Abstract
This article reviews progress in the research of methyl mercury (MeHg) and nutrient interactions during the past two decades. Special emphasis is placed on the following three major areas: a) effects on kinetics, b) effects on toxicity, and c) possible mechanisms. Dietary information is not usually collected in most epidemiologic studies examining of the effects of MeHg exposure. However, inconsistency of the MeHg toxicity observed in different populations is commonly attributed to possible effects of dietary modulation. Even though the mechanisms of interaction have not been totally elucidated, research in nutritional toxicology has provided insights into the understanding of the effects of nutrients on MeHg toxicity. Some of this information can be readily incorporated into the risk assessment of MeHg in the diets of fish-eating populations. It is also clear that there is a need for more studies designed specifically to address the role of nutrition in the metabolism and detoxification of MeHg. It is also important to collect more detailed dietary information in future epidemiologic studies of MeHg exposure.
Full text
PDF









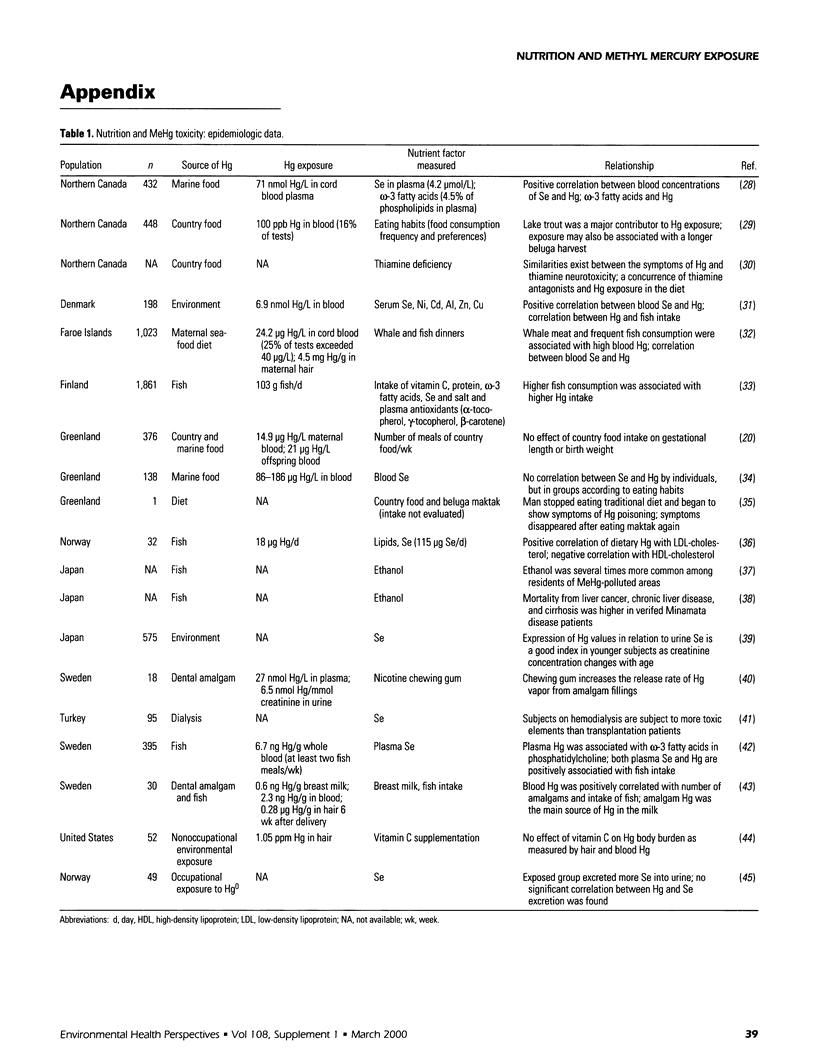
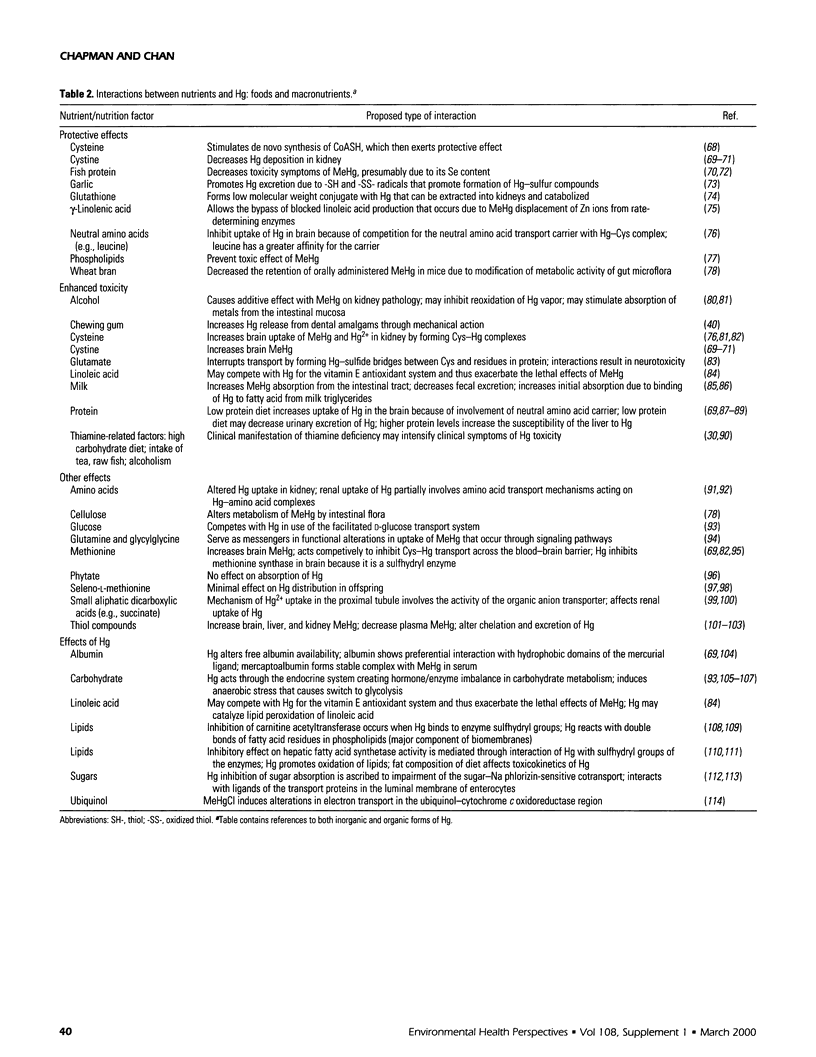

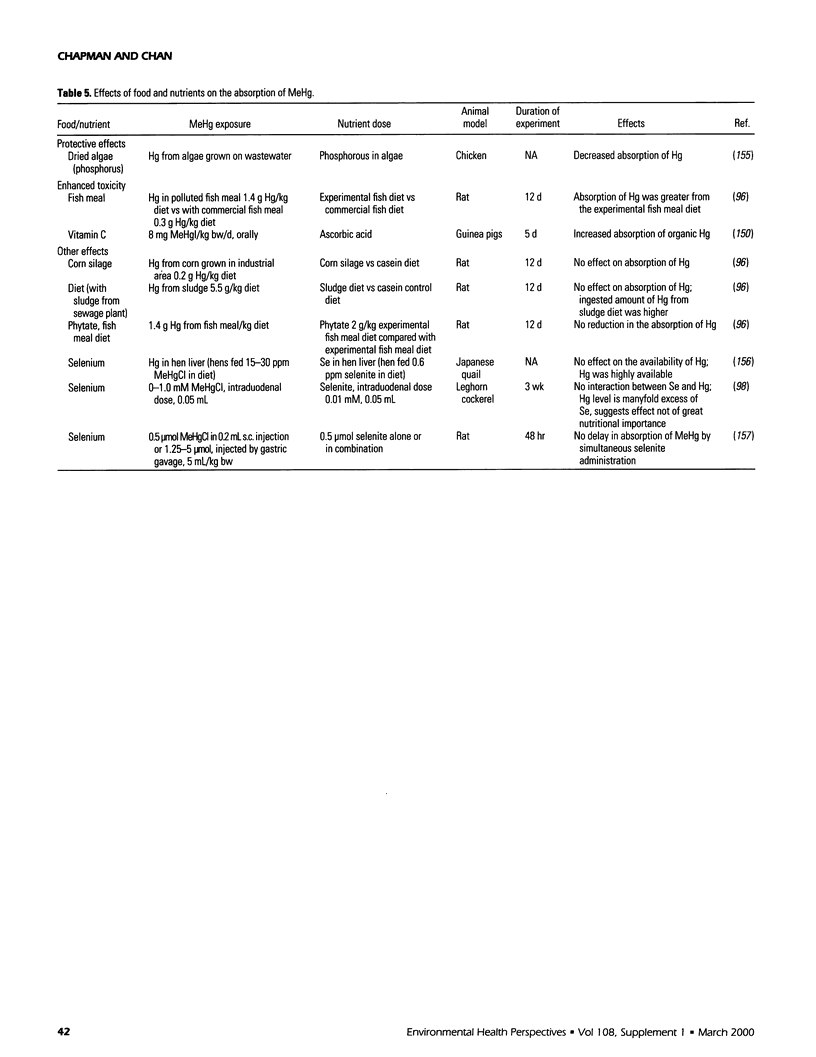
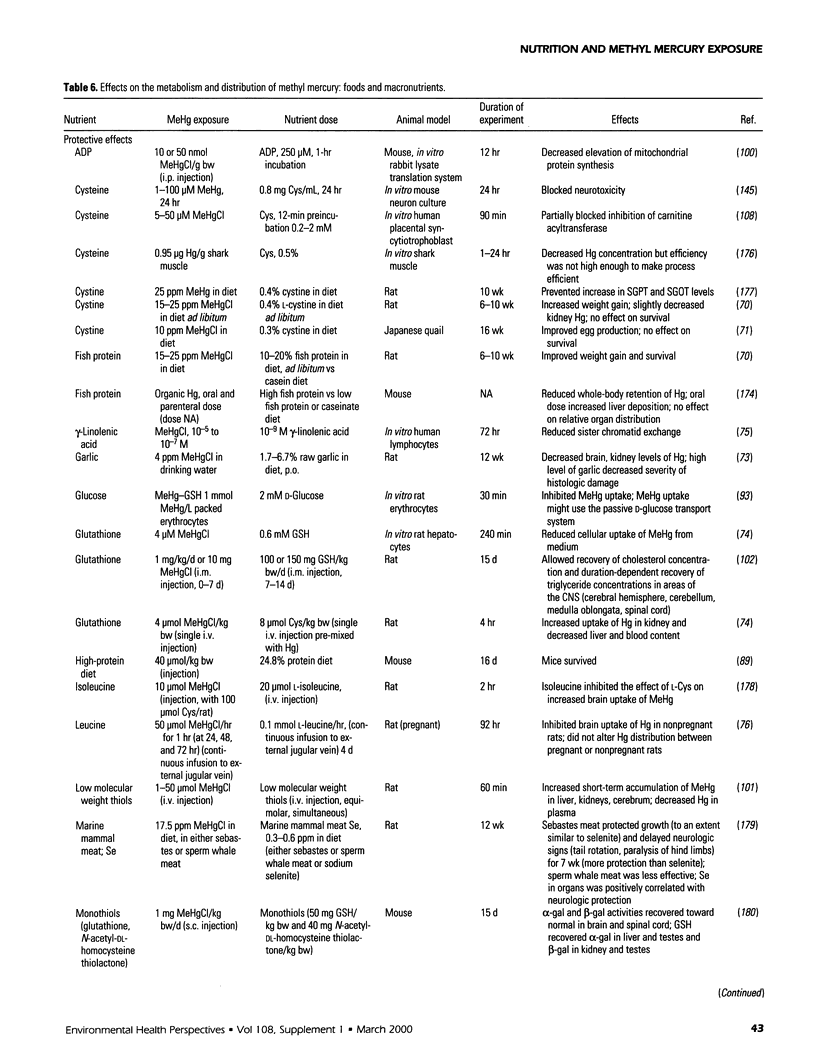
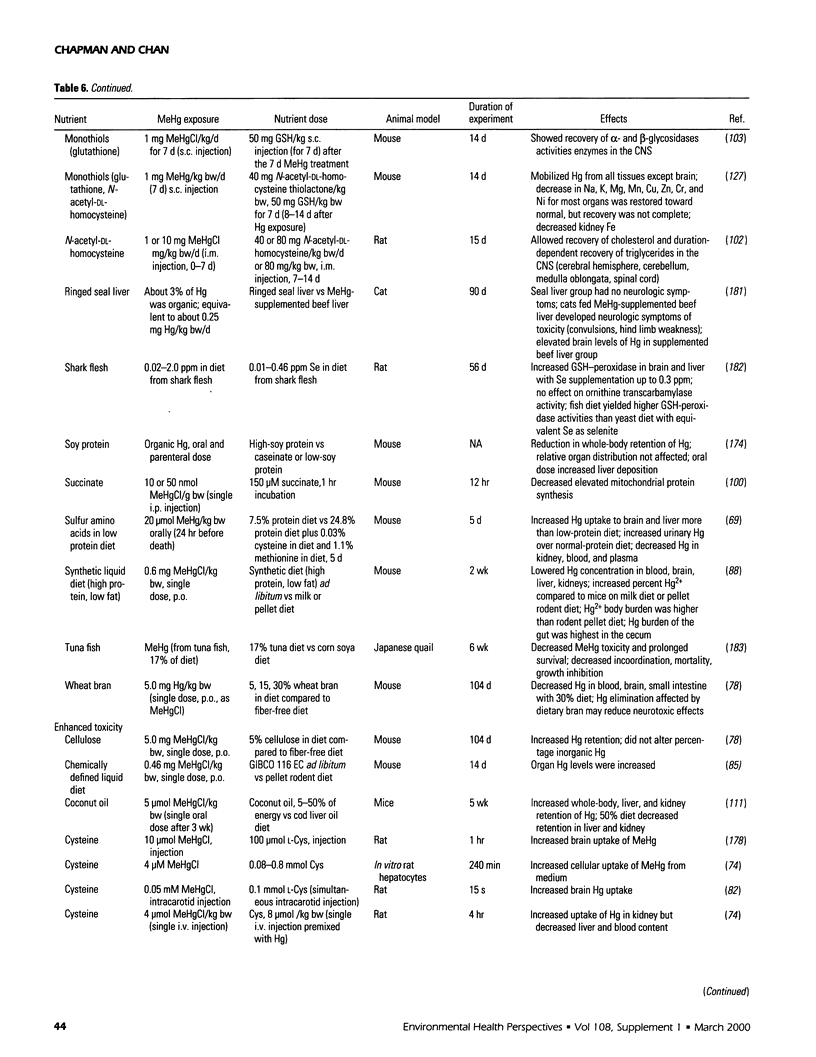
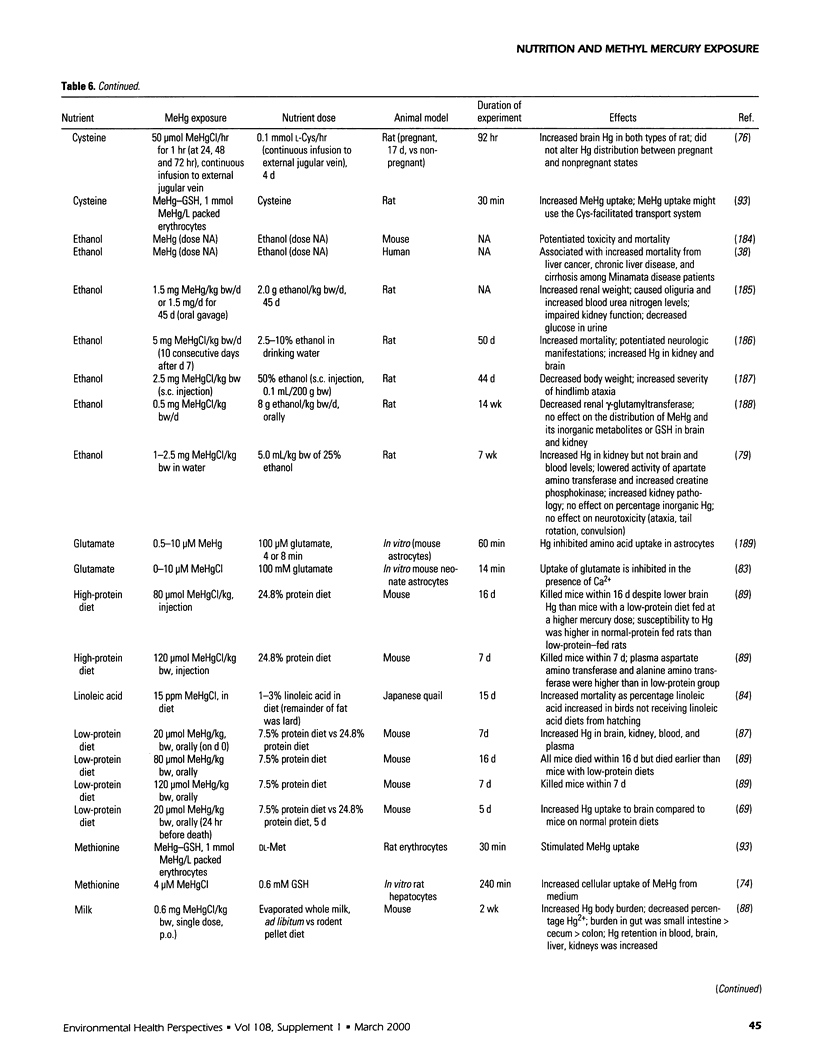
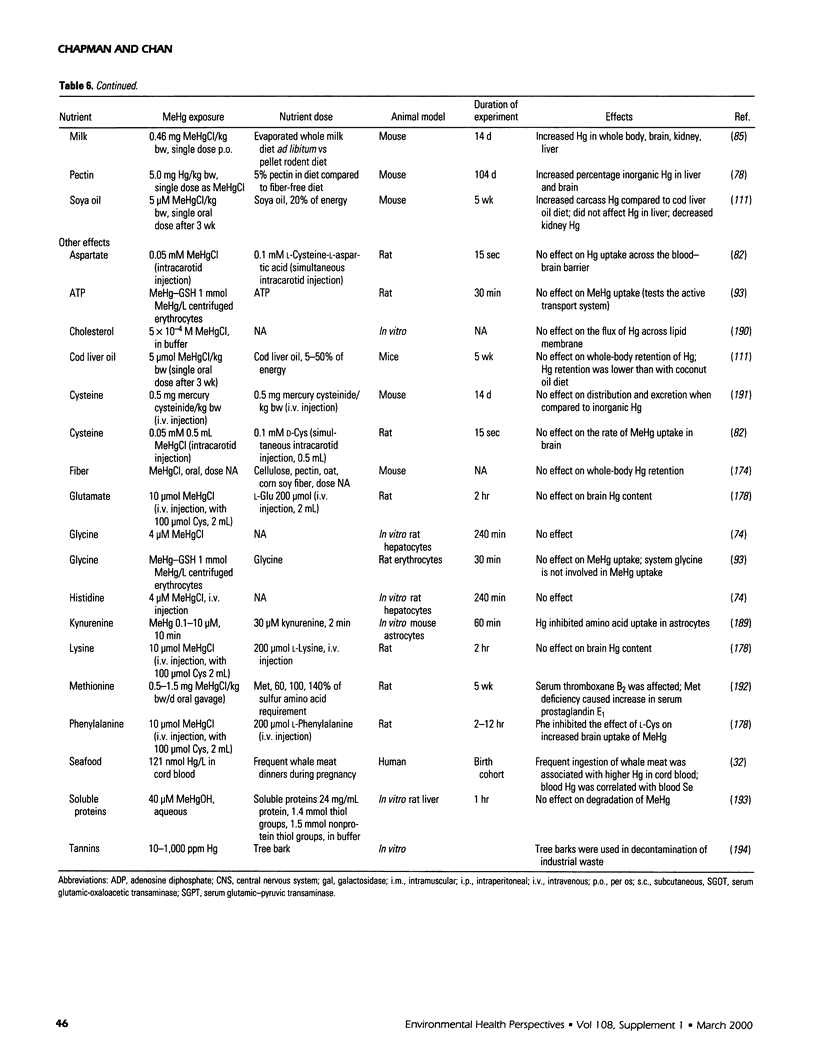
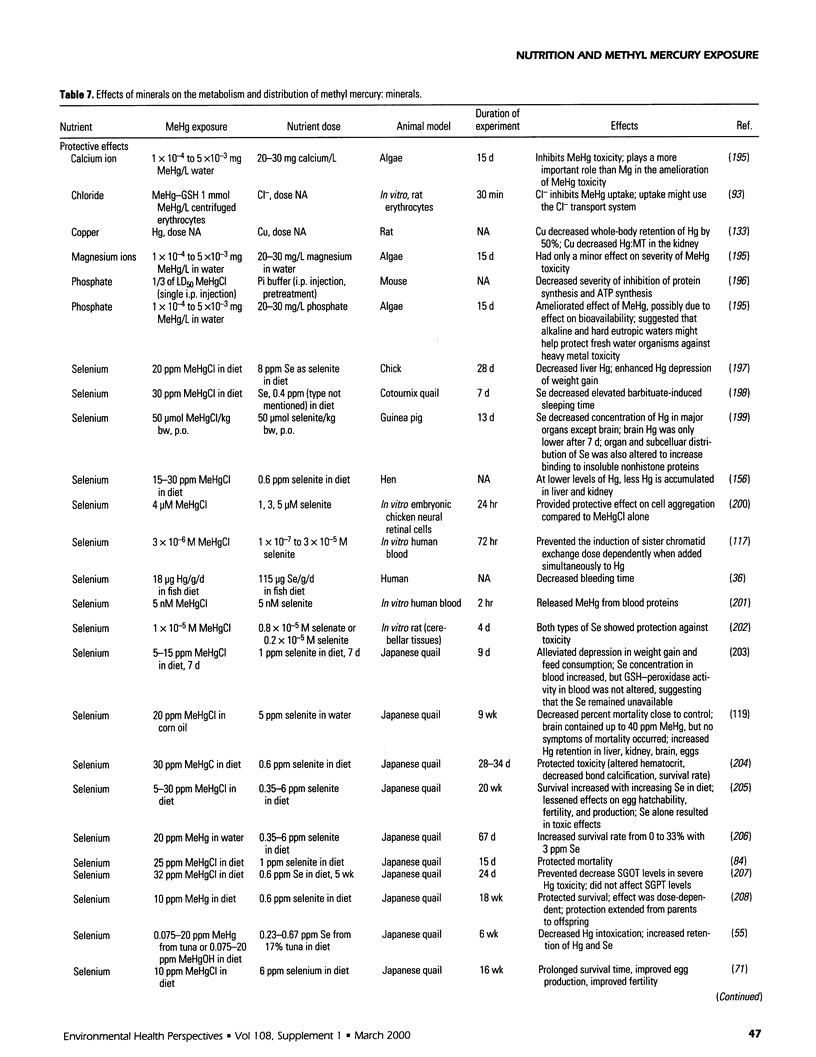
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaseth J. Recent advance in the therapy of metal poisonings with chelating agents. Hum Toxicol. 1983 Apr;2(2):257–272. doi: 10.1177/096032718300200214. [DOI] [PubMed] [Google Scholar]
- Abdulla M., Chmielnicka J. New aspects on the distribution and metabolism of essential trace elements after dietary exposure to toxic metals. Biol Trace Elem Res. 1989;23:25–53. doi: 10.1007/BF02917176. [DOI] [PubMed] [Google Scholar]
- Abramson J. J., Trimm J. L., Weden L., Salama G. Heavy metals induce rapid calcium release from sarcoplasmic reticulum vesicles isolated from skeletal muscle. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1526–1530. doi: 10.1073/pnas.80.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Yasutake A., Eto K., Hirayama K. Influence of dietary protein levels on the acute toxicity of methylmercury in mice. Toxicology. 1996 Aug 1;112(1):11–17. doi: 10.1016/0300-483x(96)03340-9. [DOI] [PubMed] [Google Scholar]
- Adachi T., Yasutake A., Hirayama K. Influence of dietary levels of protein and sulfur amino acids on the fate of methylmercury in mice. Toxicology. 1994 Nov 11;93(2-3):225–234. doi: 10.1016/0300-483x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Adachi T., Yasutake A., Hirayama K. Influence of dietary protein levels on the fate of methylmercury and glutathione metabolism in mice. Toxicology. 1992;72(1):17–26. doi: 10.1016/0300-483x(92)90082-p. [DOI] [PubMed] [Google Scholar]
- Alexander J., Aaseth J. Organ distribution and cellular uptake of methyl mercury in the rat as influenced by the intra- and extracellular glutathione concentration. Biochem Pharmacol. 1982 Mar 1;31(5):685–690. doi: 10.1016/0006-2952(82)90450-6. [DOI] [PubMed] [Google Scholar]
- Alexander J., Thomassen Y., Aaseth J. Increased urinary excretion of selenium among workers exposed to elemental mercury vapor. J Appl Toxicol. 1983 Jun;3(3):143–145. doi: 10.1002/jat.2550030308. [DOI] [PubMed] [Google Scholar]
- Ali S. F., LeBel C. P., Bondy S. C. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992 Fall;13(3):637–648. [PubMed] [Google Scholar]
- Andersen H. R., Andersen O. Effects of dietary alpha-tocopherol and beta-carotene on lipid peroxidation induced by methyl mercuric chloride in mice. Pharmacol Toxicol. 1993 Oct;73(4):192–201. doi: 10.1111/j.1600-0773.1993.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Anner B. M., Moosmayer M., Imesch E. Mercury blocks Na-K-ATPase by a ligand-dependent and reversible mechanism. Am J Physiol. 1992 May;262(5 Pt 2):F830–F836. doi: 10.1152/ajprenal.1992.262.5.F830. [DOI] [PubMed] [Google Scholar]
- Anner B. M., Moosmayer M. Mercury inhibits Na-K-ATPase primarily at the cytoplasmic side. Am J Physiol. 1992 May;262(5 Pt 2):F843–F848. doi: 10.1152/ajprenal.1992.262.5.F843. [DOI] [PubMed] [Google Scholar]
- Anttolainen M., Valsta L. M., Alfthan G., Kleemola P., Salminen I., Tamminen M. Effect of extreme fish consumption on dietary and plasma antioxidant levels and fatty acid composition. Eur J Clin Nutr. 1996 Nov;50(11):741–746. [PubMed] [Google Scholar]
- Arakawa O., Nakahiro M., Narahashi T. Mercury modulation of GABA-activated chloride channels and non-specific cation channels in rat dorsal root ganglion neurons. Brain Res. 1991 Jun 14;551(1-2):58–63. doi: 10.1016/0006-8993(91)90913-g. [DOI] [PubMed] [Google Scholar]
- Aronsson A. M., Lind B., Nylander M., Nordberg M. Dental amalgam and mercury. Biol Met. 1989;2(1):25–30. doi: 10.1007/BF01116197. [DOI] [PubMed] [Google Scholar]
- Aschner M., Aschner J. L. Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav Rev. 1990 Summer;14(2):169–176. doi: 10.1016/s0149-7634(05)80217-9. [DOI] [PubMed] [Google Scholar]
- Aschner M., Clarkson T. W. Mercury 203 distribution in pregnant and nonpregnant rats following systemic infusions with thiol-containing amino acids. Teratology. 1987 Dec;36(3):321–328. doi: 10.1002/tera.1420360308. [DOI] [PubMed] [Google Scholar]
- Aschner M., Clarkson T. W. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res. 1988 Oct 11;462(1):31–39. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Aschner M., Conklin D. R., Yao C. P., Allen J. W., Tan K. H. Induction of astrocyte metallothioneins (MTs) by zinc confers resistance against the acute cytotoxic effects of methylmercury on cell swelling, Na+ uptake, and K+ release. Brain Res. 1998 Dec 7;813(2):254–261. doi: 10.1016/s0006-8993(98)00947-0. [DOI] [PubMed] [Google Scholar]
- Aschner M., Mullaney K. J., Wagoner D., Lash L. H., Kimelberg H. K. Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)- and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocytes cultures. Brain Res. 1994 Nov 21;664(1-2):133–140. doi: 10.1016/0006-8993(94)91963-1. [DOI] [PubMed] [Google Scholar]
- Aschner M., Rising L., Mullaney K. J. Differential sensitivity of neonatal rat astrocyte cultures to mercuric chloride (MC) and methylmercury (MeHg): studies on K+ and amino acid transport and metallothionein (MT) induction. Neurotoxicology. 1996 Spring;17(1):107–116. [PubMed] [Google Scholar]
- Aschner M., Vitarella D., Allen J. W., Conklin D. R., Cowan K. S. Methylmercury-induced astrocytic swelling is associated with activation of the Na+/H+ antiporter, and is fully reversed by amiloride. Brain Res. 1998 Jul 20;799(2):207–214. doi: 10.1016/s0006-8993(98)00399-0. [DOI] [PubMed] [Google Scholar]
- Atchison W. D., Hare M. F. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994 Jun;8(9):622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- Atchison W. D., Joshi U., Thornburg J. E. Irreversible suppression of calcium entry into nerve terminals by methylmercury. J Pharmacol Exp Ther. 1986 Aug;238(2):618–624. [PubMed] [Google Scholar]
- Bala K. V., Sridevi K., Rao K. P. Inhibition of methyl mercury chloride-induced chromosomal damage by gamma-linolenic acid. Food Chem Toxicol. 1993 Jun;31(6):431–434. doi: 10.1016/0278-6915(93)90158-u. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Lieberman M. W., Wang W. N-acetylcysteine as an antidote in methylmercury poisoning. Environ Health Perspect. 1998 May;106(5):267–271. doi: 10.1289/ehp.98106267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthrop J. E., Braddon S. A. Effects of selenium and methylmercury upon glutathione and glutathione-S-transferase in mice. Arch Environ Contam Toxicol. 1985 Mar;14(2):197–202. doi: 10.1007/BF01055612. [DOI] [PubMed] [Google Scholar]
- Bano Y., Hasan M. Mercury induced time-dependent alterations in lipid profiles and lipid peroxidation in different body organs of cat-fish Heteropneustes fossilis. J Environ Sci Health B. 1989 Apr;24(2):145–166. doi: 10.1080/03601238909372641. [DOI] [PubMed] [Google Scholar]
- Bapu C., Purohit R. C., Sood P. P. Fluctuation of trace elements during methylmercury toxication and chelation therapy. Hum Exp Toxicol. 1994 Dec;13(12):815–823. doi: 10.1177/096032719401301201. [DOI] [PubMed] [Google Scholar]
- Bapu C., Vijayalakshmi K., Sood P. P. Comparison of monothiols and vitamin therapy administered alone or in combinations during methylmercury poisoning. Bull Environ Contam Toxicol. 1994 Feb;52(2):182–189. doi: 10.1007/BF00198486. [DOI] [PubMed] [Google Scholar]
- Björkman L., Mottet K., Nylander M., Vahter M., Lind B., Friberg L. Selenium concentrations in brain after exposure to methylmercury: relations between the inorganic mercury fraction and selenium. Arch Toxicol. 1995;69(4):228–234. doi: 10.1007/s002040050163. [DOI] [PubMed] [Google Scholar]
- Björkman L., Palm B., Nylander M., Nordberg M. Mercury and selenium distribution in human kidney cortex. Biol Trace Elem Res. 1994 Mar;40(3):255–265. doi: 10.1007/BF02950798. [DOI] [PubMed] [Google Scholar]
- Blackstone S., Hurley R. J., Hughes R. E. Some inter-relationships between vitamin C (L-ascorbic acid) and mercury in the guinea-pig. Food Cosmet Toxicol. 1974 Aug;12(4):511–516. doi: 10.1016/0015-6264(74)90065-0. [DOI] [PubMed] [Google Scholar]
- Boudou A., Ribeyre F. Mercury in the food web: accumulation and transfer mechanisms. Met Ions Biol Syst. 1997;34:289–319. [PubMed] [Google Scholar]
- Brookes N., Kristt D. A. Inhibition of amino acid transport and protein synthesis by HgCl2 and methylmercury in astrocytes: selectivity and reversibility. J Neurochem. 1989 Oct;53(4):1228–1237. doi: 10.1111/j.1471-4159.1989.tb07419.x. [DOI] [PubMed] [Google Scholar]
- Burger J., Sanchez J., Gochfeld M. Fishing, consumption, and risk perception in fisherfolk along an east coast estuary. Environ Res. 1998 Apr;77(1):25–35. doi: 10.1006/enrs.1997.3819. [DOI] [PubMed] [Google Scholar]
- Böhme M., Diener M., Rummel W. Chloride secretion induced by mercury and cadmium: action sites and mechanisms. Toxicol Appl Pharmacol. 1992 Jun;114(2):295–301. doi: 10.1016/0041-008x(92)90080-c. [DOI] [PubMed] [Google Scholar]
- Calabrese E. J., Stoddard A., Leonard D. A., Dinardi S. R. The effects of vitamin C supplementation on blood and hair levels of cadmium, lead, and mercury. Ann N Y Acad Sci. 1987;498:347–353. doi: 10.1111/j.1749-6632.1987.tb23773.x. [DOI] [PubMed] [Google Scholar]
- Cambar J., Boudou A., Hocquellet P., Faugére J. G. Etude de la fixation du mercure sur les différentes sous-fractions de la sérumalbumine humaine, séparées par électrophorése en gel de polyacrylamide. Eur J Toxicol Environ Hyg. 1975 Jul-Aug;8(4):201–204. [PubMed] [Google Scholar]
- Cappon C. J., Smith J. C. Chemical form and distribution of mercury and selenium in eggs from chickens fed mercury-contaminated grain. Bull Environ Contam Toxicol. 1981 Apr;26(4):472–478. doi: 10.1007/BF01622122. [DOI] [PubMed] [Google Scholar]
- Caurant F., Navarro M., Amiard J. C. Mercury in pilot whales: possible limits to the detoxification process. Sci Total Environ. 1996 Jul 16;186(1-2):95–104. doi: 10.1016/0048-9697(96)05087-5. [DOI] [PubMed] [Google Scholar]
- Cha C. W. A study on the effect of garlic to the heavy metal poisoning of rat. J Korean Med Sci. 1987 Dec;2(4):213–224. doi: 10.3346/jkms.1987.2.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. W., Gilbert M., Sprecher J. Modification of methylmercury neurotoxicity by vitamin E. Environ Res. 1978 Dec;17(3):356–366. doi: 10.1016/0013-9351(78)90040-3. [DOI] [PubMed] [Google Scholar]
- Chang L. W., Suber R. Protective effect of selenium on methylmercury toxicity: a possible mechanism. Bull Environ Contam Toxicol. 1982 Sep;29(3):285–289. doi: 10.1007/BF01706230. [DOI] [PubMed] [Google Scholar]
- Chen R. W., Lacy V. L., Whanger P. D. Effect of selenium on methylmercury binding to subcellular and soluble proteins in rat tissues. Res Commun Chem Pathol Pharmacol. 1975 Oct;12(2):297–308. [PubMed] [Google Scholar]
- Chetty C. S., Rajanna S., Hall E., Yallapragada P. R., Rajanna B. In vitro and in vivo effects of lead, methyl mercury and mercury on inositol 1,4,5-trisphosphate and 1,3,4,5-tetrakisphosphate receptor bindings in rat brain. Toxicol Lett. 1996 Sep;87(1):11–17. doi: 10.1016/0378-4274(96)03670-3. [DOI] [PubMed] [Google Scholar]
- Chmielnicka J., Komsta-Szumska E., Zareba G. Effect of interaction between 65Zn, mercury and selenium in rats (retention, metallothionein, endogenous copper). Arch Toxicol. 1983 Jun;53(2):165–175. doi: 10.1007/BF00302724. [DOI] [PubMed] [Google Scholar]
- Chowdhury B. A., Chandra R. K. Biological and health implications of toxic heavy metal and essential trace element interactions. Prog Food Nutr Sci. 1987;11(1):55–113. [PubMed] [Google Scholar]
- Cikrt M., Tichý M. Effect of some chelating agents on the biliary excretion of mercury. 1. Excretion kinetics and distribution of mercury in the organism. J Hyg Epidemiol Microbiol Immunol. 1980;24(3):346–355. [PubMed] [Google Scholar]
- Clarkson T. W. Molecular and ionic mimicry of toxic metals. Annu Rev Pharmacol Toxicol. 1993;33:545–571. doi: 10.1146/annurev.pa.33.040193.002553. [DOI] [PubMed] [Google Scholar]
- Clarkson T. W. The pharmacology of mercury compounds. Annu Rev Pharmacol. 1972;12:375–406. doi: 10.1146/annurev.pa.12.040172.002111. [DOI] [PubMed] [Google Scholar]
- Clarkson T. Methylmercury. Fundam Appl Toxicol. 1991 Jan;16(1):20–21. doi: 10.1016/0272-0590(91)90129-r. [DOI] [PubMed] [Google Scholar]
- Cloëz I., Dumont O., Piciotti M., Bourre J. M. Alterations of lipid synthesis in the normal and dysmyelinating trembler mouse sciatic nerve by heavy metals (Hg, Pb, Mn, Cu, Ni). Toxicology. 1987 Oct 12;46(1):65–71. doi: 10.1016/0300-483x(87)90138-7. [DOI] [PubMed] [Google Scholar]
- Cuvin-Aralar M. L., Furness R. W. Mercury and selenium interaction: a review. Ecotoxicol Environ Saf. 1991 Jun;21(3):348–364. doi: 10.1016/0147-6513(91)90074-y. [DOI] [PubMed] [Google Scholar]
- Danielsson B. R., Dencker L., Khayat A., Orsén I. Fetotoxicity of inorganic mercury in the mouse: distribution and effects on nutrient uptake by placenta and fetus. Biol Res Pregnancy Perinatol. 1984;5(3):102–109. [PubMed] [Google Scholar]
- Das R. M., Scott J. E. Perinatal lung development following maternal exposure to methylmercuric chloride. Pediatr Pulmonol. 1994 Jan;17(1):11–21. doi: 10.1002/ppul.1950170104. [DOI] [PubMed] [Google Scholar]
- Davidson P. W., Myers G. J., Cox C., Axtell C., Shamlaye C., Sloane-Reeves J., Cernichiari E., Needham L., Choi A., Wang Y. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998 Aug 26;280(8):701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. The control of calcium and phosphorus metabolism by the vitamin D endocrine system. Ann N Y Acad Sci. 1980;355:1–17. doi: 10.1111/j.1749-6632.1980.tb21323.x. [DOI] [PubMed] [Google Scholar]
- Di Simplicio P., Gorelli M., Vignani R., Leonzio C. The differential modulation of the enzymes of glutathione metabolism. Indication of overlapping effects of toxicity and repair in mouse liver and kidney after dietary treatment with methyl mercury and sodium selenite. Biol Trace Elem Res. 1993 Feb;36(2):167–181. doi: 10.1007/BF02783176. [DOI] [PubMed] [Google Scholar]
- Di Simplicio P., Leonzio C. Effects of selenium and mercury on glutathione and glutathione-dependent enzymes in experimental quail. Bull Environ Contam Toxicol. 1989 Jan;42(1):15–21. doi: 10.1007/BF01699198. [DOI] [PubMed] [Google Scholar]
- Donaldson W. E. Mercury inhibition of avian fatty acid synthetase complex. Chem Biol Interact. 1975 Nov;11(5):343–350. doi: 10.1016/0009-2797(75)90003-4. [DOI] [PubMed] [Google Scholar]
- Dumont C., Kosatsky T. Bush food contamination and public health policy. Arctic Med Res. 1991;Suppl:699–703. [PubMed] [Google Scholar]
- Dunn J. D., Clarkson T. W., Magos L. Ethanol reveals novel mercury detoxification step in tissues. Science. 1981 Sep 4;213(4512):1123–1125. doi: 10.1126/science.7268418. [DOI] [PubMed] [Google Scholar]
- Eaton R. D., Secord D. C., Hewitt P. An experimental assessment of the toxic potential of mercury in ringed seal liver for adult laboratory cats. Toxicol Appl Pharmacol. 1980 Sep 30;55(3):514–521. doi: 10.1016/0041-008x(80)90053-8. [DOI] [PubMed] [Google Scholar]
- El-Begearmi M. M., Ganther H. E., Sunde M. L. Dietary interaction between methylmercury, selenium, arsenic, and sulfur amino acids in Japanese quail. Poult Sci. 1982 Feb;61(2):272–279. doi: 10.3382/ps.0610272. [DOI] [PubMed] [Google Scholar]
- El-Begearmi M. M., Sunde M. L., Ganther H. E. A mutual protective effect of mercury and selenium in Japanese quail. Poult Sci. 1977 Jan;56(1):313–322. doi: 10.3382/ps.0560313. [DOI] [PubMed] [Google Scholar]
- Endo T., Nakaya S., Kimura R., Murata T. Gastrointestinal absorption of inorganic mercuric compounds in vivo and in situ. Toxicol Appl Pharmacol. 1984 Jun 30;74(2):223–229. doi: 10.1016/0041-008x(84)90146-7. [DOI] [PubMed] [Google Scholar]
- Falnoga I., Kregar I., Skreblin M., Tusek-Znidaric M., Stegnar P. Interactions of mercury in rat brain. Biol Trace Elem Res. 1993 Apr;37(1):71–83. doi: 10.1007/BF02789402. [DOI] [PubMed] [Google Scholar]
- Fang S. C. Interaction of selenium and mercury in the rat. Chem Biol Interact. 1977 Apr;17(1):25–40. doi: 10.1016/0009-2797(77)90069-2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald W. F., Clarkson T. W. Mercury and monomethylmercury: present and future concerns. Environ Health Perspect. 1991 Dec;96:159–166. doi: 10.1289/ehp.9196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldspang A., Hansen J. C. Dietary intake of methylmercury as a correlate of gestational length and birth weight among newborns in Greenland. Am J Epidemiol. 1990 Aug;132(2):310–317. doi: 10.1093/oxfordjournals.aje.a115660. [DOI] [PubMed] [Google Scholar]
- Fukino H., Hirai M., Hsueh Y. M., Moriyasu S., Yamane Y. Mechanism of protection by zinc against mercuric chloride toxicity in rats: effects of zinc and mercury on glutathionine metabolism. J Toxicol Environ Health. 1986;19(1):75–89. doi: 10.1080/15287398609530908. [DOI] [PubMed] [Google Scholar]
- Fukino H., Hirai M., Hsueh Y. M., Yamane Y. Effect of zinc pretreatment on mercuric chloride-induced lipid peroxidation in the rat kidney. Toxicol Appl Pharmacol. 1984 May;73(3):395–401. doi: 10.1016/0041-008x(84)90091-7. [DOI] [PubMed] [Google Scholar]
- Gage J. C. Mechanisms for the biodegradation of organic mercury compounds: the action of ascorbate and of soluble proteins. Toxicol Appl Pharmacol. 1975 May;32(2):225–238. doi: 10.1016/0041-008x(75)90215-x. [DOI] [PubMed] [Google Scholar]
- Galal-Gorchev H. Dietary intake of pesticide residues: cadmium, mercury, and lead. Food Addit Contam. 1991 Nov-Dec;8(6):793–806. doi: 10.1080/02652039109374038. [DOI] [PubMed] [Google Scholar]
- Galal-Gorchev H. Dietary intake, levels in food and estimated intake of lead, cadmium, and mercury. Food Addit Contam. 1993 Jan-Feb;10(1):115–128. doi: 10.1080/02652039309374135. [DOI] [PubMed] [Google Scholar]
- Gale T. F. The amelioration of mercury-induced embryotoxic effects by simultaneous treatment with zinc. Environ Res. 1984 Dec;35(2):405–412. doi: 10.1016/0013-9351(84)90147-6. [DOI] [PubMed] [Google Scholar]
- Ganther H. E., Goudie C., Sunde M. L., Kopecky M. J., Wagner P. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972 Mar 10;175(4026):1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- George J. M. Effect of mercury on response of isolated fat cells to insulin and lipolytic hormones. Endocrinology. 1971 Dec;89(6):1489–1498. doi: 10.1210/endo-89-6-1489. [DOI] [PubMed] [Google Scholar]
- Gilbert S. G., Grant-Webster K. S. Neurobehavioral effects of developmental methylmercury exposure. Environ Health Perspect. 1995 Sep;103 (Suppl 6):135–142. doi: 10.1289/ehp.95103s6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill T. S., Tewari H., Pande J. Use of the fish enzyme system in monitoring water quality: effects of mercury on tissue enzymes. Comp Biochem Physiol C. 1990;97(2):287–292. doi: 10.1016/0742-8413(90)90143-w. [DOI] [PubMed] [Google Scholar]
- Gochfeld M. Factors influencing susceptibility to metals. Environ Health Perspect. 1997 Jun;105 (Suppl 4):817–822. doi: 10.1289/ehp.97105s4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith R. H., Soares J. H., Jr Barbiturate potentiation in mercury poisoning. Bull Environ Contam Toxicol. 1975 Jun;13(6):737–740. doi: 10.1007/BF01721945. [DOI] [PubMed] [Google Scholar]
- Goyer R. A. Toxic and essential metal interactions. Annu Rev Nutr. 1997;17:37–50. doi: 10.1146/annurev.nutr.17.1.37. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Nielsen G. D., Jørgensen P. J., Hørder M. Reference intervals for trace elements in blood: significance of risk factors. Scand J Clin Lab Invest. 1992 Jun;52(4):321–337. doi: 10.1080/00365519209088366. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Weihe P., Jørgensen P. J., Clarkson T., Cernichiari E., Viderø T. Impact of maternal seafood diet on fetal exposure to mercury, selenium, and lead. Arch Environ Health. 1992 May-Jun;47(3):185–195. doi: 10.1080/00039896.1992.9938348. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Weihe P. Neurobehavioral effects of intrauterine mercury exposure: potential sources of bias. Environ Res. 1993 Apr;61(1):176–183. doi: 10.1006/enrs.1993.1062. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Weihe P., White R. F., Debes F. Cognitive performance of children prenatally exposed to "safe" levels of methylmercury. Environ Res. 1998 May;77(2):165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Gregus Z., Stein A. F., Varga F., Klaassen C. D. Effect of lipoic acid on biliary excretion of glutathione and metals. Toxicol Appl Pharmacol. 1992 May;114(1):88–96. doi: 10.1016/0041-008x(92)90100-7. [DOI] [PubMed] [Google Scholar]
- Hansen J. C., Kromann N., Wulf H. C., Albøge K. Selenium and its interrelation with mercury in wholeblood and hair in an East Greenlandic population. Sci Total Environ. 1984 Sep;38:33–40. doi: 10.1016/0048-9697(84)90205-5. [DOI] [PubMed] [Google Scholar]
- Harada M., Nakanishi J., Konuma S., Ohno K., Kimura T., Yamaguchi H., Tsuruta K., Kizaki T., Ookawara T., Ohno H. The present mercury contents of scalp hair and clinical symptoms in inhabitants of the Minamata area. Environ Res. 1998 May;77(2):160–164. doi: 10.1006/enrs.1998.3837. [DOI] [PubMed] [Google Scholar]
- Hirayama K. Effect of amino acids on brain uptake of methyl mercury. Toxicol Appl Pharmacol. 1980 Sep 15;55(2):318–323. doi: 10.1016/0041-008x(80)90093-9. [DOI] [PubMed] [Google Scholar]
- Hirayama K. Effects of combined administration of thiol compounds and methylmercury chloride on mercury distribution in rats. Biochem Pharmacol. 1985 Jun 1;34(11):2030–2032. doi: 10.1016/0006-2952(85)90328-4. [DOI] [PubMed] [Google Scholar]
- Højbjerg S., Nielsen J. B., Andersen O. Effects of dietary lipids on whole-body retention and organ distribution of organic and inorganic mercury in mice. Food Chem Toxicol. 1992 Aug;30(8):703–708. doi: 10.1016/0278-6915(92)90166-i. [DOI] [PubMed] [Google Scholar]
- Iioka H., Moriyama I., Itoh K., Hino K., Okamura Y., Itani Y., Katoh Y., Ichijo M. [The role of glutathione on placental amino acid transport (using microvillous membrane vesicles)]. Nihon Sanka Fujinka Gakkai Zasshi. 1987 Dec;39(12):2133–2136. [PubMed] [Google Scholar]
- Iturri S., Nuñez M. T. Effect of copper, cadmium, mercury, manganese and lead on Fe2+ and Fe3+ absorption in perfused mouse intestine. Digestion. 1998 Nov-Dec;59(6):671–675. doi: 10.1159/000007574. [DOI] [PubMed] [Google Scholar]
- Iwata H., Masukawa T., Kito H., Hayashi M. Degradation of methylmercury by selenium. Life Sci. 1982 Aug 30;31(9):859–866. doi: 10.1016/0024-3205(82)90541-0. [DOI] [PubMed] [Google Scholar]
- Iwata H., Masukawa T., Kito H., Hayashi M. Involvement of tissue sulfhydryls in the formation of a complex of methylmercury with selenium. Biochem Pharmacol. 1981 Dec 1;30(23):3159–3163. doi: 10.1016/0006-2952(81)90513-x. [DOI] [PubMed] [Google Scholar]
- Iwata H., Okamoto H., Ohsawa Y. Effect of selenium on methylmercury poisoning. Res Commun Chem Pathol Pharmacol. 1973 May;5(3):673–680. [PubMed] [Google Scholar]
- Janik A. Toksyczny wpływ chlorku metylorteciowego na ustrój w świetle badań układu krwiotwórczego oraz przemiany weglowodanowej i lipidowej w mieśniu sercowym i watrobie. Folia Med Cracov. 1991;32(3-4):319–332. [PubMed] [Google Scholar]
- Jugo S. Metabolism of toxic heavy metals in growing organisms: a review. Environ Res. 1977 Feb;13(1):36–46. doi: 10.1016/0013-9351(77)90002-0. [DOI] [PubMed] [Google Scholar]
- Kasuya M. Effect of selenium on the toxicity of methylmercury on nervous tissue in culture. Toxicol Appl Pharmacol. 1976 Jan;35(1):11–20. doi: 10.1016/0041-008x(76)90106-x. [DOI] [PubMed] [Google Scholar]
- Kasuya M. Effects of inorganic, aryl, alkyl and other mercury compounds on the outgrowth of cells and fibers from dorsal root ganglia in tissue culture. Toxicol Appl Pharmacol. 1972 Sep;23(1):136–146. doi: 10.1016/0041-008x(72)90213-x. [DOI] [PubMed] [Google Scholar]
- Kasuya M. The effect of vitamin E on the toxicity of alkyl mercurials on nervous tissue in culture. Toxicol Appl Pharmacol. 1975 May;32(2):347–354. doi: 10.1016/0041-008x(75)90225-2. [DOI] [PubMed] [Google Scholar]
- Kim P., Choi B. H. Selective inhibition of glutamate uptake by mercury in cultured mouse astrocytes. Yonsei Med J. 1995 Jul;36(3):299–305. doi: 10.3349/ymj.1995.36.3.299. [DOI] [PubMed] [Google Scholar]
- Kinloch D., Kuhnlein H., Muir D. C. Inuit foods and diet: a preliminary assessment of benefits and risks. Sci Total Environ. 1992 Jul 15;122(1-2):247–278. doi: 10.1016/0048-9697(92)90249-r. [DOI] [PubMed] [Google Scholar]
- Klaverkamp J. F., Macdonald W. A., Lillie W. R., Lutz A. Joint toxicity of mercury and selenium in salmonid eggs. Arch Environ Contam Toxicol. 1983 Jul;12(4):415–419. doi: 10.1007/BF01057584. [DOI] [PubMed] [Google Scholar]
- Kling L. J., Soares J. H., Jr, Haltman W. A. Effect of vitamin E and synthetic antioxidants on the survival rate of mercury-poisoned Japanese quail. Poult Sci. 1987 Feb;66(2):325–331. doi: 10.3382/ps.0660325. [DOI] [PubMed] [Google Scholar]
- Kling L. J., Soares J. H., Jr Mercury metabolism in Japanese quail. I. The effect of dietary mercury and selenium on their tissue distribution. Poult Sci. 1978 Sep;57(5):1279–1285. doi: 10.3382/ps.0571279. [DOI] [PubMed] [Google Scholar]
- Kling L. J., Soares J. H., Jr Mercury metabolism in Japanese quail. II. The effects of dietary mercury and selenium on blood and liver glutathione peroxidase activity and selenium concentration. Poult Sci. 1978 Sep;57(5):1286–1292. doi: 10.3382/ps.0571286. [DOI] [PubMed] [Google Scholar]
- Kling L. J., Soares J. H., Jr The effect of mercury and vitamin E on tissue glutathione peroxidase activity and thiobarbituric acid values. Poult Sci. 1982 Aug;61(8):1762–1765. doi: 10.3382/ps.0611762. [DOI] [PubMed] [Google Scholar]
- Kling L. J., Soares J. H., Jr Vitamin E deficiency in the Japanese quail. Poult Sci. 1980 Oct;59(10):2352–2354. doi: 10.3382/ps.0592352. [DOI] [PubMed] [Google Scholar]
- Komiya K., Kawauchi S. Properties of the mercury and selenium complex formed in rat plasma in vivo. J Pharmacobiodyn. 1981 Aug;4(8):545–551. doi: 10.1248/bpb1978.4.545. [DOI] [PubMed] [Google Scholar]
- Komsta-Szumska E., Chmielnicka J. Effect of zinc, cadmium or copper on mercury distribution in rat tissues. Toxicol Lett. 1983 Jul;17(3-4):349–354. doi: 10.1016/0378-4274(83)90249-7. [DOI] [PubMed] [Google Scholar]
- Komsta-Szumska E., Miller D. R. A kinetic analysis of the interaction between methylmercury and selenium. Toxicology. 1984 Dec;33(3-4):229–238. doi: 10.1016/0300-483x(84)90039-8. [DOI] [PubMed] [Google Scholar]
- Komsta-Szumska E., Reuhl K. R., Miller D. R. Effect of selenium on distribution, demethylation, and excretion of methylmercury by the guinea pig. J Toxicol Environ Health. 1983 Oct-Dec;12(4-6):775–785. doi: 10.1080/15287398309530469. [DOI] [PubMed] [Google Scholar]
- Kostial K., Kargacin B., Landeka M. Influence of dietary ingredients on the body retention of strontium, cadmium and mercury in suckling rats. Toxicol Lett. 1984 Nov;23(2):163–168. doi: 10.1016/0378-4274(84)90121-8. [DOI] [PubMed] [Google Scholar]
- Kostial K., Kargacin B., Simonović I. Iodine in diet increases mercury absorption in rats. J Appl Toxicol. 1982 Aug;2(4):215–216. doi: 10.1002/jat.2550020409. [DOI] [PubMed] [Google Scholar]
- Kostial K., Rabar I., Ciganovic M., Simonovic I. Effect of milk on mercury absorption and gut retention in rats. Bull Environ Contam Toxicol. 1979 Nov;23(4-5):566–571. doi: 10.1007/BF01770004. [DOI] [PubMed] [Google Scholar]
- Kronrád L., Petrboková I., Vavrejn B. A comparison of the distribution of mercury and cadmium complexes with cystein in rats and mice. Radiobiol Radiother (Berl) 1973;14(5):569–575. [PubMed] [Google Scholar]
- Kuznetsov D. A. Paradoxical effect of methyl mercury on mitochondrial protein synthesis in mouse brain tissue. Neurochem Res. 1987 Aug;12(8):751–753. doi: 10.1007/BF00970532. [DOI] [PubMed] [Google Scholar]
- Kuznetsov D. A., Zavijalov N. V., Govorkov A. V., Sibileva T. M. Methyl mercury-induced nonselective blocking of phosphorylation processes as a possible cause of protein synthesis inhibition in vitro and in vivo. Toxicol Lett. 1987 Apr;36(2):153–160. doi: 10.1016/0378-4274(87)90179-2. [DOI] [PubMed] [Google Scholar]
- Landry T. D., Doherty R. A., Gates A. H. Effects of three diets on mercury excretion after methylmercury administration. Bull Environ Contam Toxicol. 1979 May;22(1-2):151–158. doi: 10.1007/BF02026922. [DOI] [PubMed] [Google Scholar]
- LeBel C. P., Ali S. F., Bondy S. C. Deferoxamine inhibits methyl mercury-induced increases in reactive oxygen species formation in rat brain. Toxicol Appl Pharmacol. 1992 Jan;112(1):161–165. doi: 10.1016/0041-008x(92)90292-z. [DOI] [PubMed] [Google Scholar]
- Lebel J., Mergler D., Lucotte M., Amorim M., Dolbec J., Miranda D., Arantès G., Rheault I., Pichet P. Evidence of early nervous system dysfunction in Amazonian populations exposed to low-levels of methylmercury. Neurotoxicology. 1996 Spring;17(1):157–167. [PubMed] [Google Scholar]
- Leskova G. E. Zashchitnyi éffekt amida lipoevoi kisloty pri éksperimental'nom merkurializme. Gig Tr Prof Zabol. 1979 Jun;(6):27–30. [PubMed] [Google Scholar]
- Liu X., Nordberg G. F., Jin T. Increased urinary excretion of zinc and copper by mercuric chloride injection in rats. Biometals. 1992 Spring;5(1):17–22. doi: 10.1007/BF01079693. [DOI] [PubMed] [Google Scholar]
- Lodenius M., Malm O. Mercury in the Amazon. Rev Environ Contam Toxicol. 1998;157:25–52. doi: 10.1007/978-1-4612-0625-5_2. [DOI] [PubMed] [Google Scholar]
- Lugea A., Barber A., Ponz F. Inhibition of D-galactose and L-phenylalanine transport by HgCl2 in rat intestine in vitro. Rev Esp Fisiol. 1994 Sep;50(3):167–173. [PubMed] [Google Scholar]
- Léonard A., Jacquet P., Lauwerys R. R. Mutagenicity and teratogenicity of mercury compounds. Mutat Res. 1983 Jan;114(1):1–18. doi: 10.1016/0165-1110(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Magos L., Clarkson T. W., Allen J. The interrelationship between non-protein bound thiols and the biliary excretion of methylmercury. Biochem Pharmacol. 1978;27(18):2203–2208. doi: 10.1016/0006-2952(78)90078-3. [DOI] [PubMed] [Google Scholar]
- Magos L., Clarkson T. W., Hudson A. R. Differences in the effects of selenite and biological selenium on the chemical form and distribution of mercury after the simultaneous administration of HgCl2 and selenium to rats. J Pharmacol Exp Ther. 1984 Feb;228(2):478–483. [PubMed] [Google Scholar]
- Magos L., Clarkson T. W., Sparrow S., Hudson A. R. Comparison of the protection given by selenite, selenomethionine and biological selenium against the renotoxicity of mercury. Arch Toxicol. 1987 Aug;60(6):422–426. doi: 10.1007/BF00302384. [DOI] [PubMed] [Google Scholar]
- Magos L. Neurotoxicity, anorexia and the preferential choice of antidote in methylmercury intoxicated rats. Neurobehav Toxicol Teratol. 1982 Nov-Dec;4(6):643–646. [PubMed] [Google Scholar]
- Magos L. Physiology and toxicology of mercury. Met Ions Biol Syst. 1997;34:321–370. [PubMed] [Google Scholar]
- Magos L., Webb M., Hudson A. R. Complex formation between selenium and methylmercury. Chem Biol Interact. 1979 Dec;28(2-3):359–362. doi: 10.1016/0009-2797(79)90175-3. [DOI] [PubMed] [Google Scholar]
- Magos L., Webb M. The effect of selenium on the brain uptake of methylmercury. Arch Toxicol. 1977 Sep 28;38(3):201–207. doi: 10.1007/BF00293654. [DOI] [PubMed] [Google Scholar]
- Marsh D. O., Clarkson T. W., Myers G. J., Davidson P. W., Cox C., Cernichiari E., Tanner M. A., Lednar W., Shamlaye C., Choisy O. The Seychelles study of fetal methylmercury exposure and child development: introduction. Neurotoxicology. 1995 Winter;16(4):583–596. [PubMed] [Google Scholar]
- Masukawa T., Kito H., Hayashi M., Iwata H. Formation and possible role of bis(methylmercuric) selenide in rats treated with methylmercury and selenite. Biochem Pharmacol. 1982 Jan 1;31(1):75–78. doi: 10.1016/0006-2952(82)90239-8. [DOI] [PubMed] [Google Scholar]
- Matts R. L., Schatz J. R., Hurst R., Kagen R. Toxic heavy metal ions activate the heme-regulated eukaryotic initiation factor-2 alpha kinase by inhibiting the capacity of hemin-supplemented reticulocyte lysates to reduce disulfide bonds. J Biol Chem. 1991 Jul 5;266(19):12695–12702. [PubMed] [Google Scholar]
- McNeil S. I., Bhatnagar M. K., Turner C. J. Combined toxicity of ethanol and methylmercury in rat. Toxicology. 1988 Dec 30;53(2-3):345–363. doi: 10.1016/0300-483x(88)90226-0. [DOI] [PubMed] [Google Scholar]
- Meltzer H. M., Mundal H. H., Alexander J., Bibow K., Ydersbond T. A. Does dietary arsenic and mercury affect cutaneous bleeding time and blood lipids in humans? Biol Trace Elem Res. 1994 Oct-Nov;46(1-2):135–153. doi: 10.1007/BF02790074. [DOI] [PubMed] [Google Scholar]
- Menon N. K., Lopez R. R. The effects of mild congenital methylmercury intoxication on the metabolism of 3-hydroxybutyrate and glucose in the brains of suckling rats. Neurotoxicology. 1985 Spring;6(1):55–61. [PubMed] [Google Scholar]
- Meydani M., Meydani S. N., Hathcock J. N. Effects of dietary methionine, methylmercury, and atrazine on ex-vivo synthesis of prostaglandin E1 and thromboxane B2. Prostaglandins Leukot Med. 1984 May;14(2):267–278. doi: 10.1016/0262-1746(84)90210-5. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y., Kobayashi T., Doi R. In vitro effects of mercury-selenium compounds on enzymes. Toxicol Lett. 1982 Dec;14(3-4):201–206. doi: 10.1016/0378-4274(82)90052-2. [DOI] [PubMed] [Google Scholar]
- Mokrzan E. M., Kerper L. E., Ballatori N., Clarkson T. W. Methylmercury-thiol uptake into cultured brain capillary endothelial cells on amino acid system L. J Pharmacol Exp Ther. 1995 Mar;272(3):1277–1284. [PubMed] [Google Scholar]
- Morimoto K., Iijima S., Koizumi A. Selenite prevents the induction of sister-chromatid exchanges by methyl mercury and mercuric chloride in human whole-blood cultures. Mutat Res. 1982 Sep;102(2):183–192. doi: 10.1016/0165-1218(82)90118-5. [DOI] [PubMed] [Google Scholar]
- Muckle G., Dewailly E., Ayotte P. Prenatal exposure of Canadian children to polychlorinated biphenyls and mercury. Can J Public Health. 1998 May-Jun;89 (Suppl 1):S20-5, 22-7. [PubMed] [Google Scholar]
- Murray D. R., Hughes R. E. The influence of dietary ascorbic acid on the concentration of mercury in guinea-pig tissues [proceedings]. Proc Nutr Soc. 1976 Dec;35(3):118A–119A. [PubMed] [Google Scholar]
- Myers G. J., Davidson P. W., Shamlaye C. F., Axtell C. D., Cernichiari E., Choisy O., Choi A., Cox C., Clarkson T. W. Effects of prenatal methylmercury exposure from a high fish diet on developmental milestones in the Seychelles Child Development Study. Neurotoxicology. 1997;18(3):819–829. [PubMed] [Google Scholar]
- Mykkänen H. M., Metsäniitty L. Selenium-mercury interaction during intestinal absorption of 75Se compounds in chicks. J Nutr. 1987 Aug;117(8):1453–1458. doi: 10.1093/jn/117.8.1453. [DOI] [PubMed] [Google Scholar]
- Møller-Madsen B., Danscher G. Localization of mercury in CNS of the rat. IV. The effect of selenium on orally administered organic and inorganic mercury. Toxicol Appl Pharmacol. 1991 May;108(3):457–473. doi: 10.1016/0041-008x(91)90092-s. [DOI] [PubMed] [Google Scholar]
- Naganuma A., Imura N. Bis(methylmercuric) selenide as a reaction product from methylmercury and selenite in rabbit blood. Res Commun Chem Pathol Pharmacol. 1980 Jan;27(1):163–173. [PubMed] [Google Scholar]
- Naganuma A., Imura N. Changes in in vitro interaction profiles of mercuric mercury and selenite in rabbit blood under various reaction conditions. J Pharmacobiodyn. 1983 May;6(5):331–339. doi: 10.1248/bpb1978.6.331. [DOI] [PubMed] [Google Scholar]
- Naganuma A., Imura N. Species difference in biliary excretion of methylmercury. Biochem Pharmacol. 1984 Feb 15;33(4):679–682. doi: 10.1016/0006-2952(84)90325-3. [DOI] [PubMed] [Google Scholar]
- Naganuma A., Nakajima E., Shigehara E., Tanaka M., Imura N. Mercury distribution in mouse brain after i.v. administration of bis(methylmercuric) selenide. Toxicol Lett. 1983 Feb;15(2-3):175–179. doi: 10.1016/0378-4274(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Nakada S., Imura N. Uptake of methylmercury and inorganic mercury by mouse glioma and mouse neuroblastoma cells. Neurotoxicology. 1982 Dec;3(4):249–258. [PubMed] [Google Scholar]
- Nielsen J. B., Andersen O. A comparison of the lactational and transplacental deposition of mercury in offspring from methylmercury-exposed mice. Effect of seleno-L-methionine. Toxicol Lett. 1995 Mar;76(2):165–171. doi: 10.1016/0378-4274(94)03209-p. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Andersen O. The toxicokinetics of mercury in mice offspring after maternal exposure to methylmercury--effect of selenomethionine. Toxicology. 1992 Sep;74(2-3):233–241. doi: 10.1016/0300-483x(92)90142-2. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Andersen O. Transplacental passage and fetal deposition of mercury after low-level exposure to methylmercury--effect of seleno-L-methionine. J Trace Elem Electrolytes Health Dis. 1992 Dec;6(4):227–232. [PubMed] [Google Scholar]
- Nishikido N., Furuyashiki K., Naganuma A., Suzuki T., Imura N. Maternal selenium deficiency enhances the fetolethal toxicity of methyl mercury. Toxicol Appl Pharmacol. 1987 May;88(3):322–328. doi: 10.1016/0041-008x(87)90207-9. [DOI] [PubMed] [Google Scholar]
- Norseth T. Biliary excretion and intestinal reabsorption of mercury in the rat after injection of methyl mercuric cloride. Acta Pharmacol Toxicol (Copenh) 1973;33(4):280–288. doi: 10.1111/j.1600-0773.1973.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Norseth T., Clarkson T. W. Intestinal transport of 203Hg-labeled methyl mercury chloride. Role of biotransformation in rats. Arch Environ Health. 1971 May;22(5):568–577. doi: 10.1080/00039896.1971.10665903. [DOI] [PubMed] [Google Scholar]
- Norseth T., Clarkson T. W. Studies on the biotransformation of 203Hg-labeled methyl mercury chloride in rats. Arch Environ Health. 1970 Dec;21(6):717–727. doi: 10.1080/00039896.1970.10667325. [DOI] [PubMed] [Google Scholar]
- Ohi G., Nishigaki S., Seki H., Tamura Y., Maki T. Efficacy of selenium in tuna and selenite in modifying methylmercury intoxication. Environ Res. 1976 Aug;12(1):49–58. doi: 10.1016/0013-9351(76)90008-6. [DOI] [PubMed] [Google Scholar]
- Ohi G., Nishigaki S., Seki H., Tamura Y., Maki T. Efficacy of selenium in tuna and selenite in modifying methylmercury intoxication. Environ Res. 1976 Aug;12(1):49–58. doi: 10.1016/0013-9351(76)90008-6. [DOI] [PubMed] [Google Scholar]
- Ohi G., Nishigaki S., Seki H., Tamura Y., Maki T., Minowa K., Shimamura Y., Mizoguchi I., Inaba Y., Takizawa Y. The protective potency of marine animal meat against the neurotoxicity of methylmercury: its relationship with the organ distribution of mercury and selenium in the rat. Food Cosmet Toxicol. 1980 Apr;18(2):139–145. doi: 10.1016/0015-6264(80)90067-x. [DOI] [PubMed] [Google Scholar]
- Oi G., Nishigaki S., Seki H., Tamura Y., Maki T., Maeda H., Ochiai S., Yamada H., Shimamura Y., Yagyu H. Interaction of dietary methylmercury and selenium on accumulation and retention of these substances in rat organs. Toxicol Appl Pharmacol. 1975 Jun;32(3):527–533. doi: 10.1016/0041-008x(75)90117-9. [DOI] [PubMed] [Google Scholar]
- Oskarsson A., Schültz A., Skerfving S., Hallén I. P., Ohlin B., Lagerkvist B. J. Total and inorganic mercury in breast milk in relation to fish consumption and amalgam in lactating women. Arch Environ Health. 1996 May-Jun;51(3):234–241. doi: 10.1080/00039896.1996.9936021. [DOI] [PubMed] [Google Scholar]
- Peckham N. H., Choi B. H. Surface charge alterations in mouse fetal astrocytes due to methyl mercury: an ultrastructural study with cationized ferritin. Exp Mol Pathol. 1986 Apr;44(2):230–234. doi: 10.1016/0014-4800(86)90073-0. [DOI] [PubMed] [Google Scholar]
- Peraza M. A., Ayala-Fierro F., Barber D. S., Casarez E., Rael L. T. Effects of micronutrients on metal toxicity. Environ Health Perspect. 1998 Feb;106 (Suppl 1):203–216. doi: 10.1289/ehp.98106s1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter S., Matrone G. Effect of selenite on the toxicity of dietary methyl mercury and mercuric chloride in the rat. J Nutr. 1974 May;104(5):638–647. doi: 10.1093/jn/104.5.638. [DOI] [PubMed] [Google Scholar]
- Prasad K. N., Ramanujam S. Vitamin E and vitamin C alter the effect of methylmercuric chloride on neuroblastoma and glioma cells in culture. Environ Res. 1980 Apr;21(2):343–349. doi: 10.1016/0013-9351(80)90036-5. [DOI] [PubMed] [Google Scholar]
- Prohaska J. R., Ganther H. E. Interactions between selenium and methylmercury in rat brain. Chem Biol Interact. 1977 Feb;16(2):155–167. doi: 10.1016/0009-2797(77)90125-9. [DOI] [PubMed] [Google Scholar]
- Rai L. C., Gaur J. P., Kumar H. D. Protective effects of certain environmental factors on the toxicity of zinc, mercury, and methylmercury to Chlorella vulgaris. Environ Res. 1981 Aug;25(2):250–259. doi: 10.1016/0013-9351(81)90026-8. [DOI] [PubMed] [Google Scholar]
- Rana S. V., Sharma R. Co-enzyme effects of inorganic mercury in the liver of a freshwater fish Channa punctatus. J Appl Toxicol. 1982 Dec;2(6):275–277. doi: 10.1002/jat.2550020602. [DOI] [PubMed] [Google Scholar]
- Ratcliffe H. E., Swanson G. M., Fischer L. J. Human exposure to mercury: a critical assessment of the evidence of adverse health effects. J Toxicol Environ Health. 1996 Oct 25;49(3):221–270. doi: 10.1080/713851079. [DOI] [PubMed] [Google Scholar]
- Receveur O., Boulay M., Kuhnlein H. V. Decreasing traditional food use affects diet quality for adult Dene/Métis in 16 communities of the Canadian Northwest Territories. J Nutr. 1997 Nov;127(11):2179–2186. doi: 10.1093/jn/127.11.2179. [DOI] [PubMed] [Google Scholar]
- Refsvik T., Norseth T. Methyl mercuric compounds in rat bile. Acta Pharmacol Toxicol (Copenh) 1975 Jan;36(1):67–78. doi: 10.1111/j.1600-0773.1975.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Refsvik T. The influence of some thiols on biliary excretion of methyl mercury. Acta Pharmacol Toxicol (Copenh) 1983 Jan;52(1):22–29. doi: 10.1111/j.1600-0773.1983.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Richardson R. J., Wilder A. C., Murphy S. D. Uptake of mercury and mercury-amino acid complexes by rat renal cortex slices. Proc Soc Exp Biol Med. 1975 Nov;150(2):303–307. doi: 10.3181/00379727-150-39024. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Mallett A. K., Flynn J., Hargreaves R. J. The effect of various dietary fibres on tissue concentration and chemical form of mercury after methylmercury exposure in mice. Arch Toxicol. 1986 Jul;59(2):94–98. doi: 10.1007/BF00286730. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Robinson R. D., Doherty R. A. Effects of diet on mercury metabolism and excretion in mice given methylmercury: role of gut flora. Arch Environ Health. 1984 Nov-Dec;39(6):401–408. doi: 10.1080/00039896.1984.10545872. [DOI] [PubMed] [Google Scholar]
- Rowland I. The influence of the gut microflora on food toxicity. Proc Nutr Soc. 1981 Jan;40(1):67–74. doi: 10.1079/pns19810011. [DOI] [PubMed] [Google Scholar]
- Rubenstein D. A., Soares J. H., Jr The effect of selenium on the biliary excretion and tissue deposition of two forms of mercury in the broiler chick. Poult Sci. 1979 Sep;58(5):1289–1298. doi: 10.3382/ps.0581289. [DOI] [PubMed] [Google Scholar]
- Rumbeiha W. K., Gentry P. A., Bhatnagar M. K. The effects of administering methylmercury in combination with ethanol in the rat. Vet Hum Toxicol. 1992 Feb;34(1):21–25. [PubMed] [Google Scholar]
- Sakamoto M., Ikegami N., Nakano A. Protective effects of Ca2+ channel blockers against methyl mercury toxicity. Pharmacol Toxicol. 1996 Mar;78(3):193–199. doi: 10.1111/j.1600-0773.1996.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Salminen K. Alterations of rat brain lipids in methyl mercury intoxication. Acta Vet Scand. 1975;16(1):76–83. doi: 10.1186/BF03546697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian T. A., Bredesen D. E., Verity M. A. Cellular resistance to methylmercury. Neurotoxicology. 1996 Spring;17(1):27–36. [PubMed] [Google Scholar]
- Sarafian T., Verity M. A. Oxidative mechanisms underlying methyl mercury neurotoxicity. Int J Dev Neurosci. 1991;9(2):147–153. doi: 10.1016/0736-5748(91)90005-7. [DOI] [PubMed] [Google Scholar]
- Seko Y., Miura T., Takahashi M. Reduced decomposition and faecal excretion of methyl mercury in caecum-resected mice. Acta Pharmacol Toxicol (Copenh) 1982 Feb;50(2):117–120. doi: 10.1111/j.1600-0773.1982.tb00952.x. [DOI] [PubMed] [Google Scholar]
- Seppänen K., Laatikainen R., Salonen J. T., Kantola M., Lötjönen S., Harri M., Nurminen L., Kaikkonen J., Nyyssönen K. Mercury-binding capacity of organic and inorganic selenium in rat blood and liver. Biol Trace Elem Res. 1998 Dec;65(3):197–210. doi: 10.1007/BF02789096. [DOI] [PubMed] [Google Scholar]
- Shah M., Garg A. High-fat and high-carbohydrate diets and energy balance. Diabetes Care. 1996 Oct;19(10):1142–1152. doi: 10.2337/diacare.19.10.1142. [DOI] [PubMed] [Google Scholar]
- Sharma D. C. Biochemical basis of the toxicity of mercury. Med Hypotheses. 1987 Jul;23(3):259–263. doi: 10.1016/0306-9877(87)90017-x. [DOI] [PubMed] [Google Scholar]
- Sharma D. C., Davis P. S. Effect of sodium selenite & selenomethionine on the accumulation & acute toxicity of mercuric & methylmercuric chloride in the goldfish Carassius auratus. Indian J Exp Biol. 1980 Jan;18(1):82–84. [PubMed] [Google Scholar]
- Sharma D. C., Davis P. S., Sharma P. K. Effect of ascorbic acid on biotransformation and modification of the toxicity of mercurials in goldfish (Carassius auratus). Experientia. 1982 May 15;38(5):565–567. doi: 10.1007/BF02327051. [DOI] [PubMed] [Google Scholar]
- Sharma D. C., Davis P. S., Sharma P. K. Studies in search of modifiers of the toxicity of mercurials and speculations on its biochemical mechanism. Biochem Pharmacol. 1981 Nov 15;30(22):3105–3107. doi: 10.1016/0006-2952(81)90500-1. [DOI] [PubMed] [Google Scholar]
- Shoaf A. R., Jarmer S., Harbison R. D. Heavy metal inhibition of carnitine acetyltransferase activity in human placental syncytiotrophoblast: possible site of action of HgCl2, CH3HgCl, and CdCl2. Teratog Carcinog Mutagen. 1986;6(5):351–360. doi: 10.1002/tcm.1770060502. [DOI] [PubMed] [Google Scholar]
- Siegel B. Z., Siegel S. M., Correa T., Dagan C., Galvez G., LeeLoy L., Padua A., Yaeger E. The protection of invertebrates, fish, and vascular plants against inorganic mercury poisoning by sulfur and selenium derivatives. Arch Environ Contam Toxicol. 1991 Feb;20(2):241–246. doi: 10.1007/BF01055910. [DOI] [PubMed] [Google Scholar]
- Skerfving S. Mercury in women exposed to methylmercury through fish consumption, and in their newborn babies and breast milk. Bull Environ Contam Toxicol. 1988 Oct;41(4):475–482. doi: 10.1007/BF02020989. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Smith J. G. Effects of methylmercury in vitro on methionine synthase activity in various rat tissues. Bull Environ Contam Toxicol. 1990 Nov;45(5):649–654. doi: 10.1007/BF01700981. [DOI] [PubMed] [Google Scholar]
- Sood P. P., Vinay S. D. Therapeutic abilities of thiol compounds in the restoration of methylmercury-inhibited cholesterol and triglycerides of the rat's central nervous system. Arch Environ Contam Toxicol. 1991 Aug;21(2):212–217. doi: 10.1007/BF01055339. [DOI] [PubMed] [Google Scholar]
- Sorg O., Schilter B., Honegger P., Monnet-Tschudi F. Increased vulnerability of neurones and glial cells to low concentrations of methylmercury in a prooxidant situation. Acta Neuropathol. 1998 Dec;96(6):621–627. doi: 10.1007/s004010050943. [DOI] [PubMed] [Google Scholar]
- Spindle A., Matsumoto N. Enhancement of methylmercury toxicity by L-cystine in cultured mouse blastocysts. Reprod Toxicol. 1987 1988;1(4):279–284. doi: 10.1016/0890-6238(87)90019-0. [DOI] [PubMed] [Google Scholar]
- Srivastava D. K. Comparative effects of copper, cadmium and mercury on tissue glycogen of the catfish, Heteropneustes fossils (Bloch). Toxicol Lett. 1982 Apr;11(1-2):135–139. doi: 10.1016/0378-4274(82)90118-7. [DOI] [PubMed] [Google Scholar]
- Steinwall O., Olsson Y. Impairment of the blood-brain barrier in mercury poisoning. Acta Neurol Scand. 1969;45(3):351–361. doi: 10.1111/j.1600-0404.1969.tb01247.x. [DOI] [PubMed] [Google Scholar]
- Stern A. H. Re-evaluation of the reference dose for methylmercury and assessment of current exposure levels. Risk Anal. 1993 Jun;13(3):355–364. doi: 10.1111/j.1539-6924.1993.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Stoewsand G. S., Bache C. A., Lisk D. J. Dietary selenium protection of methylmercury intoxication of Japanese quail. Bull Environ Contam Toxicol. 1974 Feb;11(2):152–156. doi: 10.1007/BF01684595. [DOI] [PubMed] [Google Scholar]
- Sumino K., Yamamoto R., Kitamura S. A role of selenium against methylmercury toxicity. Nature. 1977 Jul 7;268(5615):73–74. doi: 10.1038/268073a0. [DOI] [PubMed] [Google Scholar]
- Suzuki K. T., Sasakura C., Yoneda S. Binding sites for the (Hg-Se) complex on selenoprotein P. Biochim Biophys Acta. 1998 Dec 8;1429(1):102–112. doi: 10.1016/s0167-4838(98)00221-0. [DOI] [PubMed] [Google Scholar]
- Svensson B. G., Schütz A., Nilsson A., Akesson I., Akesson B., Skerfving S. Fish as a source of exposure to mercury and selenium. Sci Total Environ. 1992 Sep 11;126(1-2):61–74. doi: 10.1016/0048-9697(92)90484-a. [DOI] [PubMed] [Google Scholar]
- Sällsten G., Thorén J., Barregård L., Schütz A., Skarping G. Long-term use of nicotine chewing gum and mercury exposure from dental amalgam fillings. J Dent Res. 1996 Jan;75(1):594–598. doi: 10.1177/00220345960750011301. [DOI] [PubMed] [Google Scholar]
- Tamashiro H., Arakaki M., Akagi H., Murao K., Hirayama K., Smolensky M. H. Effects of ethanol on methyl mercury toxicity in rats. J Toxicol Environ Health. 1986;18(4):595–605. doi: 10.1080/15287398609530897. [DOI] [PubMed] [Google Scholar]
- Thaxton P., Parkhurst C. R., Cogburn L. A., Young P. S. Adrenal function in chickens experiencing mercury toxicity. Poult Sci. 1975 Mar;54(2):578–584. doi: 10.3382/ps.0540578. [DOI] [PubMed] [Google Scholar]
- Thayer R. H., Donaldson W. E. Mercury inhibition of fatty acid synthesis in chicks. Chem Biol Interact. 1975 Oct;11(4):235–243. doi: 10.1016/0009-2797(75)90077-0. [DOI] [PubMed] [Google Scholar]
- Thomas D. J., Smith J. C. Effects of coadministered low-molecular-weight thiol compounds on short-term distribution of methyl mercury in the rat. Toxicol Appl Pharmacol. 1982 Jan;62(1):104–110. doi: 10.1016/0041-008x(82)90106-5. [DOI] [PubMed] [Google Scholar]
- Thomas D. J., Smith J. C. Effects of coadministered sodium selenite on short-term distribution of methyl mercury in the rat. Environ Res. 1984 Aug;34(2):287–294. doi: 10.1016/0013-9351(84)90097-5. [DOI] [PubMed] [Google Scholar]
- Thrower S. J., Andrewartha K. A. Glutathione peroxidase response in tissues of rats fed diets containing fish protein concentrate prepared from shark flesh of known mercury and selenium contents. Bull Environ Contam Toxicol. 1981 Jan;26(1):77–84. doi: 10.1007/BF01622058. [DOI] [PubMed] [Google Scholar]
- Trevisan R., Vedovato M., Tiengo A. The epidemiology of diabetes mellitus. Nephrol Dial Transplant. 1998;13 (Suppl 8):2–5. doi: 10.1093/ndt/13.suppl_8.2. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Hasunuma R., Kawanishi Y., Okazaki I. Urinary concentrations of heavy metals in healthy Japanese under 20 years of age: a comparison between concentrations expressed in terms of creatinine and of selenium. Tokai J Exp Clin Med. 1995 May;20(1):53–64. [PubMed] [Google Scholar]
- Turan B., Delilbaşi E., Dalay N., Sert S., Afrasyap L., Sayal A. Serum selenium and glutathione-peroxidase activities and their interaction with toxic metals in dialysis and renal transplantation patients. Biol Trace Elem Res. 1992 Apr-Jun;33:95–102. doi: 10.1007/BF02783997. [DOI] [PubMed] [Google Scholar]
- Turner C. J., Bhatnagar M. K., Speisky H. Effect of subchronic administration of ethanol and methylmercury in combination on the tissue distribution of mercury in rats. Can J Physiol Pharmacol. 1990 Dec;68(12):1558–1562. doi: 10.1139/y90-237. [DOI] [PubMed] [Google Scholar]
- Turner C. J., Bhatnagar M. K., Yamashiro S. Ethanol potentiation of methyl mercury toxicity: a preliminary report. J Toxicol Environ Health. 1981 Mar-Apr;7(3-4):665–668. doi: 10.1080/15287398109530008. [DOI] [PubMed] [Google Scholar]
- Urbach J., Boadi W., Brandes J. M., Kerner H., Yannai S. Effect of inorganic mercury on in vitro placental nutrient transfer and oxygen consumption. Reprod Toxicol. 1992;6(1):69–75. doi: 10.1016/0890-6238(92)90023-m. [DOI] [PubMed] [Google Scholar]
- Varghese G., Naik P. S., Katdare M. Respiratory responses and blood sugar level of the crab, Barytelphusa cunicularis (Westwood), exposed to mercury, copper and zinc. Indian J Exp Biol. 1992 Apr;30(4):308–312. [PubMed] [Google Scholar]
- Vijayalakshmi K., Bapu C., Sood P. P. Differential effects of methylmercury, thiols, and vitamins on galactosidases of nervous and non-nervous tissues. Bull Environ Contam Toxicol. 1992 Jul;49(1):71–77. doi: 10.1007/BF00193343. [DOI] [PubMed] [Google Scholar]
- WORKER N. A., MIGICOVSKY B. B. Effect of vitamin D on the utilization of zinc, cadmium and mercury in the chick. J Nutr. 1961 Oct;75:222–224. doi: 10.1093/jn/75.2.222. [DOI] [PubMed] [Google Scholar]
- Webb M., Cain K. Functions of metallothionein. Biochem Pharmacol. 1982 Jan 15;31(2):137–142. doi: 10.1016/0006-2952(82)90202-7. [DOI] [PubMed] [Google Scholar]
- Weiss B. Long ago and far away: a retrospective on the implications of Minamata. Neurotoxicology. 1996 Spring;17(1):257–263. [PubMed] [Google Scholar]
- Welsh S. O. The protective effect of vitamin E and N,N'-diphenyl-p-phenylenediamine (DPPD) against methyl mercury toxicity in the rat. J Nutr. 1979 Oct;109(10):1673–1681. doi: 10.1093/jn/109.10.1673. [DOI] [PubMed] [Google Scholar]
- Whanger P. D. Selenium in the treatment of heavy metal poisoning and chemical carcinogenesis. J Trace Elem Electrolytes Health Dis. 1992 Dec;6(4):209–221. [PubMed] [Google Scholar]
- Wheatley M. A. The importance of social and cultural effects of mercury on aboriginal peoples. Neurotoxicology. 1996 Spring;17(1):251–256. [PubMed] [Google Scholar]
- Wormworth J. Toxins and tradition: the impact of food-chain contamination on the Inuit of northern Quebec. CMAJ. 1995 Apr 15;152(8):1237–1240. [PMC free article] [PubMed] [Google Scholar]
- Wu G. No involvement of system N, system y+ and the oligopeptide-H+ transport system in the uptake of methylmercury in rat erythrocytes. J Appl Toxicol. 1998 Jan-Feb;18(1):55–61. doi: 10.1002/(sici)1099-1263(199801/02)18:1<55::aid-jat478>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wu G. Screening of potential transport systems for methyl mercury uptake in rat erythrocytes at 5 degrees by use of inhibitors and substrates. Pharmacol Toxicol. 1995 Sep;77(3):169–176. doi: 10.1111/j.1600-0773.1995.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto R., Suzuki T. Decreased membrane fragility of mouse erythrocytes by small dose of methylmercury and its restoration by coadministered selenite. Tohoku J Exp Med. 1982 Jul;137(3):297–303. doi: 10.1620/tjem.137.297. [DOI] [PubMed] [Google Scholar]
- Yamini B., Sleight S. D. Effects of ascorbic acid deficiency on methyl mercury dicyandiamide toxicosis in guinea pigs. J Environ Pathol Toxicol Oncol. 1984 Jul;5(4-5):139–150. [PubMed] [Google Scholar]
- Yannai S., Sachs K. M. Absorption and accumulation of cadmium, lead and mercury from foods by rats. Food Chem Toxicol. 1993 May;31(5):351–355. doi: 10.1016/0278-6915(93)90190-a. [DOI] [PubMed] [Google Scholar]
- Yasutake A., Hirayama K., Inoue M. Interaction of methylmercury compounds with albumin. Arch Toxicol. 1990;64(8):639–643. doi: 10.1007/BF01974691. [DOI] [PubMed] [Google Scholar]
- Yee S., Choi B. H. Oxidative stress in neurotoxic effects of methylmercury poisoning. Neurotoxicology. 1996 Spring;17(1):17–26. [PubMed] [Google Scholar]
- Yonemoto J., Webb M., Magos L. Methylmercury stimulates the exhalation of volatile selenium and potentiates the toxicity of selenite. Toxicol Lett. 1985 Jan;24(1):7–14. doi: 10.1016/0378-4274(85)90132-8. [DOI] [PubMed] [Google Scholar]
- Zalups R. K., Barfuss D. W. Small aliphatic dicarboxylic acids inhibit renal uptake of administered mercury. Toxicol Appl Pharmacol. 1998 Jan;148(1):183–193. doi: 10.1006/taap.1997.8320. [DOI] [PubMed] [Google Scholar]
- Zalups R. K., Lash L. H. Binding of mercury in renal brush-border and basolateral membrane-vesicles. Biochem Pharmacol. 1997 Jun 15;53(12):1889–1900. doi: 10.1016/s0006-2952(97)00138-x. [DOI] [PubMed] [Google Scholar]
- Zalups R. K., Parks L. D., Cannon V. T., Barfuss D. W. Mechanisms of action of 2,3-dimercaptopropane-1-sulfonate and the transport, disposition, and toxicity of inorganic mercury in isolated perfused segments of rabbit proximal tubules. Mol Pharmacol. 1998 Aug;54(2):353–363. doi: 10.1124/mol.54.2.353. [DOI] [PubMed] [Google Scholar]
- Zorn N. E., Smith J. T. A relationship between vitamin B12, folic acid, ascorbic acid, and mercury uptake and methylation. Life Sci. 1990;47(2):167–173. doi: 10.1016/0024-3205(90)90230-o. [DOI] [PubMed] [Google Scholar]


