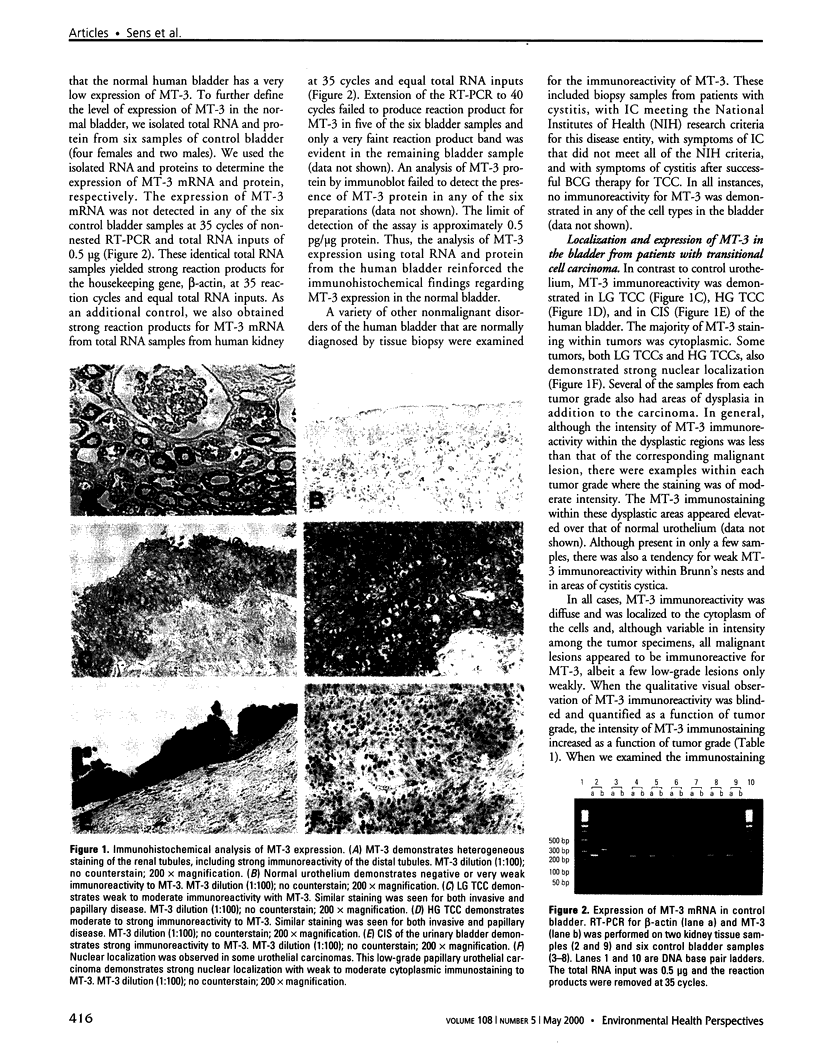
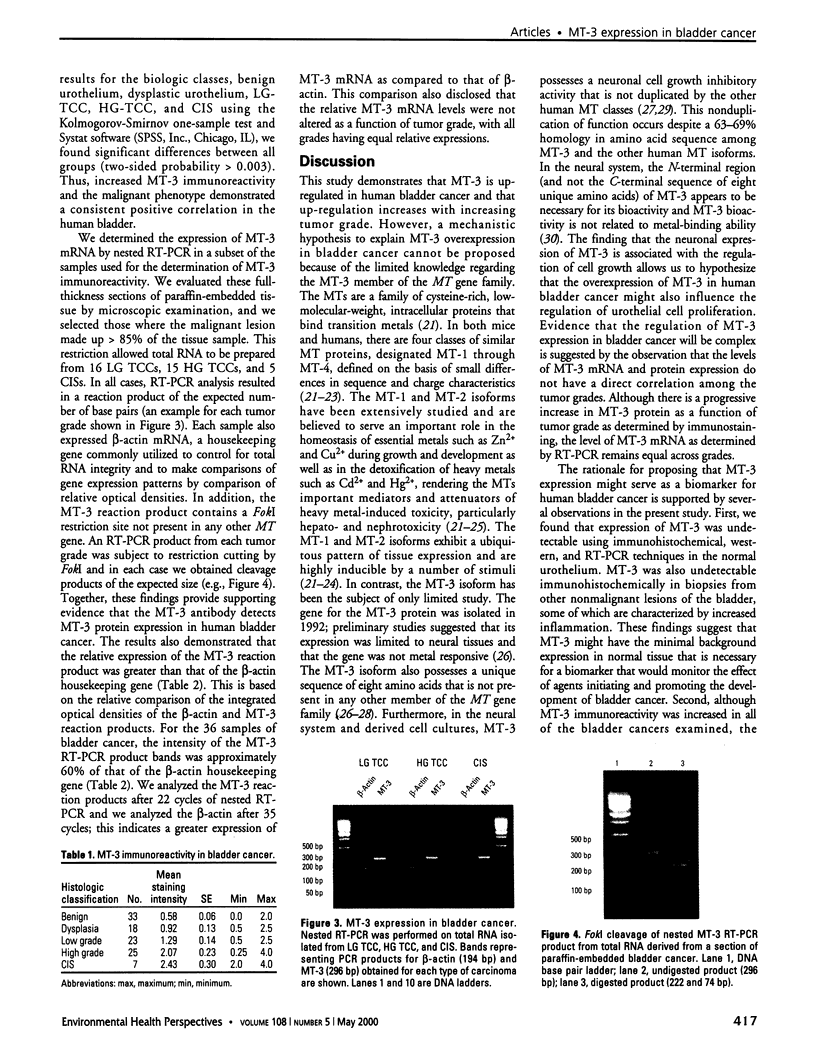
Abstract
The goal of the present study was to determine if the expression of metallothionein isoform 3 (MT-3) might serve as a biomarker for human bladder cancer. To accomplish this goal, we defined the localization and expression of MT-3 protein and mRNA using fresh and archival biopsy specimens obtained from patients undergoing differential diagnosis for a variety of bladder disorders. We used immunohistochemistry, immunoblot, and RT-PCR analysis to define the localization and expression of MT-3 protein and mRNA. Immunohistochemical analysis disclosed no immunoreactivity for MT-3 in normal bladder cells. The absence of MT-3 expression in the normal bladder was further confirmed by demonstrating that MT-3 mRNA could not be detected using reverse transcriptase-polymerase chain reaction (RT-PCR) or MT-3 protein using immunoblot. Immunohistochemistry also disclosed no immunoreactivity for MT-3 in archival biopsy specimens from patients with interstitial cystitis and related disorders. Immunohistochemical analysis demonstrated that MT-3 was expressed in carcinoma in situ (CIS), high-grade bladder cancer, low-grade bladder cancer, and dysplastic lesions. MT-3 immunostaining was intense in both CIS and high-grade bladder cancer, and low to moderate in low-grade bladder cancer and dysplastic lesions. We determined MT-3 mRNA expression in a subset of these bladder cancer specimens; expression was elevated as compared to that of the housekeeping gene, ss-actin. The cDNA from the RT-PCR reaction primed for MT-3 contained a FokI restriction site, a site unique for MT-3 as compared to other MT family members. In conclusion, this study demonstrates that MT-3 is up-regulated in human bladder cancer and that this up-regulation increases with increasing tumor grade. The finding that MT-3 expression is minimal in normal bladder suggests that MT-3 might be developed into an effective biomarker for bladder cancer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amoureux M. C., Wurch T., Pauwels P. J. Modulation of metallothionein-III mRNA content and growth rate of rat C6-glial cells by transfection with human 5-HT1D receptor genes. Biochem Biophys Res Commun. 1995 Sep 14;214(2):639–645. doi: 10.1006/bbrc.1995.2334. [DOI] [PubMed] [Google Scholar]
- CASE R. A., HOSKER M. E., McDONALD D. B., PEARSON J. T. Tumours of the urinary bladder in workmen engaged in the manufacture and use of certain dyestuff intermediates in the British chemical industry. I. The role of aniline, benzidine, alpha-naphthylamine, and beta-naphthylamine. Br J Ind Med. 1954 Apr;11(2):75–104. doi: 10.1136/oem.11.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel J., Cordier S., Boccon-Gibod L., Hemon D. Tobacco and bladder cancer in males: increased risk for inhalers and smokers of black tobacco. Int J Cancer. 1989 Oct 15;44(4):605–610. doi: 10.1002/ijc.2910440408. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Metallothionein--aspects related to copper and zinc metabolism. J Inherit Metab Dis. 1983;6 (Suppl 1):15–21. doi: 10.1007/BF01811318. [DOI] [PubMed] [Google Scholar]
- Droller M. J. Bladder cancer: state-of-the-art care. CA Cancer J Clin. 1998 Sep-Oct;48(5):269–284. doi: 10.3322/canjclin.48.5.269. [DOI] [PubMed] [Google Scholar]
- Friedline J. A., Garrett S. H., Somji S., Todd J. H., Sens D. A. Differential expression of the MT-1E gene in estrogen-receptor-positive and -negative human breast cancer cell lines. Am J Pathol. 1998 Jan;152(1):23–27. [PMC free article] [PubMed] [Google Scholar]
- Garrett S. H., Sens M. A., Shukla D., Nestor S., Somji S., Todd J. H., Sens D. A. Metallothionein isoform 3 expression in the human prostate and cancer-derived cell lines. Prostate. 1999 Nov 1;41(3):196–202. doi: 10.1002/(sici)1097-0045(19991101)41:3<196::aid-pros7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Garrett S. H., Sens M. A., Todd J. H., Somji S., Sens D. A. Expression of MT-3 protein in the human kidney. Toxicol Lett. 1999 Apr 12;105(3):207–214. doi: 10.1016/s0378-4274(99)00003-x. [DOI] [PubMed] [Google Scholar]
- Goering P. L., Klaassen C. D. Altered subcellular distribution of cadmium following cadmium pretreatment: possible mechanism of tolerance to cadmium-induced lethality. Toxicol Appl Pharmacol. 1983 Sep 15;70(2):195–203. doi: 10.1016/0041-008x(83)90095-9. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hoey J. G., Garrett S. H., Sens M. A., Todd J. H., Sens D. A. Expression of MT-3 mRNA in human kidney, proximal tubule cell cultures, and renal cell carcinoma. Toxicol Lett. 1997 Jul 21;92(2):149–160. doi: 10.1016/s0378-4274(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Hunziker P. Mammalian metallothionein. Biol Trace Elem Res. 1989 Jul-Sep;21:111–118. doi: 10.1007/BF02917243. [DOI] [PubMed] [Google Scholar]
- Lamm D. L. Bladder cancer: twenty years of progress and the challenges that remain. CA Cancer J Clin. 1998 Sep-Oct;48(5):263–268. doi: 10.3322/canjclin.48.5.263. [DOI] [PubMed] [Google Scholar]
- Landis S. H., Murray T., Bolden S., Wingo P. A. Cancer statistics, 1999. CA Cancer J Clin. 1999 Jan-Feb;49(1):8-31, 1. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- Mididoddi S., McGuirt J. P., Sens M. A., Todd J. H., Sens D. A. Isoform-specific expression of metallothionein mRNA in the developing and adult human kidney. Toxicol Lett. 1996 Apr;85(1):17–27. doi: 10.1016/0378-4274(96)03632-6. [DOI] [PubMed] [Google Scholar]
- Morrison A. S., Buring J. E., Verhoek W. G., Aoki K., Leck I., Ohno Y., Obata K. An international study of smoking and bladder cancer. J Urol. 1984 Apr;131(4):650–654. doi: 10.1016/s0022-5347(17)50559-5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Findley S. D., Whitmore T. E., Durnam D. M. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Findley S. D., Whitmore T. E., Durnam D. M. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell A. K., Jensen L. T., Erickson J. C., Palmiter R. D., Winge D. R. Bioactivity of metallothionein-3 correlates with its novel beta domain sequence rather than metal binding properties. Biochemistry. 1995 Apr 11;34(14):4740–4747. doi: 10.1021/bi00014a031. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Kobayashi H., Uchida Y., Ihara Y., Miyatake T. Molecular cloning of human growth inhibitory factor cDNA and its down-regulation in Alzheimer's disease. EMBO J. 1992 Dec;11(13):4843–4850. doi: 10.1002/j.1460-2075.1992.tb05590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y., Takio K., Titani K., Ihara Y., Tomonaga M. The growth inhibitory factor that is deficient in the Alzheimer's disease brain is a 68 amino acid metallothionein-like protein. Neuron. 1991 Aug;7(2):337–347. doi: 10.1016/0896-6273(91)90272-2. [DOI] [PubMed] [Google Scholar]
- Vineis P., Esteve J., Hartge P., Hoover R., Silverman D. T., Terracini B. Effects of timing and type of tobacco in cigarette-induced bladder cancer. Cancer Res. 1988 Jul 1;48(13):3849–3852. [PubMed] [Google Scholar]
- Whitmore W. F., Jr Toward the rational management of bladder cancer: an overview. Urology. 1988 Feb;31(2 Suppl):5–8. [PubMed] [Google Scholar]