The live-virus vector era began in 1983, when Smith, Mackett, and Moss constructed a recombinant vaccinia virus expressing hepatitis B surface antigen and demonstrated the induction of hepatitis B-specific antibodies in rabbits immunized with the recombinant virus (113). Subsequently, live-virus vectors were developed with other DNA viruses, such as adenoviruses and herpesviruses, and with positive-strand RNA viruses, such as alphaviruses and flaviviruses. The vaccine vector potential of the large group of nonsegmented negative-strand RNA viruses (NNSV) remained unexplored largely due to the lack of infectivity of their genomic RNA in cell culture and the absence of a mechanism for recombinational insertion of foreign genes. The NNSV (order Mononegavirales) comprise four families: Rhabdoviridae, represented by vesicular stomatitis virus (VSV) and rabies virus (RV); Paramyxoviridae, including Sendai virus (SeV), human parainfluenza virus types 1, 2, 3, and 4 (HPIV1 to -4), measles and mumps viruses, Newcastle disease virus (NDV), and human respiratory syncytial and metapneumoviruses (HRSV and HMPV); Filoviridae, containing Ebola and Marburg viruses; and Bornaviridae, containing Borna disease virus. In 1994, Schnell, Mebatsion, and Conzelmann developed a reverse genetic system for RV that allowed the recovery of infectious virus entirely from cloned cDNA (102). This was followed quickly by the development of reverse genetic systems for numerous other NNSV (17, 22, 33, 50, 55, 69, 73, 76, 83, 133). This review will focus on the use of NNSV, in particular members of the Rhabdoviridae and Paramyxoviridae, as live vaccine vectors.
WHY USE A VECTORED VACCINE, AND WHY CHOOSE A NNSV?
In general, a live attenuated version of a viral pathogen is the vaccine of choice, because it expresses the full complement of viral antigens, induces a broad array of local and systemic immunity, and provides durable protection. However, vectored vaccines can have advantages in certain situations, including those involving (i) viruses for which a live attenuated vaccine might not be feasible, such as human immunodeficiency virus (HIV), due to its ability to establish a persistent infection, or Ebola virus, due to its high pathogenicity, (ii) viruses that do not grow well in vitro, such as human papillomavirus and hepatitis C virus, (iii) highly pathogenic viruses that present safety challenges during vaccine development, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Ebola virus, (iv) viruses that lose infectivity due to physical instability, such as HRSV, and (v) viruses that can exchange genes with circulating viruses, such as coronaviruses, influenza viruses, and enteroviruses. An appropriate vectored vaccine can overcome these specific challenges. In addition, features of the biology of certain candidate viral vectors might provide for improved efficacy, such as the targeting of dendritic cells by the alphavirus Venezuelan equine encephalitis virus (59). Finally, a major advantage of a vectored vaccine is that it can be rapidly engineered to generate a vaccine against a newly emerged highly pathogenic virus. In this case, sequence analysis of the new pathogen would identify the likely protective antigen(s), and its cDNA clones would be inserted into a pretested vector that has an extensive clinical pedigree, permitting expedited vaccine development.
The NNSV have a number of features that make them attractive candidates as viral vectors and that also illustrate desirable properties for a vector (Fig. 1). First, there are numerous well-characterized animal, avian, and human NNSV that could serve as vectors. Some animal or avian NNSV, such as VSV and NDV, are antigenically distinct from common human pathogens and therefore essentially the entire population should be susceptible to infection and successful immunization. Some of the human NNSV, such as HPIV1, -2, and -3, also have the potential for use as vaccine vectors in susceptible populations. For example, infants and young children characteristically are immunologically naïve to HPIV1, -2, and -3; thus, attenuated versions of HPIV1 to -3 can be developed as pediatric vaccine vectors. Second, much is known about attenuating NNSV. In some cases, animal or avian viruses are attenuated in primates due to a natural host range restriction. In other cases, mutations that attenuate these viruses have been identified and can be manipulated by reverse genetics. Thus, it should be possible to attenuate each NNSV vector expressing a foreign viral protective antigen such that it achieves an acceptable balance between attenuation and immunogenicity for the target population. Third, NNSV that are being actively explored as vectors replicate efficiently in cell substrates, such as Vero cells that are qualified for human use, a requirement for vaccine manufacture. Fourth, most NNSV can infect efficiently via the intranasal (IN) route and efficiently induce local immunoglobulin A and systemic immunoglobulin G antibody and cell-mediated protective immune responses. Needle-free intranasal vaccines could be administered easily and safely, facilitating immunization in the context of an outbreak or epidemic. Intranasal vaccines are particularly useful against viruses that can infect and spread via the respiratory tract, such as SARS-CoV and influenza virus. Fifth, the NNSV being developed as vectors replicate in the cytoplasm and do not integrate into the host genome, obviating concerns about cellular transformation. Sixth, gene exchange involving NNSV is an extremely rare event (114) and has not been observed in nature, indicating that the use of NNSV vaccine viruses in open populations would not pose the threat of recombination between vaccine virus and circulating viruses. The lack of recombination also contributes to the stability of the inserted foreign gene. Seventh, NNSV encode 5 to 11 separate proteins, while large DNA viruses, such as poxviruses, encode as many as 200 proteins. Several proteins expressed by these complex viruses modulate or antagonize host immune responses, which might decrease vaccine effectiveness. It is true that most NNSV encode one or two proteins that interfere with the host interferon system (reviewed in references 18 and 32), but these often can be mutated or deleted (1, 23, 125). Measles virus is one NNSV that is significantly immunosuppressive (mediated by a mechanism other than interferon antagonism), making it a less attractive vector candidate. In addition, the use of a complex vector that expresses a large number of proteins might be less desirable, since these vector antigens might dilute the desired antibody and cell-mediated response directed against the foreign antigen and lead to a reduction of the overall memory T-cell pool (58). Eighth, the genomes of NNSV consist of genes that, for the most part, are nonoverlapping and are expressed as separate mRNAs, thus constituting a modular organization that can be readily manipulated for the insertion and stable maintenance of foreign inserts.
FIG. 1.
Features of NNSV that make them attractive as viral vectors.
CONSTRUCTION OF NNSV-VECTORED VACCINES
Reverse genetics.
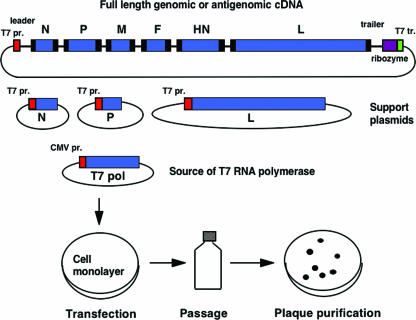
NNSV can be recovered entirely from cloned cDNA by transfecting cultured cells with plasmids encoding the viral components of a functional nucleocapsid, namely (i) full-length genomic or antigenomic (i.e., replicative intermediate) RNA and (ii) the major proteins involved in replication and transcription, namely the nucleoprotein (N or NP), phosphoprotein (P), and the large polymerase protein (L) (Fig. 2). The recovery of HRSV and Filoviridae members Marburg or Ebola virus requires, in addition, the transcription elongation factor M2-1 or transcription activator factor VP30, respectively (17, 26, 129). Plasmids are delivered by transfection into a cell line that can support replication of the particular virus. Plasmid expression is directed by bacteriophage T7 RNA polymerase that can be delivered in any of three ways: (i) coinfection with a recombinant vaccinia virus expressing T7 polymerase (135); (ii) the use of cells that constitutively express T7 polymerase, which presently are available as a baby hamster kidney line (6); or (iii) cotransfection of a plasmid encoding T7 polymerase (134). The third method (shown in Fig. 2) is preferable, because it allows the recovery of viruses in a cell line approved for vaccine production (e.g., Vero) without adding components that can complicate regulatory approval (e.g., vaccinia virus). Intracellular expression of these RNA and protein components results in the assembly (although inefficient) of a viral nucleocapsid that is competent for gene expression and genome replication, thus launching a productive infection that yields infectious virus (Fig. 2).
FIG. 2.
Recovery of a complete, infectious NNSV from cDNA. A plasmid encoding the complete antigenomic RNA of a parainfluenza virus is shown at the top. The extragenic 3′ leader and 5′ trailer regions are shown, as are the six viral genes: N, nucleoprotein; P, phosphoprotein, M, matrix protein, F, fusion protein, HN, hemagglutinin-neuraminidase, and L, large polymerase subunit. The GS and GE transcription signals are shown as black boxes at the beginning and at the end of each gene, respectively. The antigenomic cDNA is flanked to the left by a promoter for T7 RNA polymerase (T7 pr.) and on the right by a self-cleaving ribozyme and T7 terminator (T7 tr.). Three other T7 expression plasmids encode the N, P, and L support proteins needed to reconstitute a biologically active nucleocapsid. The T7 RNA polymerase is supplied from a cotransfected eukaryotic expression plasmid bearing a cytomegalovirus promoter (CMV pr.). The plasmids are transfected or electroporated into a cell monolayer in which the plasmid-expressed viral RNA and protein components assemble into a functional nucleocapsid and launch a productive infection. Recovered virus is amplified by passage and can be plaque purified.
Strategy 1. Attenuated versions of wild-type NNSV expressing foreign genes.
The simplest vector strategy is to express one or more foreign antigens from genes that have been added to a complete, replication-competent NNSV (Fig. 3A). The NNSV vector must be sufficiently attenuated to be safe for administration to humans. In the case of NNSV, such as NDV, that are attenuated in primates due to a natural host restriction (9), the wild-type virus can be used directly as the vector.
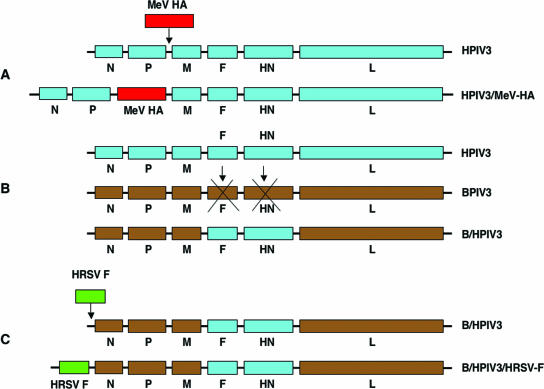
FIG. 3.
Three strategies for designing NNSV vectors. In panel A, a complete virus (in this case, HPIV3) is modified by insertion of a transcription cassette encoding a foreign antigen (measles virus [MeV] HA glycoprotein). In panel B, a NNSV (BPIV3) is modified by deleting its surface glycoprotein genes (F and HN) and replacing them with those (F and HN) from the target pathogen (HPIV3), resulting in an antigenic chimeric virus (B/HPIV3). In panel C, an antigenic chimeric virus (B/HPIV3) is modified by insertion of a transcription cassette encoding a foreign glycoprotein (HRSV F). In each case (A to C), the coding sequence of the foreign glycoprotein(s) must be under the control of GS and GE signals that are compatible with the vector backbone.
Other NNSV will require attenuation by reverse genetics before use as potential vectors in humans. For example, VSV is not a common human pathogen, but VSV infection is associated with disease, albeit usually mild, in humans (reviewed in reference 91). Thus, attenuation and careful clinical evaluation will be needed before VSV can be used as a vaccine vector. Another example is HPIV3, which can cause respiratory tract disease in humans, as already noted, and for which attenuated strains similarly must be prepared and validated as safe.
Attenuation of NNSV can be achieved by employing a variety of strategies. The viral gene order can be rearranged, which results in a suboptimal level of gene expression and thereby reduces replication efficiency (29). Attenuating nucleotide or, more commonly, amino acid point mutations can be identified and introduced in desired combinations by reverse genetics (106, 111). Point mutations also often can be “transferred” between related viruses by reverse genetics using sequence alignments as a guide (2, 62, 72). Deleting or silencing nonessential accessory genes, including those encoding interferon antagonist proteins, often yields attenuated derivatives (3, 23, 126). For rhabdoviruses, an effective attenuation of replication without a significant reduction of immunogenicity was achieved by the partial truncation of the G glycoprotein cytoplasmic tail (65, 79, 88, 98). The insertion of foreign genes for expression frequently has an attenuating effect by itself (see below). Attenuation also can be achieved by swapping internal protein genes between related human and animal or avian viruses to introduce host range restriction (78, 105).
Strategy 2. Antigenic chimeric viruses.
An alternative to expressing foreign antigens from added genes is to replace the major protective surface antigen(s) of the vector with that of the pathogenic virus of interest (Fig. 3B). Such constructs are referred to as antigenic chimeric viruses. An antigenic chimeric virus is viable (e.g., capable of efficient multicycle replication) only if the foreign surface proteins are (i) effectively incorporated into the virion particles and (ii) functional in the vector background.
Several notable examples are based on VSV in which the VSV G glycoprotein was replaced by the hemagglutinin (HA) glycoprotein of influenza virus (88) or the respective glycoprotein of Ebola, Marburg, or Lassa virus (31). The resulting chimeras were attenuated in vitro compared to the VSV parent, suggestive of a degree of incompatibility between the vector proteins and foreign glycoproteins. However, the efficiency of VSV replication is normally so high that a substantial reduction can be tolerated and, in addition, will likely increase vaccine safety. Although attenuated, the chimeras were competent for in vivo replication and were immunogenic and protective in mice or nonhuman primates (31, 35, 46, 88). However, not all VSV-based chimeras are replication competent. For example, chimeras in which the VSV G protein was replaced with the G or F protein of HRSV were not viable, even though each HRSV protein was incorporated into the VSV particle, and even though F alone is sufficient to confer efficient infectivity and growth to HRSV in vitro (47, 124).
As another example, the substitution of the HN and F glycoproteins of HPIV1 into an HPIV3 backbone resulted in a chimera (Fig. 4B) that replicated with wild-type efficiency in vitro and in vivo (111, 121). The lack of attenuation associated with chimerization in this case likely reflects the close relationship between these two viruses (they are both in the genus Respirovirus). Consistent with this idea, the substitution of the HN and F glycoproteins of the more-distantly related HPIV2 (genus Rubulavirus) into HPIV3 did not yield a viable chimeric virus. However, when the HN and F proteins of HPIV2 were modified to replace their cytoplasmic domains with the corresponding ones of HPIV3, viable antigenic chimeras that replicated efficiently in vitro could be recovered (Fig. 4C), although they were attenuated in vivo (122). Thus, perhaps not surprisingly, the ability of a heterologous glycoprotein to function efficiently in an antigenic chimera can depend on the fit of its cytoplasmic domain into the vector background.
FIG. 4.
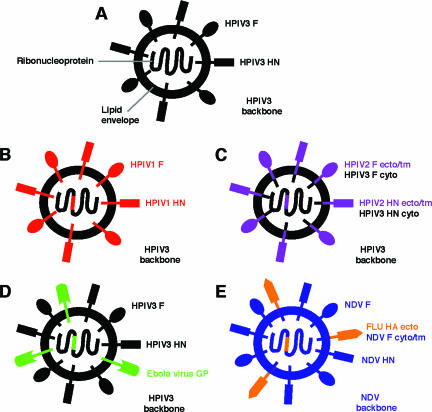
Examples of the incorporation of foreign glycoproteins into NNSV vector particles involving antigenic chimeric viruses (B and C) and foreign glycoproteins expressed from an added gene (D and E). The F and HN glycoproteins of HPIV3 (A, black) were deleted and replaced with the F and HN glycoproteins of HPIV1 (red), yielding an antigenic chimeric virus bearing the surface antigens of HPIV1 (B). A comparable derivative of HPIV3 bearing the surface antigens of HPIV2 (purple) was viable only if the HPIV2 F and HN proteins were modified to contain the cytoplasmic tails of the HPIV3 F and HN proteins, respectively (C). The expression of Ebola virus GP (green) from an added gene in HPIV3 resulted the incorporation of GP into the HPIV3 vector particle (D), whereas the incorporation of the avian influenza HA protein (FLU, orange) expressed from an added gene in NDV (blue) depended on replacing its cytoplasmic and transmembrane domains with those of the NDV F protein (E). ecto, ectodomain; tm, transmembrane domain; cyto, cytoplasmic domain.
Another noteworthy antigenic chimeric virus involved replacing the F and HN surface glycoprotein genes of bovine PIV3 (BPIV3), which is attenuated in humans due to a natural host range restriction, with the F and HN genes from its closely related human counterpart HPIV3 (Fig. 3B). This had the effect of combining the host range restriction of BPIV3 with the major antigenic determinants of HPIV3, resulting in an attenuated virus bovine-human PIV3 (B/HPIV3) that replicates with undiminished efficiency in vitro, retains the host range attenuation phenotype in nonhuman primates, and is being evaluated clinically by the National Institute of Allergy and Infectious Diseases as a vaccine against HPIV3 (37, 77, 95).
Strategy 3. Antigenic chimeric virus expressing additional foreign genes.
In addition to serving directly as a live vaccine, antigenic chimeric viruses also can be used to express additional antigens from added genes (Fig. 3C). For example, the B/HPIV3 chimera just mentioned also has been used to express protective antigens of HRSV or HMPV from added genes (96, 97, 119). These applications would yield a bivalent vaccine against HPIV3 and HRSV or HPIV3 and HMPV, respectively, each based on a single virus. B/HPIV3 expressing the HRSV F glycoprotein is currently in clinical trials by MedImmune, Inc.
Insertion of a foreign gene into the genome of a NNSV.
The modular organization of a typical NNSV genome is illustrated in Fig. 2 and 3. Transcription is initiated at the 3′ (left-hand) end, and the genes are copied sequentially by the viral polymerase guided by the gene start (GS) and gene end (GE) signals that form the upstream and downstream limits of each gene. These signals direct transcriptional initiation and termination/polyadenylation, respectively, at each gene, yielding individual mRNAs. The viral promoter present at the 3′ end of the genome (and antigenome) ranges in size from ∼44 to ∼96 nucleotides, depending on the virus, and the GS and GE signals are each usually 8 to 12 nucleotides long. Thus, the cis-acting signals are short and circumscribed, characteristics which further facilitate engineering.
A transcription cassette expressing a foreign antigen is constructed by engineering a cDNA of the foreign open reading frame so that it is flanked by vector-specific GS and GE signals. This cassette is then inserted (via the cDNA intermediate) into the gene order of the NNSV vector flanked by intergenic regions. In the recovered recombinant NNSV, the foreign insert is expressed as an additional, separate mRNA encoding the foreign protein (8, 68, 100). NNSV can tolerate and efficiently express a foreign gene inserted upstream of the first gene, into essentially any intergenic region, or downstream of the last gene (110, 127, 137).
One taxonomic group of NNSV has the unusual requirement that the nucleotide length of the genome must be an even multiple of six in order to replicate efficiently, which is thought to reflect a strict nucleocapsid organization in which each N monomer associates with exactly six nucleotides (15, 52). The “rule of six” applies exclusively to members of the Paramyxovirinae subfamily of Paramyxoviridae, which includes SeV, the HPIVs, and NDV. Furthermore, for the “rule of six” viruses, the placement of each GS signal with regard to the hexamer phasing of the genome may play a role in the efficiency of transcription. Thus, for these viruses, insert length and organization must comply with these restrictions.
Position of the insert in the gene order.
Transcription by NNSV has a polar gradient, such that promoter-proximal genes are expressed more efficiently than promoter-distal ones. Thus, foreign genes are expressed more efficiently when placed in upstream positions. For VSV, transcription decreases by approximately 30% across each gene junction (43, 132). Consistent with this, when the L1 protein of cottontail rabbit papillomavirus was expressed from a gene inserted into position 2 or 5 of a recombinant VSV vector, the upstream position provided a 4.3-fold-higher level of expression. This was associated with the induction of a 16-fold-higher titer of L1-specific serum antibodies and greater protection against papillomavirus challenge, whereas there was no difference between the two constructs with regard to the induction of serum antibodies against the VSV vector (90). Viruses such as SeV and HRSV have a less regular gradient of gene expression (40, 53). Nonetheless, a consistent relationship between the position of a foreign gene and its level of expression was noted for SeV (127).
Due to the polar nature of transcription, the insertion of one or more foreign genes also can reduce the expression of downstream vector genes, which can decrease the replication of the vector in vitro and in vivo (24, 107, 110, 127, 131, 137). Insertion of a foreign gene into a promoter-proximal position can be more attenuating than insertion into a promoter-distal position (110, 127), presumably because a promoter-proximal insertion affects a greater number of downstream vector genes. For NDV and VSV, insertion between the N and P genes had the greatest attenuating effect among the gene junctions (131, 137). This disproportionate effect appeared to occur because alteration in the molar ratio between the N and P proteins, two closely interacting proteins of the nucleocapsid, is particularly deleterious to virus transcription and RNA replication.
Vector capacity: insert size versus number.
The insertion of a foreign gene into a NNSV increases its genome length and gene number and often has an attenuating effect on virus replication in vitro and in vivo, with the effect typically being greater in vivo. This can be due to effects on the level of transcription, as already noted. RNA replication and packaging might also be affected, although this has not been studied in detail.
To investigate the effect of increased genome length on the replication of a NNSV vector in vitro and in vivo, a series of foreign sequences ranging in length from 258 nt to 3,894 nt and designed to lack a significant open reading frame were inserted individually into the downstream noncoding region of the HN gene in HPIV3, thus increasing the length of the genome without increasing the number of genes (107). Inserts of up to 1,404 nt had no effect on virus replication in vitro or in vivo, while an insert of 3,126 or 3,894 nt was well tolerated in vitro but reduced replication approximately 10-fold in vivo. In a second phase of this study, the foreign sequences were inserted as an additional gene between HN and L, thus increasing the genome length and gene number (by a single gene). In this case, an inserted transcription cassette of 1,428, 1,908, or 3,918 nt was well tolerated in vitro and reduced replication in vivo 5-, 2-, or 25-fold, respectively, whereas inserts of 168 and 678 nt had no effect (107). Thus, small inserts added either as a noncoding sequence or as an additional gene had little or no effect on replication in vitro and in vivo. As the size of the either type of insert increased to ∼1.4 kb and greater, attenuation became evident and increased with increasing size, confirming the importance of size on attenuation. Whether the foreign sequence was added as a noncoding sequence or as an additional gene made little difference, indicating that increasing the gene number by one had little effect by itself. The lack of attenuation associated with increased gene number also was observed in a study in which one, two, or three antigens of SARS-CoV were expressed from the attenuated B/HPIV3 backbone: in this case, adding one or two small SARS-CoV genes to a vector already bearing the large SARS-CoV S protein gene had little further attenuating effect on replication in vitro and in vivo (5).
In another study, up to three foreign genes were inserted into HPIV3, with an aggregate increase in genome length from 15.5 kb to 23 kb (a 49% increase) (110). The insertion of a single foreign gene of ∼2 kb had little effect on replication in vitro or in vivo, but the insertion of two such genes decreased replication 10-fold in vitro and up to 100-fold in vivo. The insertion of three genes (aggregate insert length, up to 7.5 kb) had a modest further attenuating effect on replication in vitro but reduced replication in vivo over 10,000-fold, rendering the virus overattenuated and poorly immunogenic in hamsters. The practical carrying capacity of a PIV vector thus appears to be one to three genes of an aggregate length of approximately 4 to 5 kb. NNSV, such as VSV, that replicate with high efficiency likely have a higher capacity, although the limit has not been evaluated.
Toxic inserts.
In a few cases, the expressed foreign glycoprotein appeared to be toxic and strongly attenuated the replication of the NNSV vector. During growth in vitro, these foreign genes rapidly accumulated mutations that resulted in an altered protein, a reduction of the level of expression, or the full ablation of expression. Examples of toxic combinations of insert and vector include the HPIV1 HN protein expressed from an added gene by HPIV3, which may have been inhibitory because it was packaged in the vector particle and may have interfered with the HPIV3 HN protein due to their similarity (110), and the expression of the mumps virus F protein by measles virus (130) and measles virus F protein by VSV (81), which may have been inhibitory because of their fusogenic nature or interference with vector glycoproteins.
Insert stability.
In order to be feasible for human immunization, a foreign gene in a NNSV vector must be maintained stably during all phases of manufacture and use. This is more of a challenge for added genes (as opposed to swapped genes in antigenic chimeric viruses), since they do not contribute to vector growth; thus, there is no selective pressure to maintain integrity. RNA viruses have a misincorporation rate of 10−3 to 10−5 substitutions per nucleotide per round of replication (115; reviewed in reference 21), and vectors based on positive-strand RNA viruses often exhibit rapid deletion of the foreign insert in whole or in part during passage in vitro (34). Surprisingly, inserts in NNSV appear to accumulate point mutations more slowly than anticipated and rarely sustain deletions. For example, when a recombinant VSV bearing the bacterial chloramphenicol acetyltransferase gene was subjected to 15 passages, four resulting plaque-purified viruses had no mutations in the foreign insert, whereas two others had a single nucleotide substitution each (100). In another example, a recombinant NDV expressing the VP2 protein of infectious bursal disease virus was passaged 12 times in embryonated chicken eggs; sequencing of the reverse transcription-PCR-amplified insert indicated that the consensus sequence was unchanged (41). Even though foreign genes are generally stable, it is necessary to monitor all vaccine preparations to ensure the integrity of the insert.
INCORPORATION OF THE FOREIGN GLYCOPROTEIN INTO THE VECTOR PARTICLE AND EFFECTS ON VECTOR BIOLOGY
NNSV acquire their envelope from the host cell plasma membrane during budding and have the potential to incorporate surface-expressed foreign glycoproteins into the vector particle. We have already discussed antigenic chimeric viruses, which indeed depend on the foreign glycoprotein(s) being incorporated into the vector particle and being functional in place of the deleted vector glycoprotein(s) (e.g., Fig. 3B and 4B and C). Foreign glycoproteins expressed from added genes also can be incorporated into the vector particle (Fig. 4D and E).
In the case of VSV, foreign glycoproteins expressed from added genes usually are incorporated efficiently into the virion, based on examples that include the influenza virus neuraminidase and HA proteins, the measles virus H and F proteins, and the HRSV F protein (48, 54, 99). These foreign glycoproteins were packaged at levels of up to 30% of the relative molar amount of the vector G glycoprotein (or up to 50% when corrected for the relative level of intracellular expression). The HIV type 1 (HIV-1) envelope protein was an exception that was incorporated only in a trace amount, but this amount was increased substantially (although it was still relatively low) by replacing its cytoplasmic tail with that of VSV G (44). However, the expedient of swapping the cytoplasmic tail did not increase the incorporation of the HRSV F or CD4 surface glycoproteins, indicating that the presence of the VSV G tail usually is unnecessary. The HRSV F and HIV-1 envelope proteins were shown to be functional in the VSV envelope and capable of initiating infection independent of the G protein of the vector. Thus, the VSV virion appears to be promiscuous with regard to incorporation of foreign surface proteins and, in most cases, this seems to be irrespective of structural or taxonomic relatedness or the presence of the VSV cytoplasmic tail.
Incorporation of a foreign glycoprotein expressed from an added foreign gene also has been investigated for the PIVs, with results that are somewhat more varied than those for VSV. Expression of the HRSV G protein by recombinant SeV (a murine PIV) or of the SARS-CoV spike S protein by B/HPIV3 did not result in significant incorporation of these proteins into the viral particles (10, 117). However, the HPIV1 HN protein was incorporated into the HPIV3 vector particle and indeed interfered with replication of the vector, as already noted (110). This might be a consequence of the greater relatedness of HPIV1 and HPIV3 than the other viruses. However, a close taxonomic/sequence relationship is not essential for incorporation, since the HMPV F protein expressed by HPIV1 was efficiently incorporated into the vector particle (M. H. Skiadopoulos, P. L. Collins, and B. R. Murphy, unpublished observations), and the Ebola virus GP glycoprotein expressed by HPIV3 also was incorporated into the particle (13) (Fig. 4D).
One potential consequence of incorporating a foreign glycoprotein into the vector particle is that it might alter the tropism or the ability of the vector to spread within the host. This potentially might lead to an increase in virulence in the case of a highly pathogenic agent, but the available evidence, albeit incomplete, is reassuring. Recombinant VSV expressing the HIV-1 envelope glycoprotein from an added gene (and which is packaged and functional in the vector particle) and antigenic chimeras of VSV expressing the GP of Ebola, Marburg, or Lassa virus have been evaluated in nonhuman primates and appeared to be attenuated and safe, in addition to being immunogenic and protective (35, 46, 92). This suggests that the foreign glycoproteins did not substantially increase the virulence of the vector on a gross level, although these studies were not designed to investigate possible subtle changes in vector biology. Perhaps the best-controlled study involved HPIV3 in which the Ebola virus GP was expressed from an added gene and incorporated into the vector particle (13). In this case, the HPIV3 vector became resistant to neutralization in vitro by HPIV3-specific antiserum, suggesting that the Ebola GP could function in the vector particle as an alternative attachment and penetration protein. This was confirmed by the finding that the vector also had acquired sensitivity to neutralization with Ebola virus-specific antiserum. This recombinant was evaluated by IN inoculation, in parallel with the HPIV3 parent, into guinea pigs, which are permissive to both HPIV3 and Ebola virus, and in which even a trace inoculum of Ebola virus is highly lethal. There was no difference in the health of the animals between the group receiving the HPIV3 parent versus the derivative expressing Ebola virus GP, suggesting that there was no increase in virulence, even in this very sensitive system (13).
IMMUNOGENICITY OF NNSV-VECTORED VACCINES
Several factors influence the immunogenicity of a vectored vaccine. First, the level of immunogenicity and protective efficacy of any live viral vaccine depends on its level of replication. Thus, reduced replication resulting from attenuation can result in reduced immunogenicity. Achieving a level of attenuation that renders the vaccine safe and yet retains a level of replication sufficient to be satisfactorily immunogenic can be challenging and ultimately depends on clinical evaluation.
A second factor influencing the efficacy of a vaccine is the repertoire of pathogen antigens that is expressed. A vectored vaccine typically expresses only one or two proteins of the target pathogen, usually the surface glycoprotein(s), whereas an attenuated version of a pathogen expresses the full repertoire of pathogen antigens. Although the expression of neutralization antigens can provide an efficient induction of virus-neutralizing antibodies, the reduced repertoire of pathogen antigens likely leads to a reduced cell-mediated response relative to a comparably attenuated version of the complete pathogen. For example, an HPIV1 vector expressing the HMPV F fusion protein from an added gene induced a high level of HMPV-neutralizing serum antibodies, one that was similar in magnitude to that induced by infection with wild-type or attenuated strains of HMPV (103, 104). However, there was a substantial difference in protective efficacy against a subsequent HMPV challenge. One or two immunizations (with a one-month interval) with the HPIV1-vectored vaccine reduced challenge HMPV replication by 1.8 log10 or 2.8 log10, respectively. In contrast, a single immunization with wild-type or attenuated HMPV reduced challenge HMPV replication by 5.0 log10 even though the HPIV1 vector replicated much more efficiently than the highly attenuated HMPV strains (4, 78, 103, 104). The idea that internal proteins make a substantial contribution to protective immunity was illustrated with the already-mentioned antigenic chimeric virus in which the F and HN surface antigens of HPIV3 were replaced by those of HPIV1 (Fig. 4B). This antigenic chimeric virus induced neutralizing antibodies only to HPIV1, but induced protection against both HPIV1 and HPIV3, the latter presumably due to cell-mediated immunity induced by the internal proteins (123).
The impact of the reduced repertoire of antigens probably varies with different situations. In the case of VSV vectors against HIV-1, for which a strong cell-mediated response is desired, the HIV-1 Gag proteins often are included along with the HIV-1 envelope glycoprotein to provide a greater repertoire of antigens for cell-mediated responses. In other applications, such as for vaccines against SARS-CoV, serum antibodies are highly effective in controlling the infection, and an incomplete repertoire of antigens is less likely to compromise vaccine efficacy (116). In contrast, protection against the pediatric respiratory pathogen HRSV is difficult to achieve due to the immunologic immaturity of the young infant, the immunosuppressive effect of maternal serum antibodies on the humoral response (20), and the mucosal site of replication. Thus, for HRSV, it might be preferable to stimulate immunity as broadly as possible.
The immunogenicity of a vectored foreign glycoprotein also is affected by whether it is incorporated into the vector particle. This reflects the greater immunogenicity of particles than soluble proteins, particularly particles that can mediate binding to and (in some cases) infection of antigen-presenting cells, as well as the more rapid release from infected cells of virion-bound proteins versus those released by necrosis and apoptosis. For example, the replacement of the cytoplasmic tail and the transmembrane domain of the HA glycoprotein of avian H7 influenza virus expressed by a recombinant NDV with that of the vector F protein resulted in an enhanced incorporation of the protein into virion particles (Fig. 4E), an enhanced immune response, and an increased protective efficacy against influenza virus compared to the construct expressing the unmodified HA protein (75). As another example, when the incorporation of the HIV envelope protein into recombinant VSV was increased by swapping its cytoplasmic domain with that of VSV G, its immunogenicity was increased (44, 79).
The immunogenicity of a NNSV vector can be increased by the coexpression of an immunostimulatory molecule such as gamma interferon, interleukin-2, granulocyte macrophage colony stimulating factor, or cytochrome c. In some cases, coexpression of the immunostimulatory molecule attenuated the NNSV vector yet allowed it to retain its immunogenicity (7, 12, 84). In other cases, the magnitude of the immune response was increased due to the coexpressed molecule, such as with an antigenic chimeric B/HPIV3 vector expressing granulocyte macrophage colony stimulating factor (11) and a RV vector expressing interleukin-2 (64). The expression of a proapoptotic protein cytochrome c by a recombinant RV resulted in attenuation of the virus but enhanced its immunogenicity, an effect attributed to increased capture of apoptotic cells by antigen-presenting cells (80).
NNSV vectors also provide strategies for improved booster immunizations. Reimmunization with a vectored vaccine, as with a live attenuated vaccine, can have reduced effectiveness because the immune response to the initial immunization restricts the replication and immunogenicity of subsequent doses. However, the effectiveness of reimmunization can be improved if it is performed using a different vector. For example, the use of RV- and VSV-based vectors expressing HIV-1 envelope protein for the first and second immunizations, respectively, resulted in a dramatic enhancement of cellular and humoral immune responses compared to two immunizations with one virus (118). The availability of four serotypes of HPIV, HPIV1 to -4, provides the potential for sequential immunizations in the pediatric population.
Alternatively, the vector can be modified to bear a different set of surface antigens. For example, three versions of recombinant VSV have been made, each bearing the G glycoprotein from a different VSV serotype. Sequential immunization with these three vectors, each expressing the HIV-1 envelope protein or the envelope and Gag proteins in combination, was shown to provide improved boosts (92, 93). The HN and F surface proteins of HPIV3 also have been exchanged with those of HPIV1 and -2, as already noted (121, 122). However, with the use of glycoprotein exchange vectors bearing a constant set of internal proteins, cell-mediated immunity raised against the internal proteins also can confer resistance to reinfection, although this protection can diminish within a few weeks to a few months and, thus, is another factor that must be considered for sequential immunization (123).
A NNSV-vectored vaccine also can be used to boost the immune response induced by a live-virus vaccine. For example, immunization with an attenuated strain of HRSV followed by a boost with an HPIV1 vector expressing the HRSV F protein was more immunogenic than two doses of the attenuated strain (Skiadopoulos et al., unpublished). Booster immunization might also be more effective if sequential immunizations employ different routes of administration, such as the use of a VSV vector administered intramuscularly (IM) followed by an NDV vector administered IN.
SPECIFIC VACCINE VECTORS
Vesicular stomatitis virus.
VSV (family Rhabdoviridae, genus Vesiculovirus) is a natural pathogen of cattle, horses, and swine that causes disease characterized by severe vesiculation and/or ulceration of tongue, oral tissues, feet, and teats (reviewed in reference 56). The infection of mice and cotton rats with VSV by the IN route results in significant replication of the virus in brain, encephalitis, and high mortality (30, 85, 94). VSV is not usually a human pathogen. Most human infections that do occur (from contact with infected animals) are believed to be asymptomatic but sometimes result in illness characterized by chills, myalgia, and nausea (28, 39, 45); in addition, a case of encephalitis associated with VSV infection in a 3-year-old child has been documented (82).
VSV has a number of very advantageous features for use as a vaccine vector. It is the simplest NNSV and has a very high level of gene expression as well as rapid and extremely efficient replication in vitro. The general population in most areas of the world lacks immunity to VSV and thus, presumably, is susceptible to immunization. Versions of recombinant VSV bearing the G glycoprotein from three different serotypes are available for sequential immunization (92, 93). In experimental animals, VSV vectors have been very effective when administered by the IN, IM, intradermal, or oral routes (25, 86, 93). The virus particle has a regular structure and is stable, facilitating purification, manufacture, and use.
The major drawback for VSV is that, at present, there is little or no experience with its administration to humans and, thus, it is not clear which tissues are infected, how much the infection spreads, what titers are achieved, and whether serious complications may occur in some individuals. The central nervous system involvement observed with rodents and with a human case warrants caution. It also is not known how immunogenic and protective VSV vectors will be in humans, either as antigenic chimeric viruses or as vectors expressing added foreign genes, although results with experimental animals are very promising. Recombinant VSV appears to be inherently attenuated, presumably due to chance mutations, and a number of strategies exist for further attenuating the virus, as already noted. It seems clear that VSV-based vaccines will be evaluated clinically in the near future and, thus, detailed information on its replication, pathogenesis, and immunogenicity in humans will be forthcoming.
Given that VSV is somewhat of an unknown entity at present with regard to human administration, we believe that its most feasible first use will be as a vector to control outbreaks of highly pathogenic agents. This is because these are situations in which (i) the vaccinees are mostly healthy adults, (ii) the number of vaccinees will be relatively small, and (iii) the high risk of the pathogen would outweigh some potential risk of residual virulence of the vector. As already noted, antigenic chimeras based on VSV expressing the GP glycoprotein of the hemorrhagic fever agents Ebola, Marburg, or Lassa viruses have been constructed. A single IM immunization of cynomolgus macaques with the constructs was well tolerated and induced antibody and cellular immune responses specific to the foreign GP, which protected the animals against a subsequent challenge with a high dose of each respective virus (35, 46). VSV also has been used to express the SARS-CoV S spike glycoprotein from an added gene, and a single IN immunization of mice provided essentially complete protection against an IN challenge with SARS-CoV (49). As experience with VSV in humans is gained and safety and immunogenicity are established, another use in outbreak control would be to immunize against highly pathogenic avian influenza virus in areas where that virus is endemic. VSV bearing the HA glycoprotein of human influenza A virus was highly immunogenic and protective against an otherwise lethal challenge in mice (89). In this application, the lack of recombination associated with NNSV provides a major safety advantage by avoiding exchanging genes with circulating influenza virus strains, thus making prospective immunization feasible.
VSV also has been evaluated as a vaccine vector against a number of prevalent human viruses, including papillomavirus (86, 90), hepatitis C virus (14), and HIV (25, 36, 92, 93). In particular, the three VSV glycoprotein exchange vectors were each engineered to express the simian immunodeficiency virus (SIV) Gag protein and HIV-1 envelope protein (bearing the cytoplasmic tail of the VSV G protein to increase its efficiency of incorporation into the vector particle) from added genes. Three sequential immunizations of rhesus monkeys by the combined IM and oral routes induced a vigorous HIV-specific cytotoxic T lymphocyte (CTL) response and protection against a subsequent challenge with simian-human immunodeficiency virus (SHIV) (92). This is a particularly important line of experiments, since effective immunization against HIV poses the most daunting challenge to vaccinology.
VSV also has been developed as a vector against common pediatric pathogens, such as HRSV (47) and measles virus (94, 99). This use seems less likely for the VSV vector, since it would be preferable to avoid mass inoculation of infants with a virus that they otherwise would rarely experience and for which immunoprophylaxis is not needed. This view might change if VSV is found to be a highly effective and very benign vector in humans and if such VSV-based vaccines are found to be substantially more effective in humans than live attenuated versions for these pediatric pathogens. However, the use of VSV in a mass vaccination probably would interfere with its subsequent use against other pathogens.
Human parainfluenza viruses.
The HPIVs belong to family Paramyxoviridae, subfamily Paramyxovirinae, genus Respirovirus (HPIV1 and -3), and genus Rubulavirus (HPIV2 and -4). The HPIVs infect nearly everyone worldwide and cause respiratory illness in infants and children ranging from the common cold to pneumonia. Live attenuated derivatives of HPIV1, -2, and -3 are actively being developed by reverse genetics for use as intranasal pediatric vaccines, whereas HPIV4 is a less serious pathogen (2, 74, 106; reviewed in reference 16). Thus, in contrast to VSV, there is extensive experience with infection of humans by the HPIVs in naturally occurring disease, in experimental administration of wild-type virus, and in clinical administration of vaccine candidates. HPIVs have a somewhat larger genome (15 kb versus 11 kb) than VSV and encode two or more additional proteins. They replicate at least 10- to 100-fold less efficiently in cell culture and are larger and pleomorphic. The HPIVs infect and cause disease in the respiratory tract and do not spread significantly to other tissues. This limits HPIV-based vectors to IN administration, but it also provides an inherent safety feature. IN immunization induces both strong systemic and local immunity.
The HPIVs seem ideally suited as vectors for pediatric pathogens, especially for ones that use the respiratory tract as a portal of entry, such as measles virus, HRSV, and HMPV. Since vaccines for HPIVs are needed, the expression of a protective antigen of another pediatric virus from an added gene creates a bivalent vaccine from a single virus, thus simplifying vaccine development and administration. For example, topical immunization of the respiratory tract of rhesus monkeys with an attenuated version of HPIV3 expressing the HA glycoprotein of measles virus efficiently induced systemic neutralizing antibody responses specific for both measles virus and HPIV3 (24, 108). This particular use provides two specific benefits. First, the existing measles vaccine is a live attenuated virus that is administered IM and is very sensitive to neutralization by maternal antibodies. This necessitates a delay in administration until 12 to 15 months of age with the result that some individuals become susceptible before the vaccination is received. An HPIV3-vectored vaccine is not neutralized by measles virus-specific antibodies and can efficiently infect and immunize young infants at an earlier age. The second benefit is that the use of a vectored measles virus vaccine eliminates concerns of persistence or partial reversion of the live attenuated measles vaccine virus, facilitating a global effort to eradicate measles virus. However, an established, successful vaccine like the current live attenuated measles vaccine is not readily replaced.
As another example, attenuated chimeric B/HPIV3 expressing the protective F or G protein of HRSV or the F protein of HMPV from added genes was highly immunogenic against both the vector and the vectored antigen in rodents and nonhuman primates (96, 97, 119, 120). In this case, the use of an HPIV-vectored vaccine provides a higher level of antigen expression and avoids the poor growth and instability of HRSV and HMPV. As already noted, the attenuated B/HPIV3 virus expressing HRSV F is presently in clinical trials as a live intranasal vaccine against HPIV3 and HRSV. Based on the time in infancy when infections with HPIV1, -2, and -3 occur, vectors based on HPIV3 would be most suited for immunization within the first six months of life, whereas vectors based on HPIV1 and -2 would be most suited for immunization between six months and one year of life.
The HPIVs also have been evaluated as vectors against highly pathogenic viruses that can infect via the respiratory tract. IN and IT immunization of African green monkeys with an attenuated B/HPIV3 virus expressing the spike (S) protein of the respiratory pathogen SARS-CoV from an added gene resulted in the induction of a systemic neutralizing antibody response against SARS-CoV and conferred protection against IN and IT challenge with SARS-CoV (10). HPIV3 also was used to express the GP glycoprotein of Ebola virus from an added gene to evaluate the efficacy of IN immunization against a virus that causes a severe systemic infection. IN immunization of guinea pigs with this recombinant induced a systemic Ebola virus-specific antibody response and conferred complete protection against a lethal dose of guinea pig-adapted Ebola virus administered intraperitoneally (13); the construct now is being evaluated in nonhuman primates. These vaccine candidates could be developed for use as pediatric vaccines against SARS-CoV and Ebola virus. However, it is likely that their efficacy in adults will be greatly reduced due to the prevalence of immunity against HPIV3 acquired by natural infection, which would restrict the replication of the vector and reduce immunogenicity. One solution of the problem of preexisting immunity would be the use vectors based on VSV, as discussed above, or nonhuman paramyxoviruses, such as NDV, as discussed below.
Newcastle disease virus.
NDV (family Paramyxoviridae, subfamily Paramyxovirinae, genus Avulavirus) is an avian pathogen whose naturally occurring strains exhibit a wide range of disease severity. They are categorized into three pathotypes: velogenic strains, which cause systemic infections with a high level of mortality; mesogenic strains, which cause systemic infections of intermediate severity; and lentogenic strains, which cause mild infections that are largely limited to the respiratory tract and which are used as live attenuated vaccines against NDV for poultry (reviewed in reference 38). One of the primary determinants of virulence is the cleavage activation phenotype of the F protein precursor, which in highly virulent strains is cleaved by ubiquitous intracellular proteases, providing the potential for pantropic replication, and which in nonvirulent strains is cleaved by a secretory protease, restricting replication to mucosal surfaces. NDV usually does not infect humans, but bird handlers can be infected, and occasional cases of conjunctivitis have been documented (57, 71). IN and IT inoculation of the respiratory tract of African green and rhesus monkeys with a mesogenic or lentogenic strain of NDV resulted in a low-level, asymptomatic infection of the respiratory tract with little or no virus shedding (9), evidence that both pathotypes are attenuated in primates due to a natural host-range restriction.
NDV shares only a low level of amino acid sequence identity with human paramyxoviruses and is distinct antigenically, suggesting that the human population would be susceptible to vaccination with an NDV-based vector. Studies with mice showed that NDV expressing protective antigens of influenza A virus, HRSV, or SIV were immunogenic and, in the first two cases, induced protection against challenge (60, 69, 70). However, rodents are not good models for evaluating a host range-restricted vector against human pathogens, because their permissiveness to both the vector and the pathogen probably do not accurately reflect that of the human due to the phylogenetic distance. Recently, recombinant lentogenic and mesogenic strains of NDV expressing the HPIV3 HN protein from an added gene were evaluated by IN and IT immunization of the respiratory tract of African green monkeys and rhesus monkeys, which likely provide more predictive models of humans (9). After one dose, an efficient HPIV3-specific serum antibody response was detected; after the second dose, it became equal to or greater than that induced by HPIV3 infection (9). Thus, NDV warrants further evaluation as a potential vector for human use. NDV also is being actively developed as a vaccine vector for veterinary use in which the vector itself serves as a needed poultry vaccine (41, 75, 128), but that is outside the scope of this review. NDV is classified as serotype 1 of the avian paramyxoviruses of genus Avulavirus; eight other serotypes, which cause disease of various levels of severity in different species of birds, have been identified (reviewed in reference 87). These also can be evaluated as possible vectors for human use.
Rabies virus.
RV (family Rhabdoviridae, genus Lyssavirus) causes deadly neurological disease in numerous animal species and humans. Several highly attenuated strains of the virus lacking neurovirulence in experimental animals have been developed (19, 65, 67, 136). Studies with mice have demonstrated the immunogenicity of RV vectors expressing the HIV envelope or Gag protein, or the SARS-CoV S protein, from added genes (27, 63, 66, 101, 118). The immunization of rhesus monkeys with highly attenuated rabies-based vaccine vectors expressing SIV Gag protein and HIV-1 envelope protein followed by a boost with similar vaccine constructs in which RV G protein was replaced with that of VSV resulted in a strong HIV-specific CTL and antibody response and protection against SHIV (P. McKenna, M. Koser, K. R. Carlson et al., Abstr. 13th Int. Conf. on Negative Strand Viruses, abstr. 297, 2006). Since RV infection in humans is rare, most of the population would be susceptible to immunization with an RV-based vector. However, whether attenuated derivatives could be used in humans is unclear, given the high neurovirulence of the parent virus.
Sendai virus.
SeV (family Paramyxoviridae, subfamily Paramyxovirinae, genus Respirovirus) is a parainfluenza virus that is virulent in mice and shares a moderate level of sequence and antigenic relatedness with HPIV1. Because of this antigenic relatedness, SeV is being developed as a pediatric vaccine against HPIV1 on the premise that SeV will be attenuated in humans due to a host range restriction (42, 109, 112). In addition, SeV has been evaluated as a vector to express the HRSV G glycoprotein from an added gene. IN inoculation of cotton rats with this recombinant induced HRSV-neutralizing antibodies and a high level of protection against a subsequent challenge with HRSV (117). This would provide a bivalent vaccine against HPIV1 and HRSV. However, SeV replicated nearly as efficiently as wild-type HPIV1 in African green monkeys and chimpanzees, raising doubts as to whether it will be satisfactorily attenuated in unmodified form for use in seronegative humans (109).
Several studies evaluated recombinant SeV expressing the SIV Gag gene for immunogenicity and protective efficacy against SIV challenge. A group of macaques primed with a plasmid DNA expressing SIV Gag was boosted with the SeV-Gag construct and challenged with SIV by the intravenous route; five out of eight vaccinated animals controlled the viral load and had undetectable plasma viremia 5 weeks after vaccination (61). The use of a recombinant SeV expressing SIV Gag for immunization of macaques chronically infected with SIV resulted in the induction of an effective Gag-specific CTL response (51). However, the use of SeV as a vaccine vector for the adult human population might be compromised by its antigenic relatedness to HPIV1. Indeed, IN immunization of healthy human adult volunteers with SeV resulted in an increase in virus-specific antibodies in only three out of nine individuals, suggesting that replication was restricted due to previous exposure to HPIV1 (112). Thus, further studies are needed to evaluate the level of attenuation and immunogenicity of SeV-based vaccines in HPIV1 seronegative and seropositive humans.
SUMMARY AND FUTURE DIRECTIONS
NNSV-based vectors can be useful for the development of vaccines for several particular situations. One use is mass immunization of the pediatric population against major respiratory viral pathogens. Particularly promising examples are attenuated versions of HPIV1, -2, and -3 expressing protective antigens of other pediatric respiratory viruses, such as HRSV and HMPV, from added genes. This converts a single virus into a bi- or multivalent vaccine. At present, recombinant B/HPIV3 expressing the HRSV F protein is being evaluated in clinical trials. The effectiveness of this strategy will depend on whether the PIV-vectored vaccine is as immunogenic and protective in infants and young children as a complete attenuated version of HRSV. A second use is immunization and outbreak control against emerging respiratory and systemic viral infections, such as those caused by SARS, H5N1 avian influenza, Ebola, Marburg, Machupo, Lassa, Sin Nombre, Nipah, and Hendra viruses. These vaccines will be based on nonhuman NNSV, such as NDV and VSV, that are antigenically unrelated to common human pathogens so that essentially the entire population will be susceptible to immunization. The effectiveness of this strategy will depend on the level of safety and immunogenicity of these vectored vaccines in humans. A third use is against prevalent viral infections, such as HIV or hepatitis C, using vectors based on nonhuman NNSV, such as VSV. These vaccines would be used in preventive immunization and possibly also to boost immune responses in infected individuals. NNSV-based vectors also are being developed against veterinary pathogens, as in the use of attenuated vaccine strains of BPIV3 and NDV as vectors against additional diseases of cattle and poultry, respectively.
Acknowledgments
The authors were supported by the NIAID Intramural Research Program.
REFERENCES
- 1.Arimilli, S., M. A. Alexander-Miller, and G. D. Parks. 2006. A simian virus 5 (SV5) P/V mutant is less cytopathic than wild-type SV5 in human dendritic cells and is a more effective activator of dendritic cell maturation and function. J. Virol. 80:3416-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett, E. J., E. Amaro-Carambot, S. R. Surman, J. T. Newman, P. L. Collins, B. R. Murphy, and M. H. Skiadopoulos. 2005. Human parainfluenza virus type I (HPIV1) vaccine candidates designed by reverse genetics are attenuated and efficacious in African green monkeys. Vaccine 23:4631-4646. [DOI] [PubMed] [Google Scholar]
- 3.Bermingham, A., and P. L. Collins. 1999. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. USA 96:11259-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchholz, U. J., S. Biacchesi, Q. N. Pham, K. C. Tran, L. Yang, C. L. Luongo, M. H. Skiadopoulos, B. R. Murphy, and P. L. Collins. 2005. Deletion of M2 gene open reading frames 1 and 2 of human metapneumovirus: effects on RNA synthesis, attenuation, and immunogenicity. J. Virol. 79:6588-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchholz, U. J., A. Bukreyev, L. Yang, E. W. Lamirande, B. R. Murphy, K. Subbarao, and P. L. Collins. 2004. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. USA 101:9804-9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukreyev, A., I. M. Belyakov, J. A. Berzofsky, B. R. Murphy, and P. L. Collins. 2001. Granulocyte-macrophage colony-stimulating factor expressed by recombinant respiratory syncytial virus attenuates viral replication and increases the level of pulmonary antigen-presenting cells. J. Virol. 75:12128-12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukreyev, A., E. Camargo, and P. L. Collins. 1996. Recovery of infectious respiratory syncytial virus expressing an additional, foreign gene. J. Virol. 70:6634-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukreyev, A., Z. Huang, L. Yang, S. Elankumaran, M. St. Claire, B. R. Murphy, S. K. Samal, and P. L. Collins. 2005. Recombinant Newcastle disease virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J. Virol. 79:13275-13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukreyev, A., E. W. Lamirande, U. J. Buchholz, L. N. Vogel, W. R. Elkins, M. St. Claire, B. R. Murphy, K. Subbarao, and P. L. Collins. 2004. Mucosal immunisation of African green monkeys (Cercopithecus aethiops) with an attenuated parainfluenza virus expressing the SARS coronavirus spike protein for the prevention of SARS. Lancet 363:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukreyev, A., M. H. Skiadopoulos, J. McAuliffe, B. R. Murphy, P. L. Collins, and A. C. Schmidt. 2002. More antibody with less antigen: can immunogenicity of attenuated live virus vaccines be improved? Proc. Natl. Acad. Sci. USA 99:16987-16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bukreyev, A., S. S. Whitehead, N. Bukreyeva, B. R. Murphy, and P. L. Collins. 1999. Interferon gamma expressed by a recombinant respiratory syncytial virus attenuates virus replication in mice without compromising immunogenicity. Proc. Natl. Acad. Sci. USA 96:2367-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukreyev, A., L. Yang, S. R. Zaki, W. J. Shieh, P. E. Rollin, B. R. Murphy, P. L. Collins, and A. Sanchez. 2006. A single intranasal inoculation with a paramyxovirus-vectored vaccine protects guinea pigs against a lethal-dose Ebola virus challenge. J. Virol. 80:2267-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonocore, L., K. J. Blight, C. M. Rice, and J. K. Rose. 2002. Characterization of vesicular stomatitis virus recombinants that express and incorporate high levels of hepatitis C virus glycoproteins. J. Virol. 76:6865-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calain, P., and L. Roux. 1993. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 67:4822-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chanock, R. M., B. R. Murphy, and P. L. Collins. 2001. Parainfluenza viruses, p. 1341-1379. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 17.Collins, P. L., M. G. Hill, E. Camargo, H. Grosfeld, R. M. Chanock, and B. R. Murphy. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc. Natl. Acad. Sci. USA 92:11563-11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conzelmann, K. K. 2005. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. J. Virol. 79:5241-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coulon, P., J. P. Ternaux, A. Flamand, and C. Tuffereau. 1998. An avirulent mutant of rabies virus is unable to infect motoneurons in vivo and in vitro. J. Virol. 72:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowe, J. E., Jr., C. Y. Firestone, and B. R. Murphy. 2001. Passively acquired antibodies suppress humoral but not cell-mediated immunity in mice immunized with live attenuated respiratory syncytial virus vaccines. J. Immunol. 167:3910-3918. [DOI] [PubMed] [Google Scholar]
- 21.Domingo, E., and J. J. Holland. 1997. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51:151-178. [DOI] [PubMed] [Google Scholar]
- 22.Durbin, A. P., S. L. Hall, J. W. Siew, S. S. Whitehead, P. L. Collins, and B. R. Murphy. 1997. Recovery of infectious human parainfluenza virus type 3 from cDNA. Virology 235:323-332. [DOI] [PubMed] [Google Scholar]
- 23.Durbin, A. P., J. M. McAuliffe, P. L. Collins, and B. R. Murphy. 1999. Mutations in the C, D, and V open reading frames of human parainfluenza virus type 3 attenuate replication in rodents and primates. Virology 261:319-330. [DOI] [PubMed] [Google Scholar]
- 24.Durbin, A. P., M. H. Skiadopoulos, J. M. McAuliffe, J. M. Riggs, S. R. Surman, P. L. Collins, and B. R. Murphy. 2000. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. J. Virol. 74:6821-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egan, M. A., S. Y. Chong, N. F. Rose, S. Megati, K. J. Lopez, E. B. Schadeck, J. E. Johnson, A. Masood, P. Piacente, R. E. Druilhet, P. W. Barras, D. L. Hasselschwert, P. Reilly, E. M. Mishkin, D. C. Montefiori, M. G. Lewis, D. K. Clarke, R. M. Hendry, P. A. Marx, J. H. Eldridge, S. A. Udem, Z. R. Israel, and J. K. Rose. 2004. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res. Hum. Retrovir. 20:989-1004. [DOI] [PubMed] [Google Scholar]
- 26.Enterlein, S., V. Volchkov, M. Weik, L. Kolesnikova, V. Volchkova, H. D. Klenk, and E. Muhlberger. 2006. Rescue of recombinant Marburg virus from cDNA is dependent on nucleocapsid protein VP30. J. Virol. 80:1038-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faber, M., E. W. Lamirande, A. Roberts, A. B. Rice, H. Koprowski, B. Dietzschold, and M. J. Schnell. 2005. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J. Gen. Virol. 86:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fellowes, O. N., G. T. Dimopoullos, and J. J. Callis. 1955. Isolation of vesicular stomatitis virus from an infected laboratory worker. Am. J. Vet. Res. 16:623-626. [PubMed] [Google Scholar]
- 29.Flanagan, E. B., J. M. Zamparo, L. A. Ball, L. L. Rodriguez, and G. W. Wertz. 2001. Rearrangement of the genes of vesicular stomatitis virus eliminates clinical disease in the natural host: new strategy for vaccine development. J. Virol. 75:6107-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forger, J. M., III, R. T. Bronson, A. S. Huang, and C. S. Reiss. 1991. Murine infection by vesicular stomatitis virus: initial characterization of the H-2d system. J. Virol. 65:4950-4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garbutt, M., R. Liebscher, V. Wahl-Jensen, S. Jones, P. Moller, R. Wagner, V. Volchkov, H. D. Klenk, H. Feldmann, and U. Stroher. 2004. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 78:5458-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Sastre, A. 2004. Identification and characterization of viral antagonists of type I interferon in negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:249-280. [DOI] [PubMed] [Google Scholar]
- 33.Garcin, D., T. Pelet, P. Calain, L. Roux, J. Curran, and D. Kolakofsky. 1995. A highly recombinogenic system for the recovery of infectious Sendai paramyxovirus from cDNA: generation of a novel copy-back nondefective interfering virus. EMBO J. 14:6087-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gehrke, R., F. X. Heinz, N. L. Davis, and C. W. Mandl. 2005. Heterologous gene expression by infectious and replicon vectors derived from tick-borne encephalitis virus and direct comparison of this flavivirus system with an alphavirus replicon. J. Gen. Virol. 86:1045-1053. [DOI] [PubMed] [Google Scholar]
- 35.Geisbert, T. W., S. Jones, E. A. Fritz, A. C. Shurtleff, J. B. Geisbert, R. Liebscher, A. Grolla, U. Stroher, L. Fernando, K. M. Daddario, M. C. Guttieri, B. R. Mothe, T. Larsen, L. E. Hensley, P. B. Jahrling, and H. Feldmann. 2005. Development of a new vaccine for the prevention of Lassa fever. PLoS Med. 2:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. High-level primary CD8+ T-cell response to human immunodeficiency virus type 1 Gag and Env generated by vaccination with recombinant vesicular stomatitis viruses. J. Virol. 76:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haller, A. A., T. Miller, M. Mitiku, and K. Coelingh. 2000. Expression of the surface glycoproteins of human parainfluenza virus type 3 by bovine parainfluenza virus type 3, a novel attenuated virus vaccine vector. J. Virol. 74:11626-11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson, R. P. 1978. Newcastle disease, 7th ed. Iowa State University Press, Ames, Iowa.
- 39.Hanson, R. P., A. F. Rasmussen, Jr., C. A. Brandly, and J. W. Brown. 1950. Human infection with the virus of vesicular stomatitis. J. Lab. Clin. Med. 36:754-758. [PubMed] [Google Scholar]
- 40.Homann, H. E., P. H. Hofschneider, and W. J. Neubert. 1990. Sendai virus gene expression in lytically and persistently infected cells. Virology 177:131-140. [DOI] [PubMed] [Google Scholar]
- 41.Huang, Z., S. Elankumaran, A. S. Yunus, and S. K. Samal. 2004. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J. Virol. 78:10054-10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurwitz, J. L., K. F. Soike, M. Y. Sangster, A. Portner, R. E. Sealy, D. H. Dawson, and C. Coleclough. 1997. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 15:533-540. [DOI] [PubMed] [Google Scholar]
- 43.Iverson, L. E., and J. K. Rose. 1981. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell 23:477-484. [DOI] [PubMed] [Google Scholar]
- 44.Johnson, J. E., M. J. Schnell, L. Buonocore, and J. K. Rose. 1997. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J. Virol. 71:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson, K. M., J. E. Vogel, and P. H. Peralta. 1966. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV). Am. J. Trop. Med. Hyg. 15:244-246. [DOI] [PubMed] [Google Scholar]
- 46.Jones, S. M., H. Feldmann, U. Stroher, J. B. Geisbert, L. Fernando, A. Grolla, H. D. Klenk, N. J. Sullivan, V. E. Volchkov, E. A. Fritz, K. M. Daddario, L. E. Hensley, P. B. Jahrling, and T. W. Geisbert. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11:786-790. [DOI] [PubMed] [Google Scholar]
- 47.Kahn, J. S., A. Roberts, C. Weibel, L. Buonocore, and J. K. Rose. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 75:11079-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kahn, J. S., M. J. Schnell, L. Buonocore, and J. K. Rose. 1999. Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion. Virology 254:81-91. [DOI] [PubMed] [Google Scholar]
- 49.Kapadia, S. U., J. K. Rose, E. Lamirande, L. Vogel, K. Subbarao, and A. Roberts. 2005. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology 340:174-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kato, A., Y. Sakai, T. Shioda, T. Kondo, M. Nakanishi, and Y. Nagai. 1996. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells 1:569-579. [DOI] [PubMed] [Google Scholar]
- 51.Kato, M., H. Igarashi, A. Takeda, Y. Sasaki, H. Nakamura, M. Kano, T. Sata, A. Iida, M. Hasegawa, S. Horie, E. Higashihara, Y. Nagai, and T. Matano. 2005. Induction of Gag-specific T-cell responses by therapeutic immunization with a Gag-expressing Sendai virus vector in macaques chronically infected with simian-human immunodeficiency virus. Vaccine 23:3166-3173. [DOI] [PubMed] [Google Scholar]
- 52.Kolakofsky, D., T. Pelet, D. Garcin, S. Hausmann, J. Curran, and L. Roux. 1998. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J. Virol. 72:891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krempl, C., B. R. Murphy, and P. L. Collins. 2002. Recombinant respiratory syncytial virus with the G and F genes shifted to the promoter-proximal positions. J. Virol. 76:11931-11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kretzschmar, E., L. Buonocore, M. J. Schnell, and J. K. Rose. 1997. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J. Virol. 71:5982-5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Letchworth, G. J., L. L. Rodriguez, and J. Del Cbarrera. 1999. Vesicular stomatitis. Vet. J. 157:239-260. [DOI] [PubMed] [Google Scholar]
- 57.Lippmann, O. 1952. Human conjunctivitis due to the Newcastle-disease virus of fowls. Am. J. Ophthalmol. 35:1021-1028. [DOI] [PubMed] [Google Scholar]
- 58.Liu, H., S. Andreansky, G. Diaz, S. J. Turner, D. Wodarz, and P. C. Doherty. 2003. Quantitative analysis of long-term virus-specific CD8+-T-cell memory in mice challenged with unrelated pathogens. J. Virol. 77:7756-7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez-Sobrido, L., N. Gitiban, A. Fernandez-Sesma, J. Cros, S. E. Mertz, N. A. Jewell, S. Hammond, E. Flano, R. K. Durbin, A. Garcia-Sastre, and J. E. Durbin. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J. Virol. 80:1130-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 199:1709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McAuliffe, J. M., S. R. Surman, J. T. Newman, J. M. Riggs, P. L. Collins, B. R. Murphy, and M. H. Skiadopoulos. 2004. Codon substitution mutations at two positions in the L polymerase protein of human parainfluenza virus type 1 yield viruses with a spectrum of attenuation in vivo and increased phenotypic stability in vitro. J. Virol. 78:2029-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGettigan, J. P., H. D. Foley, I. M. Belyakov, J. A. Berzofsky, R. J. Pomerantz, and M. J. Schnell. 2001. Rabies virus-based vectors expressing human immunodeficiency virus type 1 (HIV-1) envelope protein induce a strong, cross-reactive cytotoxic T-lymphocyte response against envelope proteins from different HIV-1 isolates. J. Virol. 75:4430-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGettigan, J. P., M. L. Koser, P. M. McKenna, M. E. Smith, J. M. Marvin, L. C. Eisenlohr, B. Dietzschold, and M. J. Schnell. 2006. Enhanced humoral HIV-1-specific immune responses generated from recombinant rhabdoviral-based vaccine vectors co-expressing HIV-1 proteins and IL-2. Virology 344:363-377. [DOI] [PubMed] [Google Scholar]
- 65.McGettigan, J. P., R. J. Pomerantz, C. A. Siler, P. M. McKenna, H. D. Foley, B. Dietzschold, and M. J. Schnell. 2003. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 Gag have greatly reduced pathogenicity but are highly immunogenic. J. Virol. 77:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGettigan, J. P., S. Sarma, J. M. Orenstein, R. J. Pomerantz, and M. J. Schnell. 2001. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J. Virol. 75:8724-8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mebatsion, T. 2001. Extensive attenuation of rabies virus by simultaneously modifying the dynein light chain binding site in the P protein and replacing Arg333 in the G protein. J. Virol. 75:11496-11502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mebatsion, T., M. J. Schnell, J. H. Cox, S. Finke, and K. K. Conzelmann. 1996. Highly stable expression of a foreign gene from rabies virus vectors. Proc. Natl. Acad. Sci. USA 93:7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakaya, T., J. Cros, M. S. Park, Y. Nakaya, H. Zheng, A. Sagrera, E. Villar, A. Garcia-Sastre, and P. Palese. 2001. Recombinant Newcastle disease virus as a vaccine vector. J. Virol. 75:11868-11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakaya, Y., T. Nakaya, M. S. Park, J. Cros, J. Imanishi, P. Palese, and A. Garcia-Sastre. 2004. Induction of cellular immune responses to simian immunodeficiency virus Gag by two recombinant negative-strand RNA virus vectors. J. Virol. 78:9366-9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson, C. B., B. S. Pomeroy, K. Schrall, W. E. Park, and R. J. Lindeman. 1952. An outbreak of conjunctivitis due to Newcastle disease virus (NDV) occurring in poultry workers. Am. J. Public Health 42:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Newman, J. T., J. M. Riggs, S. R. Surman, J. M. McAuliffe, T. A. Mulaikal, P. L. Collins, B. R. Murphy, and M. H. Skiadopoulos. 2004. Generation of recombinant human parainfluenza virus type 1 vaccine candidates by importation of temperature-sensitive and attenuating mutations from heterologous paramyxoviruses. J. Virol. 78:2017-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newman, J. T., S. R. Surman, J. M. Riggs, C. T. Hansen, P. L. Collins, B. R. Murphy, and M. H. Skiadopoulos. 2002. Sequence analysis of the Washington/1964 strain of human parainfluenza virus type 1 (HPIV1) and recovery and characterization of wild-type recombinant HPIV1 produced by reverse genetics. Virus Genes 24:77-92. [DOI] [PubMed] [Google Scholar]
- 74.Nolan, S. M., S. R. Surman, E. Amaro-Carambot, P. L. Collins, B. R. Murphy, and M. H. Skiadopoulos. 2005. Live-attenuated intranasal parainfluenza virus type 2 vaccine candidates developed by reverse genetics containing L polymerase protein mutations imported from heterologous paramyxoviruses. Vaccine 23:4765-4774. [DOI] [PubMed] [Google Scholar]
- 75.Park, M. S., J. Steel, A. Garcia-Sastre, D. Swayne, and P. Palese. 2006. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc. Natl. Acad. Sci. USA 103:8203-8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peeters, B. P., O. S. de Leeuw, G. Koch, and A. L. Gielkens. 1999. Rescue of Newcastle disease virus from cloned cDNA: evidence that cleavability of the fusion protein is a major determinant for virulence. J. Virol. 73:5001-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pennathur, S., A. A. Haller, M. MacPhail, T. Rizzi, S. Kaderi, F. Fernandes, L. Bicha, J. H. Schickli, R. S. Tang, W. Chen, N. Nguyen, S. Mathie, H. Mehta, and K. L. Coelingh. 2003. Evaluation of attenuation, immunogenicity and efficacy of a bovine parainfluenza virus type 3 (PIV-3) vaccine and a recombinant chimeric bovine/human PIV-3 vaccine vector in rhesus monkeys. J. Gen. Virol. 84:3253-3261. [DOI] [PubMed] [Google Scholar]
- 78.Pham, Q. N., S. Biacchesi, M. H. Skiadopoulos, B. R. Murphy, P. L. Collins, and U. J. Buchholz. 2005. Chimeric recombinant human metapneumoviruses with the nucleoprotein or phosphoprotein open reading frame replaced by that of avian metapneumovirus exhibit improved growth in vitro and attenuation in vivo. J. Virol. 79:15114-15122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Publicover, J., E. Ramsburg, and J. K. Rose. 2004. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J. Virol. 78:9317-9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pulmanausahakul, R., M. Faber, K. Morimoto, S. Spitsin, E. Weihe, C. Hooper, M. J. Schnell, and B. Dietzschold. 2001. Overexpression of cytochrome c by a recombinant rabies virus attenuates pathogenicity and enhances antiviral immunity. J. Virol. 75:10800-10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quiñones-Kochs, M. I., M. J. Schnell, L. Buonocore, and J. K. Rose. 2001. Mechanisms of loss of foreign gene expression in recombinant vesicular stomatitis viruses. Virology 287:427-435. [DOI] [PubMed] [Google Scholar]
- 82.Quiroz, E., N. Moreno, P. H. Peralta, and R. B. Tesh. 1988. A human case of encephalitis associated with vesicular stomatitis virus (Indiana serotype) infection. Am. J. Trop. Med. Hyg. 39:312-314. [DOI] [PubMed] [Google Scholar]
- 83.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramsburg, E., J. Publicover, L. Buonocore, A. Poholek, M. Robek, A. Palin, and J. K. Rose. 2005. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J. Virol. 79:15043-15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reiss, C. S., I. V. Plakhov, and T. Komatsu. 1998. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann. N. Y. Acad. Sci. 855:751-761. [DOI] [PubMed] [Google Scholar]
- 86.Reuter, J. D., B. E. Vivas-Gonzalez, D. Gomez, J. H. Wilson, J. L. Brandsma, H. L. Greenstone, J. K. Rose, and A. Roberts. 2002. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J. Virol. 76:8900-8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ritchie, B., G. Harrison, and L. Harrison. 1993. Avian medicine: principles and application. Wingers Publishing, Lake Worth, Fla.
- 88.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts, A., J. D. Reuter, J. H. Wilson, S. Baldwin, and J. K. Rose. 2004. Complete protection from papillomavirus challenge after a single vaccination with a vesicular stomatitis virus vector expressing high levels of L1 protein. J. Virol. 78:3196-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields Virology, 4th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 92.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 93.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schlereth, B., L. Buonocore, A. Tietz, V. ter Meulen, J. K. Rose, and S. Niewiesk. 2003. Successful mucosal immunization of cotton rats in the presence of measles virus-specific antibodies depends on degree of attenuation of vaccine vector and virus dose. J. Gen. Virol. 84:2145-2151. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt, A. C., J. M. McAuliffe, A. Huang, S. R. Surman, J. E. Bailly, W. R. Elkins, P. L. Collins, B. R. Murphy, and M. H. Skiadopoulos. 2000. Bovine parainfluenza virus type 3 (BPIV3) fusion and hemagglutinin-neuraminidase glycoproteins make an important contribution to the restricted replication of BPIV3 in primates. J. Virol. 74:8922-8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmidt, A. C., J. M. McAuliffe, B. R. Murphy, and P. L. Collins. 2001. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J. Virol. 2001:4594-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidt, A. C., D. R. Wenzke, J. M. McAuliffe, M. St. Claire, W. R. Elkins, B. R. Murphy, and P. L. Collins. 2002. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J. Virol. 76:1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schnell, M. J., L. Buonocore, E. Boritz, H. P. Ghosh, R. Chernish, and J. K. Rose. 1998. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 17:1289-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schnell, M. J., L. Buonocore, E. Kretzschmar, E. Johnson, and J. K. Rose. 1996. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 93:11359-11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schnell, M. J., L. Buonocore, M. A. Whitt, and J. K. Rose. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schnell, M. J., H. D. Foley, C. A. Siler, J. P. McGettigan, B. Dietzschold, and R. J. Pomerantz. 2000. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc. Natl. Acad. Sci. USA 97:3544-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schnell, M. J., T. Mebatsion, and K. K. Conzelmann. 1994. Infectious rabies viruses from cloned cDNA. EMBO J. 13:4195-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, E. Amaro-Carambot, S. R. Surman, P. L. Collins, and B. R. Murphy. 2006. Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity. Virology 345:492-501. [DOI] [PubMed] [Google Scholar]
- 104.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, J. M. Riggs, S. R. Surman, E. Amaro-Carambot, J. M. McAuliffe, W. R. Elkins, M. St. Claire, P. L. Collins, and B. R. Murphy. 2004. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J. Virol. 78:6927-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skiadopoulos, M. H., A. C. Schmidt, J. M. Riggs, S. R. Surman, W. R. Elkins, M. St. Claire, P. L. Collins, and B. R. Murphy. 2003. Determinants of the host range restriction of replication of bovine parainfluenza virus type 3 in rhesus monkeys are polygenic. J. Virol. 77:1141-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skiadopoulos, M. H., S. Surman, J. M. Tatem, M. Paschalis, S. L. Wu, S. A. Udem, A. P. Durbin, P. L. Collins, and B. R. Murphy. 1999. Identification of mutations contributing to the temperature-sensitive, cold-adapted, and attenuation phenotypes of the live-attenuated cold-passage 45 (cp45) human parainfluenza virus 3 candidate vaccine. J. Virol. 73:1374-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Skiadopoulos, M. H., S. R. Surman, A. P. Durbin, P. L. Collins, and B. R. Murphy. 2000. Long nucleotide insertions between the HN and L protein coding regions of human parainfluenza virus type 3 yield viruses with temperature-sensitive and attenuation phenotypes. Virology 272:225-234. [DOI] [PubMed] [Google Scholar]
- 108.Skiadopoulos, M. H., S. R. Surman, J. M. Riggs, P. L. Collins, and B. R. Murphy. 2001. A chimeric human-bovine parainfluenza virus type 3 expressing measles virus hemagglutinin is attenuated for replication but is still immunogenic in rhesus monkeys. J. Virol. 75:10498-10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Skiadopoulos, M. H., S. R. Surman, J. M. Riggs, W. R. Elkins, M. St. Claire, M. Nishio, D. Garcin, D. Kolakofsky, P. L. Collins, and B. R. Murphy. 2002. Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology 297:153-160. [DOI] [PubMed] [Google Scholar]
- 110.Skiadopoulos, M. H., S. R. Surman, J. M. Riggs, C. Orvell, P. L. Collins, and B. R. Murphy. 2002. Evaluation of the replication and immunogenicity of recombinant human parainfluenza virus type 3 vectors expressing up to three foreign glycoproteins. Virology 297:136-152. [DOI] [PubMed] [Google Scholar]
- 111.Skiadopoulos, M. H., T. Tao, S. R. Surman, P. L. Collins, and B. R. Murphy. 1999. Generation of a parainfluenza virus type 1 vaccine candidate by replacing the HN and F glycoproteins of the live-attenuated PIV3 cp45 vaccine virus with their PIV1 counterparts. Vaccine 18:503-510. [DOI] [PubMed] [Google Scholar]
- 112.Slobod, K. S., J. L. Shenep, J. Lujan-Zilbermann, K. Allison, B. Brown, R. A. Scroggs, A. Portner, C. Coleclough, and J. L. Hurwitz. 2004. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 22:3182-3186. [DOI] [PubMed] [Google Scholar]
- 113.Smith, G. L., M. Mackett, and B. Moss. 1983. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature 302:490-495. [DOI] [PubMed] [Google Scholar]
- 114.Spann, K. M., P. L. Collins, and M. N. Teng. 2003. Genetic recombination during coinfection of two mutants of human respiratory syncytial virus. J. Virol. 77:11201-11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steinhauer, D. A., E. Domingo, and J. J. Holland. 1992. Lack of evidence for proofreading mechanisms associated with an RNA virus polymerase. Gene 122:281-288. [DOI] [PubMed] [Google Scholar]
- 116.Subbarao, K., J. McAuliffe, L. Vogel, G. Fahle, S. Fischer, K. Tatti, M. Packard, W.-J. Shieh, S. Zaki, and B. Murphy. 2004. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J. Virol. 78:3572-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takimoto, T., J. L. Hurwitz, C. Coleclough, C. Prouser, S. Krishnamurthy, X. Zhan, K. Boyd, R. A. Scroggs, B. Brown, Y. Nagai, A. Portner, and K. S. Slobod. 2004. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J. Virol. 78:6043-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tan, G. S., P. M. McKenna, M. L. Koser, R. McLinden, J. H. Kim, J. P. McGettigan, and M. J. Schnell. 2005. Strong cellular and humoral anti-HIV Env immune responses induced by a heterologous rhabdoviral prime-boost approach. Virology 331:82-93. [DOI] [PubMed] [Google Scholar]
- 119.Tang, R. S., K. Mahmood, M. Macphail, J. M. Guzzetta, A. A. Haller, H. Liu, J. Kaur, H. A. Lawlor, E. A. Stillman, J. H. Schickli, R. A. Fouchier, A. D. Osterhaus, and R. R. Spaete. 2005. A host-range restricted parainfluenza virus type 3 (PIV3) expressing the human metapneumovirus (hMPV) fusion protein elicits protective immunity in African green monkeys. Vaccine 23:1657-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang, R. S., J. H. Schickli, M. MacPhail, F. Fernandes, L. Bicha, J. Spaete, R. A. Fouchier, A. D. Osterhaus, R. Spaete, and A. A. Haller. 2003. Effects of human metapneumovirus and respiratory syncytial virus antigen insertion in two 3′ proximal genome positions of bovine/human parainfluenza virus type 3 on virus replication and immunogenicity. J. Virol. 77:10819-10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tao, T., A. P. Durbin, S. S. Whitehead, F. Davoodi, P. L. Collins, and B. R. Murphy. 1998. Recovery of a fully viable chimeric human parainfluenza virus (PIV) type 3 in which the hemagglutinin-neuraminidase and fusion glycoproteins have been replaced by those of PIV type 1. J. Virol. 72:2955-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tao, T., M. H. Skiadopoulos, F. Davoodi, J. M. Riggs, P. L. Collins, and B. R. Murphy. 2000. Replacement of the ectodomains of the hemagglutinin-neuraminidase and fusion glycoproteins of recombinant parainfluenza virus type 3 (PIV3) with their counterparts from PIV2 yields attenuated PIV2 vaccine candidates. J. Virol. 74:6448-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tao, T., M. H. Skiadopoulos, A. P. Durbin, F. Davoodi, P. L. Collins, and B. R. Murphy. 1999. A live attenuated chimeric recombinant parainfluenza virus (PIV) encoding the internal proteins of PIV type 3 and the surface glycoproteins of PIV type 1 induces complete resistance to PIV1 challenge and partial resistance to PIV3 challenge. Vaccine 17:1100-1108. [DOI] [PubMed] [Google Scholar]
- 124.Techaarpornkul, S., P. L. Collins, and M. E. Peeples. 2002. Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus. Virology 294:296-304. [DOI] [PubMed] [Google Scholar]
- 125.Teng, M. N., and P. L. Collins. 1999. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J. Virol. 73:466-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Teng, M. N., S. S. Whitehead, A. Bermingham, M. St Claire, W. R. Elkins, B. R. Murphy, and P. L. Collins. 2000. Recombinant respiratory syncytial virus that does not express the NS1 or M2-2 protein is highly attenuated and immunogenic in chimpanzees. J. Virol. 74:9317-9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tokusumi, T., A. Iida, T. Hirata, A. Kato, Y. Nagai, and M. Hasegawa. 2002. Recombinant Sendai viruses expressing different levels of a foreign reporter gene. Virus Res. 86:33-38. [DOI] [PubMed] [Google Scholar]
- 128.Veits, J., D. Wiesner, W. Fuchs, B. Hoffmann, H. Granzow, E. Starick, E. Mundt, H. Schirrmeier, T. Mebatsion, T. C. Mettenleiter, and A. Romer-Oberdorfer. 2006. Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc. Natl. Acad. Sci. USA 103:8197-8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Volchkov, V. E., V. A. Volchkova, E. Muhlberger, L. V. Kolesnikova, M. Weik, O. Dolnik, and H. D. Klenk. 2001. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity. Science 291:1965-1969. [DOI] [PubMed] [Google Scholar]
- 130.Wang, Z., L. Hangartner, T. I. Cornu, L. R. Martin, A. Zuniga, M. A. Billeter, and H. Y. Naim. 2001. Recombinant measles viruses expressing heterologous antigens of mumps and simian immunodeficiency viruses. Vaccine 19:2329-2336. [DOI] [PubMed] [Google Scholar]
- 131.Wertz, G. W., R. Moudy, and L. A. Ball. 2002. Adding genes to the RNA genome of vesicular stomatitis virus: positional effects on stability of expression. J. Virol. 76:7642-7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wertz, G. W., V. P. Perepelitsa, and L. A. Ball. 1998. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc. Natl. Acad. Sci. USA 95:3501-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whelan, S. P., L. A. Ball, J. N. Barr, and G. T. Wertz. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 92:8388-8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Witko, S. E., C. S. Kotash, R. M. Nowak, J. E. Johnson, L. A. C. Boutilier, K. J. Melville, S. G. Heron, D. K. Clarke, A. S. Abramovitz, R. M. Hendry, M. S. Sidhu, S. A. Udem, and C. L. Parks. 2006. An efficient helper-virus-free method for rescue of recombinant paramyxoviruses and rhabdoviruses from a cell line suitable for vaccine development. J. Virol. Methods 135:91-101. [DOI] [PubMed] [Google Scholar]
- 135.Wyatt, L. S., B. Moss, and S. Rozenblatt. 1995. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology 210:202-205. [DOI] [PubMed] [Google Scholar]
- 136.Yan, X., P. S. Mohankumar, B. Dietzschold, M. J. Schnell, and Z. F. Fu. 2002. The rabies virus glycoprotein determines the distribution of different rabies virus strains in the brain. J. Neurovirol. 8:345-352. [DOI] [PubMed] [Google Scholar]
- 137.Zhao, H., and B. P. Peeters. 2003. Recombinant Newcastle disease virus as a viral vector: effect of genomic location of foreign gene on gene expression and virus replication. J. Gen. Virol. 84:781-788. [DOI] [PubMed] [Google Scholar]