Scholar]
- Wolfgang G. H., Gandolfi A. J., Stevens J. L., Brendel K. In vitro and in vivo nephrotoxicity of the L and D isomers of S-(1,2-dichlorovinyl)-cysteine. Toxicology. 1989 Sep;58(1):33–42. doi: 10.1016/0300-483x(89)90102-9. [DOI] [PubMed] [Google Scholar]
- Wolfgang G. H., Gandolfi A. J., Stevens J. L., Brendel K. N-acetyl S-(1,2-dichlorovinyl)-L-cysteine produces a similar toxicity to S-(1,2-dichlorovinyl)-L-cysteine in rabbit renal slices: differential transport and metabolism. Toxicol Appl Pharmacol. 1989 Nov;101(2):205–219. doi: 10.1016/0041-008x(89)90270-6. [DOI] [PubMed] [Google Scholar]
- Wolk A., Gridley G., Niwa S., Lindblad P., McCredie M., Mellemgaard A., Mandel J. S., Wahrendorf J., McLaughlin J. K., Adami H. O. International renal cell cancer study. VII. Role of diet. Int J Cancer. 1996 Jan 3;65(1):67–73. doi: 10.1002/(SICI)1097-0215(19960103)65:1<67::AID-IJC12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Wong O., Raabe G. K. Critical review of cancer epidemiology in petroleum industry employees, with a quantitative meta-analysis by cancer site. Am J Ind Med. 1989;15(3):283–310. doi: 10.1002/ajim.4700150305. [DOI] [PubMed] [Google Scholar]
- Wu D., Cederbaum A. I. Characterization of pyrazole and 4-methylpyrazole induction of cytochrome P4502E1 in rat kidney. J Pharmacol Exp Ther. 1994 Jul;270(1):407–413. [PubMed] [Google Scholar]
- Yu K., Chen Q., Liu H., Zhan Y., Stevens J. L. Signalling the molecular stress response to nephrotoxic and mutagenic cysteine conjugates: differential roles for protein synthesis and calcium in the induction of c-fos and c-myc mRNA in LLC-PK1 cells. J Cell Physiol. 1994 Nov;161(2):303–311. doi: 10.1002/jcp.1041610215. [DOI] [PubMed] [Google Scholar]
- de la Iglesia F. A., Gough A. W., Sigler R. E. alpha2u-Globulin nephropathy and ravens: do ravens of a different feather flock together? Environ Health Perspect. 1997 Sep;105(9):903–904. doi: 10.1289/ehp.105-1470350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Welie R. T., van Dijck R. G., Vermeulen N. P., van Sittert N. J. Mercapturic acids, protein adducts, and DNA adducts as biomarkers of electrophilic chemicals. Crit Rev Toxicol. 1992;22(5-6):271–306. doi: 10.3109/10408449209146310. [DOI] [PubMed] [Google Scholar]
- van de Water B., Zoeteweij J. P., de Bont H. J., Mulder G. J., Nagelkerke J. F. Role of mitochondrial Ca2+ in the oxidative stress-induced dissipation of the mitochondrial membrane potential. Studies in isolated proximal tubular cells using the nephrotoxin 1,2-dichlorovinyl-L-cysteine. J Biol Chem. 1994 May 20;269(20):14546–14552. [PubMed] [Google Scholar]
- van de Water B., Zoeteweij J. P., de Bont H. J., Nagelkerke J. F. Inhibition of succinate:ubiquinone reductase and decrease of ubiquinol in nephrotoxic cysteine S-conjugate-induced oxidative cell injury. Mol Pharmacol. 1995 Nov;48(5):928–937. [PubMed] [Google Scholar]
- van de Water B., Zoetewey J. P., de Bont H. J., Mulder G. J., Nagelkerke J. F. The relationship between intracellular Ca2+ and the mitochondrial membrane potential in isolated proximal tubular cells from rat kidney exposed to the nephrotoxin 1,2-dichlorovinyl-cysteine. Biochem Pharmacol. 1993 Jun 9;45(11):2259–2267. doi: 10.1016/0006-2952(93)90197-5. [DOI] [PubMed] [Google Scholar]


