Abstract
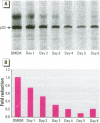
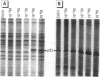
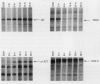
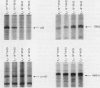
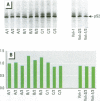
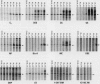
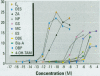
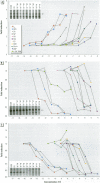
Scientific evidence suggests that humans and wildlife species may experience adverse health consequences from exposure to environmental chemicals that interact with the endocrine system. Reliable short-term assays are needed to identify hormone-disrupting chemicals. In this study we demonstrate that the estrogenic activity of a chemical can be evaluated by assaying induction or repression of endogenous estrogen-regulated "marker genes" in human breast cancer MCF-7 cells. We included four marker genes in the assay--pS2, transforming growth factor beta3 (TGFbeta3), monoamine oxidase A, and [alpha]1-antichymotrypsin--and we evaluated estrogenic activity for 17beta-estradiol (E(2)), diethylstilbestrol, [alpha]-zearalanol, nonylphenol, genistein, methoxychlor, endosulphan, o,p-DDE, bisphenol A, dibutylphthalate, 4-hydroxy tamoxifen, and ICI 182.780. All four marker genes responded strongly to the three high-potency estrogens (E(2), diethylstilbestrol, and [alpha]-zearalanol), whereas the potency of the other chemicals was 10(3)- to 10(6)-fold lower than that of E(2). There were some marker gene-dependent differences in the relative potencies of the tested chemicals. TGFbeta3 was equally sensitive to the three high-potency estrogens, whereas the sensitivity to [alpha]-zearalanol was approximately 10-fold lower than the sensitivity to E(2) and diethylstilbestrol when assayed with the other three marker genes. The potency of nonylphenol was equal to that of genistein when assayed with pS2 and TGFbeta3, but 10- to 100-fold higher/lower with monoamine oxidase A and [alpha]1-antichymotrypsin, respectively. The results are in agreement with results obtained by other methods and suggest that an assay based on endogenous gene expression may offer an attractive alternative to other E-SCREEN methods.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adami H. O., Bergström R., Möhner M., Zatoński W., Storm H., Ekbom A., Tretli S., Teppo L., Ziegler H., Rahu M. Testicular cancer in nine northern European countries. Int J Cancer. 1994 Oct 1;59(1):33–38. doi: 10.1002/ijc.2910590108. [DOI] [PubMed] [Google Scholar]
- Andersen H. R., Andersson A. M., Arnold S. F., Autrup H., Barfoed M., Beresford N. A., Bjerregaard P., Christiansen L. B., Gissel B., Hummel R. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999 Feb;107 (Suppl 1):89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A. M., Skakkebaek N. E. Exposure to exogenous estrogens in food: possible impact on human development and health. Eur J Endocrinol. 1999 Jun;140(6):477–485. doi: 10.1530/eje.0.1400477. [DOI] [PubMed] [Google Scholar]
- Bates S. E., Davidson N. E., Valverius E. M., Freter C. E., Dickson R. B., Tam J. P., Kudlow J. E., Lippman M. E., Salomon D. S. Expression of transforming growth factor alpha and its messenger ribonucleic acid in human breast cancer: its regulation by estrogen and its possible functional significance. Mol Endocrinol. 1988 Jun;2(6):543–555. doi: 10.1210/mend-2-6-543. [DOI] [PubMed] [Google Scholar]
- Berry M., Nunez A. M., Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1218–1222. doi: 10.1073/pnas.86.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B., Evans R. M. Orphan nuclear receptors--new ligands and new possibilities. Genes Dev. 1998 Oct 15;12(20):3149–3155. doi: 10.1101/gad.12.20.3149. [DOI] [PubMed] [Google Scholar]
- Bolger R., Wiese T. E., Ervin K., Nestich S., Checovich W. Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect. 1998 Sep;106(9):551–557. doi: 10.1289/ehp.98106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D. Options available--from start to finish--for obtaining expression data by microarray. Nat Genet. 1999 Jan;21(1 Suppl):25–32. doi: 10.1038/4455. [DOI] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N. E. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992 Sep 12;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S. G., Halbreich U. The influence of estrogen on monoamine oxidase activity. Psychopharmacol Bull. 1997;33(2):229–233. [PubMed] [Google Scholar]
- Dannecker C., Possinger K., Classen S. Induction of TGF-beta by an antiprogestin in the human breast cancer cell line T-47D. Ann Oncol. 1996 Apr;7(4):391–395. doi: 10.1093/oxfordjournals.annonc.a010606. [DOI] [PubMed] [Google Scholar]
- Darbre P., Yates J., Curtis S., King R. J. Effect of estradiol on human breast cancer cells in culture. Cancer Res. 1983 Jan;43(1):349–354. [PubMed] [Google Scholar]
- Davis D. L., Bradlow H. L., Wolff M., Woodruff T., Hoel D. G., Anton-Culver H. Medical hypothesis: xenoestrogens as preventable causes of breast cancer. Environ Health Perspect. 1993 Oct;101(5):372–377. doi: 10.1289/ehp.93101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck C., Goodfellow P. N. DNA microarrays in drug discovery and development. Nat Genet. 1999 Jan;21(1 Suppl):48–50. doi: 10.1038/4475. [DOI] [PubMed] [Google Scholar]
- Derynck R., Lindquist P. B., Lee A., Wen D., Tamm J., Graycar J. L., Rhee L., Mason A. J., Miller D. A., Coffey R. J. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 1988 Dec 1;7(12):3737–3743. doi: 10.1002/j.1460-2075.1988.tb03257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M., Macaulay V., Gledhill J., Ryde C., Nicholls J., Ashworth A., McKinna J. A., Smith I. E. Control of aromatase in breast cancer cells and its importance for tumor growth. J Steroid Biochem Mol Biol. 1993 Mar;44(4-6):605–609. doi: 10.1016/0960-0760(93)90266-y. [DOI] [PubMed] [Google Scholar]
- El-Ashry D., Chrysogelos S. A., Lippman M. E., Kern F. G. Estrogen induction of TGF-alpha is mediated by an estrogen response element composed of two imperfect palindromes. J Steroid Biochem Mol Biol. 1996 Nov;59(3-4):261–269. doi: 10.1016/s0960-0760(96)00118-5. [DOI] [PubMed] [Google Scholar]
- Fields C., Adams M. D., White O., Venter J. C. How many genes in the human genome? Nat Genet. 1994 Jul;7(3):345–346. doi: 10.1038/ng0794-345. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Leonard L. S., Lovell S., Gould J. C., Babaï D., Portier C. J., McDonnell D. P. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997 Mar;143(1):205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- Giwercman A., Skakkebaek N. E. The human testis--an organ at risk? Int J Androl. 1992 Oct;15(5):373–375. doi: 10.1111/j.1365-2605.1992.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Gray L. E., Jr, Ostby J., Ferrell J., Rehnberg G., Linder R., Cooper R., Goldman J., Slott V., Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol. 1989 Jan;12(1):92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Ross R. K., Pike M. C., Casagrande J. T. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982 Aug;42(8):3232–3239. [PubMed] [Google Scholar]
- Holschneider D. P., Kumazawa T., Chen K., Shih J. C. Tissue-specific effects of estrogen on monoamine oxidase A and B in the rat. Life Sci. 1998;63(3):155–160. doi: 10.1016/s0024-3205(98)00255-0. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Breathnach R., Jeltsch J. M., Masiakowski P., Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984 Mar 26;12(6):2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch J. M., Roberts M., Schatz C., Garnier J. M., Brown A. M., Chambon P. Structure of the human oestrogen-responsive gene pS2. Nucleic Acids Res. 1987 Feb 25;15(4):1401–1414. doi: 10.1093/nar/15.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S., Reynolds T., White R., Parker M. G., Sumpter J. P. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995 Jun;103(6):582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen M., Bévort M., Kledal T. S., Hansen B. V., Dalgaard M., Leffers H. Differential display competitive polymerase chain reaction: an optimal tool for assaying gene expression. Electrophoresis. 1999 Feb;20(2):230–240. doi: 10.1002/(SICI)1522-2683(19990201)20:2<230::AID-ELPS230>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Jørgensen M., Hummel R., Bévort M., Andersson A. M., Skakkebaek N. E., Leffers H. Detection of oestrogenic chemicals by assaying the expression level of oestrogen regulated genes. APMIS. 1998 Jan;106(1):245–251. doi: 10.1111/j.1699-0463.1998.tb01343.x. [DOI] [PubMed] [Google Scholar]
- Kavlock R. J., Daston G. P., DeRosa C., Fenner-Crisp P., Gray L. E., Kaattari S., Lucier G., Luster M., Mac M. J., Maczka C. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996 Aug;104 (Suppl 4):715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiber E. L., Broverman D. M., Vogel W., Peterson L. G., Snyder M. B. Individual differences in changes in mood and platelet monoamine oxidase (MAO) activity during hormonal replacement therapy in menopausal women. Psychoneuroendocrinology. 1996 Oct;21(7):575–592. doi: 10.1016/s0306-4530(96)00023-6. [DOI] [PubMed] [Google Scholar]
- Klaiber E. L., Broverman D. M., Vogel W., Peterson L. G., Snyder M. B. Relationships of serum estradiol levels, menopausal duration, and mood during hormonal replacement therapy. Psychoneuroendocrinology. 1997 Oct;22(7):549–558. doi: 10.1016/s0306-4530(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Klein K. O., Baron J., Colli M. J., McDonnell D. P., Cutler G. B., Jr Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest. 1994 Dec;94(6):2475–2480. doi: 10.1172/JCI117616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Kraus W. L., Montano M. M., Katzenellenbogen B. S. Identification of multiple, widely spaced estrogen-responsive regions in the rat progesterone receptor gene. Mol Endocrinol. 1994 Aug;8(8):952–969. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- Krøll J., Briand P. Estrogen-dependent release of serum proteins from MCF-7 breast carcinoma cells in vitro. Anticancer Res. 1988 Jan-Feb;8(1):89–91. [PubMed] [Google Scholar]
- Lan N. C., Katzenellenbogen B. S. Temporal relationships between hormone receptor binding and biological responses in the uterus: studies with short- and long-acting derivatives of estriol. Endocrinology. 1976 Jan;98(1):220–227. doi: 10.1210/endo-98-1-220. [DOI] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Ma Z. Q., Bondiolotti G. P., Olasmaa M., Violani E., Patrone C., Picotti G. B., Maggi A. Estrogen modulation of catecholamine synthesis and monoamine oxidase A activity in the human neuroblastoma cell line SK-ER3. J Steroid Biochem Mol Biol. 1993 Dec;47(1-6):207–211. doi: 10.1016/0960-0760(93)90076-9. [DOI] [PubMed] [Google Scholar]
- Ma Z. Q., Violani E., Villa F., Picotti G. B., Maggi A. Estrogenic control of monoamine oxidase A activity in human neuroblastoma cells expressing physiological concentrations of estrogen receptor. Eur J Pharmacol. 1995 Sep 15;284(1-2):171–176. doi: 10.1016/0014-2999(95)00387-z. [DOI] [PubMed] [Google Scholar]
- Masamura S., Santner S. J., Heitjan D. F., Santen R. J. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab. 1995 Oct;80(10):2918–2925. doi: 10.1210/jcem.80.10.7559875. [DOI] [PubMed] [Google Scholar]
- Massot O., Baskevitch P. P., Capony F., Garcia M., Rochefort H. Estradiol increases the production of alpha 1-antichymotrypsin in MCF7 and T47D human breast cancer cell lines. Mol Cell Endocrinol. 1985 Oct;42(3):207–214. doi: 10.1016/0303-7207(85)90050-4. [DOI] [PubMed] [Google Scholar]
- Meyer T., Koop R., von Angerer E., Schönenberger H., Holler E. A rapid luciferase transfection assay for transcription activation effects and stability control of estrogenic drugs in cell cultures. J Cancer Res Clin Oncol. 1994;120(6):359–364. doi: 10.1007/BF01247461. [DOI] [PubMed] [Google Scholar]
- Nishikawa J., Saito K., Goto J., Dakeyama F., Matsuo M., Nishihara T. New screening methods for chemicals with hormonal activities using interaction of nuclear hormone receptor with coactivator. Toxicol Appl Pharmacol. 1999 Jan 1;154(1):76–83. doi: 10.1006/taap.1998.8557. [DOI] [PubMed] [Google Scholar]
- Orlando C., Pinzani P., Pazzagli M. Developments in quantitative PCR. Clin Chem Lab Med. 1998 May;36(5):255–269. doi: 10.1515/CCLM.1998.045. [DOI] [PubMed] [Google Scholar]
- Petit F., Le Goff P., Cravédi J. P., Valotaire Y., Pakdel F. Two complementary bioassays for screening the estrogenic potency of xenobiotics: recombinant yeast for trout estrogen receptor and trout hepatocyte cultures. J Mol Endocrinol. 1997 Dec;19(3):321–335. doi: 10.1677/jme.0.0190321. [DOI] [PubMed] [Google Scholar]
- Rio M. C. Quelle(s) fonction(s) pour les peptides dits "en feuille de trèfle"? Bull Cancer. 1997 Apr;84(4):443–446. [PubMed] [Google Scholar]
- Santagati S., Garnier M., Carlo P., Violani E., Picotti G. B., Maggi A. Quantitation of low abundance mRNAs in glial cells using different polymerase chain reaction (PCR)-based methods. Brain Res Brain Res Protoc. 1997 Aug;1(3):217–223. doi: 10.1016/s1385-299x(96)00033-5. [DOI] [PubMed] [Google Scholar]
- Sarabia S. F., Liehr J. G. Induction of monoamine oxidase B by 17 beta-estradiol in the hamster kidney preceding carcinogenesis. Arch Biochem Biophys. 1998 Jul 15;355(2):249–253. doi: 10.1006/abbi.1998.0727. [DOI] [PubMed] [Google Scholar]
- Shafie S., Brooks S. C. Characteristics of the dextran-coated charcoal assay for estradiol receptor in breast cancer preparations. J Lab Clin Med. 1979 Nov;94(5):784–798. [PubMed] [Google Scholar]
- Sharpe R. M., Skakkebaek N. E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. 1993 May 29;341(8857):1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995 Oct;103 (Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpter J. P., Jobling S. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995 Oct;103 (Suppl 7):173–178. doi: 10.1289/ehp.95103s7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Eitzman B., Bossert N. L., Walmer D., Sparrow K., Flanders K. C., McLachlan J., Nelson K. G. Transforming growth factors beta 1, beta 2, and beta 3 messenger RNA and protein expression in mouse uterus and vagina during estrogen-induced growth: a comparison to other estrogen-regulated genes. Cell Growth Differ. 1994 Sep;5(9):919–935. [PubMed] [Google Scholar]
- Taupin D., Wu D. C., Jeon W. K., Devaney K., Wang T. C., Podolsky D. K. The trefoil gene family are coordinately expressed immediate-early genes: EGF receptor- and MAP kinase-dependent interregulation. J Clin Invest. 1999 May;103(9):R31–R38. doi: 10.1172/JCI3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. M., Blanchard B., Zava D. T. A simple method to determine whole cell uptake of radiolabelled oestrogen and progesterone and their subcellular localization in breast cancer cell lines in monolayer culture. J Steroid Biochem. 1984 May;20(5):1083–1088. doi: 10.1016/0022-4731(84)90347-9. [DOI] [PubMed] [Google Scholar]
- Toppari J., Larsen J. C., Christiansen P., Giwercman A., Grandjean P., Guillette L. J., Jr, Jégou B., Jensen T. K., Jouannet P., Keiding N. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996 Aug;104 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderKuur J. A., Hafner M. S., Christman J. K., Brooks S. C. Effects of estradiol-17 beta analogues on activation of estrogen response element regulated chloramphenicol acetyltransferase expression. Biochemistry. 1993 Jul 13;32(27):7016–7021. doi: 10.1021/bi00078a029. [DOI] [PubMed] [Google Scholar]
- Vinggaard A. M., Joergensen E. C., Larsen J. C. Rapid and sensitive reporter gene assays for detection of antiandrogenic and estrogenic effects of environmental chemicals. Toxicol Appl Pharmacol. 1999 Mar 1;155(2):150–160. doi: 10.1006/taap.1998.8598. [DOI] [PubMed] [Google Scholar]
- Voss H., Nentwich U., Duthie S., Wiemann S., Benes V., Zimmermann J., Ansorge W. Automated cycle sequencing with Taquenase: protocols for internal labeling, dye primer and "doublex" simultaneous sequencing. Biotechniques. 1997 Aug;23(2):312–318. [PubMed] [Google Scholar]
- Wahli W., Martinez E., Corthésy B., Cardinaux J. R. cis- and trans-acting elements of the estrogen-regulated vitellogenin gene B1 of Xenopus laevis. J Steroid Biochem. 1989;34(1-6):17–32. doi: 10.1016/0022-4731(89)90062-9. [DOI] [PubMed] [Google Scholar]
- Wang F., Porter W., Xing W., Archer T. K., Safe S. Identification of a functional imperfect estrogen-responsive element in the 5'-promoter region of the human cathepsin D gene. Biochemistry. 1997 Jun 24;36(25):7793–7801. doi: 10.1021/bi963100j. [DOI] [PubMed] [Google Scholar]
- Zava D. T., Blen M., Duwe G. Estrogenic activity of natural and synthetic estrogens in human breast cancer cells in culture. Environ Health Perspect. 1997 Apr;105 (Suppl 3):637–645. doi: 10.1289/ehp.97105s3637. [DOI] [PMC free article] [PubMed] [Google Scholar]