Abstract
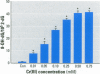
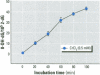
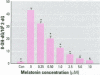
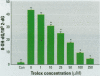
Chromium compounds are well documented carcinogens. Cr(III) is more reactive than Cr(VI) toward DNA under in vitro conditions. In the present study, we investigated the ability of Cr(III) to induce oxidative DNA damage by examining the formation of 8-hydroxydeoxyguanosine (8-OH-dG) in calf thymus DNA incubated with CrCl(3) plus H(2)O(2). We measured 8-OH-dG using HPLC with electrochemical detection. In the presence of H(2)O(2), we observed that Cr(III)-induced formation of 8-OH-dG in isolated DNA was dose and time dependent. Melatonin, ascorbate, and vitamin E (Trolox), all of which are free radical scavengers, markedly inhibited the formation of 8-OH-dG in a concentration-dependent manner. The concentration that reduced DNA damage by 50% was 0.51, 30.4, and 36.2 microM for melatonin, ascorbate, and Trolox, respectively. The results show that melatonin is 60- and 70-fold more effective than ascorbate or vitamin E, respectively, in reducing oxidative DNA damage in this in vitro model. These findings also are consistent with the conclusion that the carcinogenic mechanism of Cr(III) is possibly due to Cr(III)-mediated Fenton-type reactions and that melatonin's highly protective effects against Cr(III) relate, at least in part, to its direct hydroxyl radical scavenging ability.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi V., Celotti L., Lanfranchi G., Majone F., Marin G., Montaldi A., Sponza G., Tamino G., Venier P., Zantedeschi A. Genetic effects of chromium compounds. Mutat Res. 1983 May-Jun;117(3-4):279–300. doi: 10.1016/0165-1218(83)90128-3. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- De Flora S., Bagnasco M., Serra D., Zanacchi P. Genotoxicity of chromium compounds. A review. Mutat Res. 1990 Mar;238(2):99–172. doi: 10.1016/0165-1110(90)90007-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Aruoma O. I. DNA damage by oxygen-derived species. Its mechanism and measurement in mammalian systems. FEBS Lett. 1991 Apr 9;281(1-2):9–19. doi: 10.1016/0014-5793(91)80347-6. [DOI] [PubMed] [Google Scholar]
- Hneihen A. S., Standeven A. M., Wetterhahn K. E. Differential binding of chromium(VI) and chromium(III) complexes to salmon sperm nuclei and nuclear DNA and isolated calf thymus DNA. Carcinogenesis. 1993 Sep;14(9):1795–1803. doi: 10.1093/carcin/14.9.1795. [DOI] [PubMed] [Google Scholar]
- Langård S. One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am J Ind Med. 1990;17(2):189–215. doi: 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- Limson J., Nyokong T., Daya S. The interaction of melatonin and its precursors with aluminium, cadmium, copper, iron, lead, and zinc: an adsorptive voltammetric study. J Pineal Res. 1998 Jan;24(1):15–21. doi: 10.1111/j.1600-079x.1998.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Lorentzen R. J., Ts'o P. O. Benzo[a]yrenedione/benzo[a]pyrenediol oxidation-reduction couples and the generation of reactive reduced molecular oxygen. Biochemistry. 1977 Apr 5;16(7):1467–1473. doi: 10.1021/bi00626a035. [DOI] [PubMed] [Google Scholar]
- Lu Y. Y., Yang J. L. Long-term exposure to chromium(VI) oxide leads to defects in sulfate transport system in Chinese hamster ovary cells. J Cell Biochem. 1995 Apr;57(4):655–665. doi: 10.1002/jcb.240570410. [DOI] [PubMed] [Google Scholar]
- Léonard A., Lauwerys R. R. Carcinogenicity and mutagenicity of chromium. Mutat Res. 1980 Nov;76(3):227–239. doi: 10.1016/0165-1110(80)90018-4. [DOI] [PubMed] [Google Scholar]
- Manning F. C., Blankenship L. J., Wise J. P., Xu J., Bridgewater L. C., Patierno S. R. Induction of internucleosomal DNA fragmentation by carcinogenic chromate: relationship to DNA damage, genotoxicity, and inhibition of macromolecular synthesis. Environ Health Perspect. 1994 Sep;102 (Suppl 3):159–167. doi: 10.1289/ehp.94102s3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Pelaez A., Poeggeler B., Reiter R. J., Barlow-Walden L., Pablos M. I., Tan D. X. Nuclear localization of melatonin in different mammalian tissues: immunocytochemical and radioimmunoassay evidence. J Cell Biochem. 1993 Dec;53(4):373–382. doi: 10.1002/jcb.240530415. [DOI] [PubMed] [Google Scholar]
- Norseth T. The carcinogenicity of chromium and its salts. Br J Ind Med. 1986 Oct;43(10):649–651. doi: 10.1136/oem.43.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parshad R., Sanford K. K., Jones G. M., Tarone R. E. Fluorescent light-induced chromosome damage and its prevention in mouse cells in culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1830–1833. doi: 10.1073/pnas.75.4.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pähkla R., Zilmer M., Kullisaar T., Rägo L. Comparison of the antioxidant activity of melatonin and pinoline in vitro. J Pineal Res. 1998 Mar;24(2):96–101. doi: 10.1111/j.1600-079x.1998.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Qi W., Reiter R. J., Tan D. X., Manchester L. C., Kim S. J., Garcia J. J. Inhibitory effects of melatonin on ferric nitrilotriacetate-induced lipid peroxidation and oxidative DNA damage in the rat kidney. Toxicology. 1999 Nov 29;139(1-2):81–91. doi: 10.1016/s0300-483x(99)00100-6. [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Melchiorri D., Sewerynek E., Poeggeler B., Barlow-Walden L., Chuang J., Ortiz G. G., Acuña-Castroviejo D. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995 Jan;18(1):1–11. doi: 10.1111/j.1600-079x.1995.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Reiter R. J., Tan D. X., Kim S. J., Qi W. Melatonin as a pharmacological agent against oxidative damage to lipids and DNA. Proc West Pharmacol Soc. 1998;41:229–236. [PubMed] [Google Scholar]
- Reiter R. J., Tan D. X., Qi W. B. Suppression of oxygen toxicity by melatonin. Zhongguo Yao Li Xue Bao. 1998 Nov;19(6):575–581. [PubMed] [Google Scholar]
- Reiter R., Tang L., Garcia J. J., Muñoz-Hoyos A. Pharmacological actions of melatonin in oxygen radical pathophysiology. Life Sci. 1997;60(25):2255–2271. doi: 10.1016/s0024-3205(97)00030-1. [DOI] [PubMed] [Google Scholar]
- Romero M. P., Osuna C., García-Pergañeda A., Carrillo-Vico A., Guerrero J. M. The pineal secretory product melatonin reduces hydrogen peroxide-induced DNA damage in U-937 cells. J Pineal Res. 1999 May;26(4):227–235. doi: 10.1111/j.1600-079x.1999.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Sewerynek E., Ortiz G. G., Reiter R. J., Pablos M. I., Melchiorri D., Daniels W. M. Lipopolysaccharide-induced DNA damage is greatly reduced in rats treated with the pineal hormone melatonin. Mol Cell Endocrinol. 1996 Mar 25;117(2):183–188. doi: 10.1016/0303-7207(95)03742-x. [DOI] [PubMed] [Google Scholar]
- Shamberger R. J. Genetic toxicology of ascorbic acid. Mutat Res. 1984 Mar;133(2):135–159. doi: 10.1016/0165-1110(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Shi X., Chiu A., Chen C. T., Halliwell B., Castranova V., Vallyathan V. Reduction of chromium(VI) and its relationship to carcinogenesis. J Toxicol Environ Health B Crit Rev. 1999 Jan-Mar;2(1):87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- Shi X., Dalal N. S., Kasprzak K. S. Generation of free radicals from hydrogen peroxide and lipid hydroperoxides in the presence of Cr(III). Arch Biochem Biophys. 1993 Apr;302(1):294–299. doi: 10.1006/abbi.1993.1213. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Shida C. S., Castrucci A. M., Lamy-Freund M. T. High melatonin solubility in aqueous medium. J Pineal Res. 1994 May;16(4):198–201. doi: 10.1111/j.1600-079x.1994.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Shigenaga M. K., Aboujaoude E. N., Chen Q., Ames B. N. Assays of oxidative DNA damage biomarkers 8-oxo-2'-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Singh J., Carlisle D. L., Pritchard D. E., Patierno S. R. Chromium-induced genotoxicity and apoptosis: relationship to chromium carcinogenesis (review). Oncol Rep. 1998 Nov-Dec;5(6):1307–1318. doi: 10.3892/or.5.6.1307. [DOI] [PubMed] [Google Scholar]
- Stasica P., Ulanski P., Rosiak J. M. Melatonin as a hydroxyl radical scavenger. J Pineal Res. 1998 Aug;25(1):65–66. doi: 10.1111/j.1600-079x.1998.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Stearns D. M., Courtney K. D., Giangrande P. H., Phieffer L. S., Wetterhahn K. E. Chromium(VI) reduction by ascorbate: role of reactive intermediates in DNA damage in vitro. Environ Health Perspect. 1994 Sep;102 (Suppl 3):21–25. doi: 10.1289/ehp.94102s321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns D. M., Kennedy L. J., Courtney K. D., Giangrande P. H., Phieffer L. S., Wetterhahn K. E. Reduction of chromium(VI) by ascorbate leads to chromium-DNA binding and DNA strand breaks in vitro. Biochemistry. 1995 Jan 24;34(3):910–919. doi: 10.1021/bi00003a025. [DOI] [PubMed] [Google Scholar]
- Stohs S. J., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995 Feb;18(2):321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Sugiyama M. Role of physiological antioxidants in chromium(VI)-induced cellular injury. Free Radic Biol Med. 1992;12(5):397–407. doi: 10.1016/0891-5849(92)90089-y. [DOI] [PubMed] [Google Scholar]
- Susa N., Ueno S., Furukawa Y., Ueda J., Sugiyama M. Potent protective effect of melatonin on chromium(VI)-induced DNA single-strand breaks, cytotoxicity, and lipid peroxidation in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol. 1997 Jun;144(2):377–384. doi: 10.1006/taap.1997.8151. [DOI] [PubMed] [Google Scholar]
- Tan D. X., Manchester L. C., Reiter R. J., Plummer B. F., Hardies L. J., Weintraub S. T., Vijayalaxmi, Shepherd A. M. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker of in vivo hydroxyl radical generation. Biochem Biophys Res Commun. 1998 Dec 30;253(3):614–620. doi: 10.1006/bbrc.1998.9826. [DOI] [PubMed] [Google Scholar]
- Tan D., Reiter R. J., Chen L. D., Poeggeler B., Manchester L. C., Barlow-Walden L. R. Both physiological and pharmacological levels of melatonin reduce DNA adduct formation induced by the carcinogen safrole. Carcinogenesis. 1994 Feb;15(2):215–218. doi: 10.1093/carcin/15.2.215. [DOI] [PubMed] [Google Scholar]
- Tang L., Reiter R. J., Li Z. R., Ortiz G. G., Yu B. P., Garcia J. J. Melatonin reduces the increase in 8-hydroxy-deoxyguanosine levels in the brain and liver of kainic acid-treated rats. Mol Cell Biochem. 1998 Jan;178(1-2):299–303. doi: 10.1023/a:1006815530519. [DOI] [PubMed] [Google Scholar]
- Tsou T. C., Chen C. L., Liu T. Y., Yang J. L. Induction of 8-hydroxydeoxyguanosine in DNA by chromium(III) plus hydrogen peroxide and its prevention by scavengers. Carcinogenesis. 1996 Jan;17(1):103–108. doi: 10.1093/carcin/17.1.103. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Kato K. Chromosomal aberrations and morphological transformation in hamster embryonic cells treated with potassium dichromate in vitro. Mutat Res. 1977 Apr;46(2):87–94. doi: 10.1016/0165-1161(77)90115-7. [DOI] [PubMed] [Google Scholar]
- Verschoor M. A., Bragt P. C., Herber R. F., Zielhuis R. L., Zwennis W. C. Renal function of chrome-plating workers and welders. Int Arch Occup Environ Health. 1988;60(1):67–70. doi: 10.1007/BF00409381. [DOI] [PubMed] [Google Scholar]
- Wakatsuki A., Okatani Y., Izumiya C., Ikenoue N. Melatonin protects against ischemia and reperfusion-induced oxidative lipid and DNA damage in fetal rat brain. J Pineal Res. 1999 Apr;26(3):147–152. doi: 10.1111/j.1600-079x.1999.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Wolf T., Kasemann R., Ottenwälder H. Molecular interaction of different chromium species with nucleotides and nucleic acids. Carcinogenesis. 1989 Apr;10(4):655–659. doi: 10.1093/carcin/10.4.655. [DOI] [PubMed] [Google Scholar]
- Yang J. L., Hsieh Y. C., Wu C. W., Lee T. C. Mutational specificity of chromium(VI) compounds in the hprt locus of Chinese hamster ovary-K1 cells. Carcinogenesis. 1992 Nov;13(11):2053–2057. doi: 10.1093/carcin/13.11.2053. [DOI] [PubMed] [Google Scholar]