Abstract
The human cytomegalovirus (HCMV) TRS1 and IRS1 genes block the phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α) and the consequent shutoff of cellular protein synthesis that occur during infection with vaccinia virus (VV) deleted of the double-stranded RNA binding protein gene E3L (VVΔE3L). To further define the underlying mechanism, we first evaluated the effect of pTRS1 on protein kinase R (PKR), the double-stranded RNA (dsRNA)-dependent eIF2α kinase. Immunoblot analyses revealed that pTRS1 expression in the context of a VVΔE3L recombinant decreased levels of PKR in the cytoplasm and increased its levels in the nucleus of infected cells, an effect not seen with wild-type VV or a VVΔE3L recombinant virus expressing E3L. This effect of pTRS1 was confirmed by visualizing the nuclear relocalization of PKR-EGFP expressed by transient transfection. PKR present in both the nuclear and cytoplasmic fractions was nonphosphorylated, indicating that it was unactivated when TRS1 was present. PKR also accumulated in the nucleus during HCMV infection as determined by indirect immunofluorescence and immunoblot analysis. Binding assays revealed that pTRS1 interacted with PKR in mammalian cells and in vitro. This interaction required the same carboxy-terminal region of pTRS1 that is necessary to rescue VVΔE3L replication in HeLa cells. The carboxy terminus of pIRS1 was also required for rescue of VVΔE3L and for mediating an interaction of pIRS1 with PKR. These results suggest that these HCMV genes directly interact with PKR and inhibit its activation by sequestering it in the nucleus, away from both its activator, cytoplasmic dsRNA, and its substrate, eIF2α.
Double-stranded RNA (dsRNA) activates the host cell response to viral infection in numerous ways, one of which involves the interferon-induced, dsRNA-dependent protein kinase R (PKR) (reviewed in reference 43). After binding to dsRNA, PKR dimerizes, autophosphorylates, and then phosphorylates the eukaryotic initiation factor 2 (eIF2) on its α subunit. Phosphorylated eIF2α sequesters the guanine nucleotide exchange factor eIF2B, resulting in the inhibition of protein synthesis at the level of translation initiation. Since viruses depend on the cellular translational machinery, the shutoff of host cell protein synthesis inhibits viral replication and spread.
Many viruses have mechanisms for inhibiting the PKR-mediated phosphorylation of eIF2α (56). The vaccinia virus (VV) E3L protein prevents the activation of PKR by binding to and sequestering dsRNA via a carboxy-terminal double-stranded RNA binding domain (dsRBD) (39). VV from which the E3L gene has been deleted (VVΔE3L) exhibits a dsRNA-dependent restriction of host cell range, and this phenotype can be reversed by expression of the dsRBD from pE3L or dsRNA-binding proteins from other viruses (4, 47, 62). We previously found that the products of the human cytomegalovirus (HCMV) genes TRS1 and IRS1 restore the host cell range of VVΔE3L, inhibit the phosphorylation of eIF2α, and prevent the shutoff of cellular protein synthesis that occur upon infection of HeLa and human fibroblast (HF) cells with VVΔE3L (17). We went on to determine that pTRS1 binds dsRNA via an unconventional amino-terminal dsRBD but that, unlike the case with pE3L, the dsRNA-binding activity of pTRS1 was not sufficient for restoring VVΔE3L host cell range, as the carboxy terminus of pTRS1 was also required (36).
In order to gain a more complete understanding of how these HCMV dsRNA-binding proteins inhibit eIF2α phosphorylation during viral infection, we focused our investigations on the effects of pTRS1 on the PKR pathway. We found that pTRS1 expression resulted in the nuclear accumulation of PKR during viral infection and that the PKR observed in the nuclear and cytoplasmic fractions was nonphosphorylated, indicative of unactivated PKR. Additionally, we determined that both pTRS1 and pIRS1 interact with PKR and that this interaction requires the carboxy termini of both proteins and appears necessary for these proteins to inhibit PKR activity and rescue VVΔE3L replication.
MATERIALS AND METHODS
Cells, viruses, and infections.
HeLa and HF cells were maintained at 37°C in a 5% CO2 atmosphere in Dulbecco's modified Eagle's medium supplemented with 10% NuSerum (Collaborative Biomedical), penicillin-streptomycin (100 U/ml), and 2 mM l-glutamine. VVΔE3L, the E3L deletion mutant, and its wild-type parent VV Copenhagen strain (wtVV; VC-2, vP1080) were obtained from Bertram Jacobs (Arizona State University). VVeq855 (a VVΔE3L recombinant virus expressing pE3L) and VVeq904 (a VVΔE3L recombinant virus expressing pTRS1) have been previously described (17). HCMV infections used rTowne-1, a strain derived by cotransfection of HCMV (Towne)-derived cosmids (2). All infections used a multiplicity of infection (MOI) of 3 PFU/cell.
Plasmids.
Plasmids pEQ981 (full-length pTRS1, amino acids 1 to 795), pEQ979 (TRS1 amino acids 1 to 738), pEQ1001 (TRS1 amino acids 1 to 703), pEQ978 (TRS1 amino acids 1 to 648), and pEQ879 (empty vector) have been previously described (36). pEQ1100, which expresses enhanced green fluorescent protein (EGFP) with a carboxy-terminal six-His tag, was made by ligating the HindIII-BsrGI fragment from pEGFP-1 (Clontech) into the HindIII-EcoRV sites of pcDNA3.1/V5-His TOPO TA (Invitrogen) containing additional 3′ sequences encoding the biotinylation target amino acid sequence, MAGGLNDIFEAQKIEWHE (5).
A series of plasmids expressing full-length and truncated pIRS1 with a carboxy-terminal six-His tag were derived from pEQ890, a plasmid containing the full-length pIRS1 gene cloned into pcDNA3.1/V5-His TOPO TA as previously described (17). pEQ1007 (full-length pIRS1, amino acids 1 to 847) was constructed by PCR amplification of pEQ890 using oligonucleotides 500 (CACGCTCGAGCGATGATGAACGTGGTGAGGGGCGTGT) and 501 (GCGCCGCGCTGTGGGCGCGCGA). The PCR product was digested with BlpI and XhoI and ligated into pEQ890 digested with the same enzymes. pEQ1002 (amino acids 1 to 656) was made by digesting pEQ890 with BlpI and AgeI, blunting with DNA polymerase (Klenow fragment), and religating. pEQ1010 (amino acids 1 to 668) was constructed by PCR amplification of pEQ890 using oligonucleotides 359 (CAGGCGCTGACGGAACTGGA) and 504 (CACGCTCGAGCGGTCGAGATCCAACCAGGCTTTGTGGTCG). The resulting PCR product was digested with BstEII and XhoI and inserted into the same sites of pEQ890. The same steps were used to construct pEQ1033 (amino acids 1 to 692), except that PCR amplification used oligonucleotides 359 and 512 (CACGCTCGAGCGTTGAGTGGCCTTCAGCAGTCTGCG).
PKR-EGFP was obtained from Michael Mathews (UMDNJ-NJMS) (66). Glutathione S-transferase (GST)-PKRK296R was obtained from Antonis E. Koromilas (McGill University) (23).
Preparation and analysis of cytoplasmic and nuclear extracts.
At various times after infection, cells were washed twice in ice-cold phosphate-buffered saline (PBS), scraped off the tissue culture plates, and centrifuged at 500 × g for 10 min. The cell pellets were lysed in a volume of buffer A (100 mM NaCl, 50 mM Na2HPO4 [pH 8.0], 10% glycerol [vol/vol], 1 mM dithiothreitol [Sigma], 1% NP-40 [Calbiochem], 0.1 mM phenylmethylsulfonyl fluoride [PMSF; Sigma], 1 mM benzamidine [Sigma], 1 mM sodium orthovanadate [Sigma], 1 mM l-homoarginine [Sigma]) equal to that of the cell pellet, incubated on ice for 15 min, and centrifuged at 4,000 × g for 10 min at 4°C. The supernatants were transferred to new tubes, and the remaining pellets were lysed in a volume of 2% sodium dodecyl sulfate (SDS) equivalent to the remaining pellet and passed through a 27-gauge needle several times.
To assess the phosphorylation state of PKR present in the nuclear and cytoplasmic fractions, HeLa cells were mock infected or infected with VVΔE3L or VVeq904 for 24 h and then washed, lysed, and fractionated as described above, with the exception that 30 mM sodium fluoride (Sigma) was added to lysis buffer A.
Protein concentrations were determined by fluoraldehyde o-phthalaldehyde (Pierce) assay (35). Equivalent amounts of protein from each fraction were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to polyvinylidene difluoride membranes (PVDF; GE Lifesciences) by electroblotting. Total PKR was detected using the PKR K-17 rabbit polyclonal antibody (Santa Cruz) or PKR B-10 mouse monoclonal antibody (Santa Cruz) and Western Star chemiluminescent detection system (Tropix, Inc.) according to the manufacturers' recommendations. Antiactin rabbit polyclonal antibody (catalog no. A2066; Sigma) was used according to the manufacturer's instructions. As controls for fractionation, nuclear proteins were analyzed by immunoblotting using antibodies against lamin B (catalog no. 101-B7; Calbiochem) or activating transcription factor 4 (ATF4; Santa Cruz), and cytoplasmic proteins were analyzed using antibodies against superoxide dismutase 4 (catalog no. ab16834; Abcam) and lactate dehydrogenase (LDH; Cortex Biochemicals). Phosphorylated PKR was assessed using p-PKR(Thr446) rabbit polyclonal antibody (Santa Cruz).
Visualization of PKR subcellular localization.
To visualize the subcellular localization of PKR-EGFP, subconfluent HeLa cells on coverslips were transfected with PKR-EGFP using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Twenty-four hours after transfection, cells were mock infected or infected with VVeq855 or VVeq904. Twenty-four hours postinfection (hpi), the cells were washed once with PBS, fixed for 30 min in 4% paraformaldehyde in PBS at room temperature, and permeabilized in 0.2% Triton X-100 (in PBS) for 10 min at room temperature. Staining with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma; 0.5 μg/ml in PBS) was performed for 5 min at room temperature. The cells were then washed three times in PBS and mounted onto glass slides using ProLong Gold antifade reagent (Molecular Probes/Invitrogen).
Indirect immunofluorescence was performed on HFs grown to 90% confluence on coverslips which were mock infected or infected with HCMV. Twenty-four and 72 hpi, cells were fixed for 30 min at room temperature in 4% paraformaldehyde in PBS and then permeabilized for 10 min in 0.1% Triton X-100 (in PBS) at room temperature. After overnight blocking at 4°C in 5% bovine serum albumin (BSA) in PBS, the cells were incubated with PKR B-10 antibody (1:50 dilution in PBS plus 1% BSA) for 1 hour at room temperature, washed three times in PBS, and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G secondary antibody (Santa Cruz; 1:100 dilution in PBS plus 1% BSA) for 1 hour at room temperature. After staining with DAPI (0.5 μg/ml) for 10 min, the cells were washed three times in PBS and mounted onto glass slides using ProLong Gold antifade reagent.
All images were obtained at ×100 magnification using a Nikon Eclipse TE300 microscope with CoolSNAP CF Photometrics and MetaVue Image acquisition system (Universal Imaging Corporation), and additional processing was performed using Adobe Photoshop.
Immunoprecipitation.
HeLa cells were transfected with plasmid DNA using Lipofectamine Plus (Invitrogen) according to the manufacturer's instructions. Forty-eight hours after transfection, cells were infected with VVΔE3L or mock infected. At 24 hpi, cells were washed twice with ice-cold PBS and then lysed in RIPA buffer (150 mM NaCl, 50 mM Tris [pH 8.0], 1% NP-40, 0.1% SDS, 0.5% deoxycholate, 0.1 mM PMSF, 1 mM benzamidine, 1 mM sodium orthovanadate, 1 mM l-homoarginine), followed by centrifugation at 4,000 × g for 10 min at 4°C to pellet the nuclei. The supernatants were incubated with protein A-agarose (Sigma) on a rotator for 1 hour at 4°C and centrifuged at 13,000 × g for 2 min at 4°C, and a small aliquot of the supernatant was set aside (“input”). The remaining portion of each supernatant was incubated with penta-His mouse monoclonal antibody (1 μg/ml; QIAGEN) overnight on a rotator followed by incubation with protein A-agarose for 3 h, all at 4°C. Samples were washed four times in RIPA buffer, and after boiling in loading buffer for 5 min, proteins were separated by SDS-PAGE and transferred to PVDF membranes by electroblotting. Detection of His-tagged proteins and PKR was carried out using penta-His and PKR K-17 antibodies and the Western Star chemiluminescent detection system (Tropix, Inc.) according to the manufacturers' instructions.
In vitro translation.
Synthetic pTRS1 was produced by programming rabbit reticulocyte lysates (TnT Quick-Coupled Transcription/Translation System [Promega]) with pEQ981 in the presence of l-[35S]methionine (Translabel; MP Biomedicals, Inc.) according to the manufacturer's instructions.
GST pull-down assay.
Transformants of Escherichia coli BL21(DE3) expressing GST (pGEX2T; Pharmacia) and GST-PKRK296R (23) were grown overnight at 37°C. The next day, they were diluted (1:50), grown for 1.5 h at 37°C, and then induced with isopropyl-1-thio-β-d-galactopyranoside (0.1 mM) for 4 h. The cell pellets from 1.5 ml of each induced culture were collected by centrifugation at 14,000 × g for 1 minute at room temperature, resuspended in 0.75 ml of lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 0.1% Triton X-100, 1 mM PMSF, 1 mM l-homoarginine, 1 mM benzamidine, 1 mM sodium orthovanadate), and sonicated for 1 min. The supernatants were incubated overnight at 4°C with 200 μl (50% slurry) glutathione-agarose beads (Sigma), washed three times with 1 ml lysis buffer, and resuspended in a volume of lysis buffer equal to that of the agarose beads. Thirty-microliter aliquots were then incubated overnight at 4°C with 3.5 μl of in vitro-translated, radiolabeled pTRS1 in 41.5 μl lysis buffer (total volume of 75 μl). The samples were washed three times in 1 ml lysis buffer, boiled in 2× SDS-PAGE loading buffer, and analyzed by SDS-PAGE, fluorographic enhancement (EN3HANCE; Perkin-Elmer), and autoradiography.
VVΔE3L rescue by IRS1.
Twenty-four hours posttransfection of plasmid DNA using Lipofectamine Plus, 293T cells were infected with VVΔE3L. Twenty four hours postinfection, viral replication was measured by a β-galactosidase (β-Gal) assay as previously described (36). Cells were lysed in 2% SDS, and proteins were detected by immunoblotting using a penta-His antibody (QIAGEN) and the Western Star chemiluminescent detection system (Tropix, Inc.).
RESULTS
pTRS1 alters the subcellular localization of PKR during viral infection.
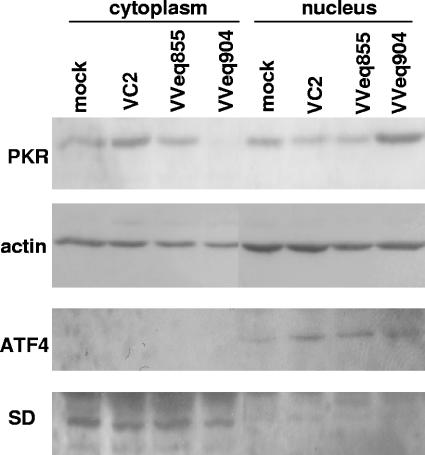
pTRS1 and pIRS1 inhibit the phosphorylation of eIF2α and the shutoff of cellular protein synthesis that occurs upon infection of HeLa and HFs with VVΔE3L (17). To further define the mechanism of action, we focused our investigations on the effect of pTRS1 on the PKR pathway. Surprisingly, we found that infection of HeLa cells with VVeq904 (a VVΔE3L recombinant virus expressing pTRS1) resulted in reduced levels of PKR in cytoplasmic lysates compared to mock infection or infection with wtVV and VVeq855 (a VVΔE3L recombinant expressing pE3L) (Fig. 1). Analysis of the nuclear lysates demonstrated an increase in PKR levels only with VVeq904 infection. Immunoblotting for actin demonstrated comparable protein loading, while the degree of cross-contamination between the cytoplasmic and nuclear samples was minimal as determined by analysis of the distribution of the cytoplasmic marker superoxide dismutase 4 (70) and the nuclear protein ATF4 (58).
FIG. 1.
pTRS1 expression during VV infection reduces cytoplasmic PKR and increases nuclear PKR. HeLa cells were mock infected or infected with wild-type VV (VC2), a VVΔE3L recombinant expressing pE3L (VVeq855), or a VVΔE3L recombinant expressing pTRS1 (VVeq904) at an MOI of 3. Twenty-four hours postinfection, cells were fractionated into cytoplasmic and nuclear components as described in Materials and Methods. PKR present in each fraction was assessed by immunoblot analysis using PKR K-17 rabbit polyclonal antibody. Analysis of actin by immunoblotting served as a loading control, while superoxide dismutase 4 (SD) and ATF4 represent cytoplasmic and nuclear controls, respectively.
We transiently expressed a PKR-EGFP fusion protein that has been shown to exhibit a cellular distribution similar to that of endogenous PKR (66) to directly visualize the effect of pTRS1 on PKR localization. Twenty-four hours after transfecting PKR-EGFP into HeLa cells, cells were mock infected or infected with VVeq855 or VVeq904. Fluorescence microscopy at 24 hpi demonstrated considerable nuclear relocalization of PKR-EGFP in the cells infected with VVeq904, while infection with VVeq855 did not alter the predominantly cytoplasmic localization detected in mock-infected cells (Fig. 2).
FIG. 2.
Fluorescence microscopy demonstrates the nuclear accumulation of PKR during infection with a VVΔE3L recombinant expressing pTRS1. HeLa cells were transfected with PKR-EGFP and 24 h later were mock infected or infected with VVeq855 or VVeq904. Twenty-four hours postinfection, the localization of PKR-EGFP was assessed by fluorescence microscopy.
These results indicated that pTRS1 causes a redistribution of PKR from the cytoplasm to the nucleus. This effect is dependent on pTRS1, as no redistribution of PKR was observed during infection with wild-type VV or with VVeq855, both of which are replication competent in HeLa cells (17).
TRS1 prevents PKR activation.
pTRS1-mediated relocalization of PKR to the nucleus during VV infection may prevent the initial activation of PKR by dsRNA in the cytoplasm or, alternatively, it may prevent the interaction of activated PKR with cytoplasmic eIF2α. Activation of PKR by dsRNA results in dimerization and autophosphorylation. While there are several phosphorylation sites present in PKR, the functional significance of many of these is unclear (43). However, phosphorylation at residue Thr446 appears to be a key mediator of PKR function. Autophosphorylation at this site has been demonstrated in yeast, in mammalian cells treated with dsRNA, and in a dimerizing PKR crystallization construct (24, 28, 59, 73). Mutation of Thr446 impairs PKR autophosphorylation and eIF2α kinase activity, demonstrating the functional relevance of this residue (28, 59).
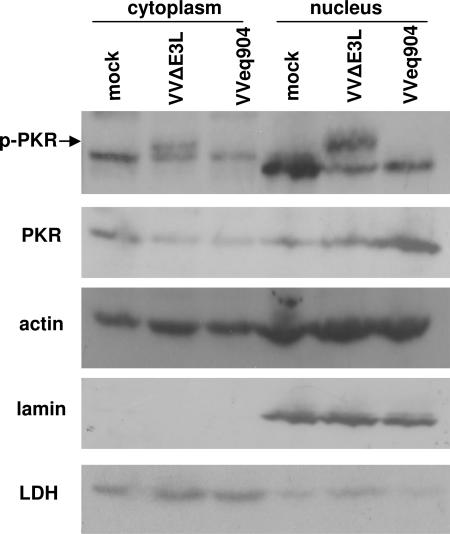
We assessed the activation state of PKR in the cytoplasmic and nuclear fractions of VVeq904-infected cells using antibodies directed against PKR phosphorylated at residue Thr446 (p-PKR). Under our assay conditions, these antibodies also detected nonspecific proteins, but the p-PKR-specific band was identifiable by its appearance in cells infected with VVΔE3L compared to mock-infected cells in both cytoplasmic and nuclear fractions (Fig. 3) (16). The amount of total PKR in the cytoplasmic extracts of VVΔE3L-infected cells was reduced compared to mock-infected cells, possibly because of the strong shutoff of overall protein synthesis observed during VVΔE3L infection of HeLa cells (45). Regardless, in clear contrast to VVΔE3L-infected cells extracts, VVeq904 extracts contained no detectable p-PKR in the cytoplasmic or nuclear fractions. Consistent with the results shown in Fig. 1 and 2, total PKR relocalized to the nucleus following VVeq904 infection. In this experiment, immunoblot analyses of the cytoplasmic protein lactate dehydrogenase (10) and the nuclear protein lamin (50) demonstrated minimal cross-contamination of these fractions. These results suggest that pTRS1 inhibits dsRNA-mediated activation of PKR in the cytoplasm and results in its translocation of PKR, where it accumulates but remains unactivated.
FIG. 3.
PKR is not activated during VVeq904 infection. HeLa cells were mock infected or infected with VVΔE3L or VVeq904, and 24 hpi, cell lysates were separated into cytoplasmic and nuclear fractions. Immunoblot analyses were performed using antibodies specific for PKR phosphorylated at Thr446 (p-PKR; indicated by the arrow above closely comigrating background bands in each fraction), total PKR (PKR B-10 mouse monoclonal antibody), and actin. Immunoblot analyses of the nuclear protein lamin B and the cytoplasmic protein lactate dehydrogenase (LDH) served as controls for the fractionation procedure.
PKR accumulates in the nucleus during HCMV infection.
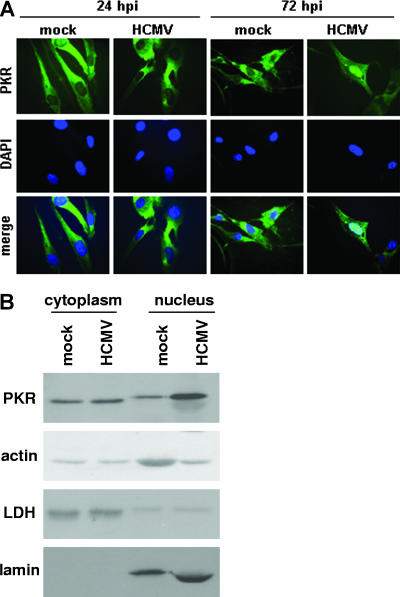
As the experiments described above examined the effects of TRS1 in the context of VV infection, we next investigated the effect of HCMV infection on PKR subcellular localization. HFs were mock infected or infected with HCMV, and indirect immunofluorescence was performed to visualize the localization of PKR. The distribution of PKR at 24 hpi did not appreciably differ from mock-infected cells, but nuclear accumulation of PKR was detected by 72 hpi (Fig. 4A).
FIG. 4.
PKR accumulates in the nucleus during HCMV infection. (A) HFs were mock infected or infected with HCMV (MOI = 3). At the indicated times postinfection, indirect immunofluorescence for endogenous PKR was performed using a PKR mouse monoclonal antibody, and PKR was visualized using fluorescence microscopy. (B) HFs were mock infected or infected with HCMV. At 72 hpi, cell lysates were fractionated into cytoplasmic and nuclear components, and immunoblot analyses of PKR (PKR B-10 mouse monoclonal antibody), actin, lactate dehydrogenase (LDH), and lamin B were performed.
We also assessed the distribution of PKR during HCMV infection by cell fractionation and immunoblot analysis. Consistent with the nuclear accumulation of PKR at 72 hpi observed by microscopy, immunoblot analysis of HF cytoplasmic and nuclear fractions at 72 hpi demonstrated an increase in PKR in the nucleus during HCMV infection compared to mock infection (Fig. 4B). Actin served as a loading control, while LDH and lamin served as cytoplasmic and nuclear markers, respectively. We did not observe a decrease in cytoplasmic PKR levels during HCMV infection as was seen during VVeq904 infection and, thus, the total amount of PKR as assessed by immunoblot analysis was greater during HCMV infection than in the mock-infected cells. This effect may be due to several factors. HCMV infection has been reported to increase PKR mRNA levels at early times postinfection (13). Additionally, HCMV infection does not shut off the synthesis of cellular proteins (38), while VV infection does (39).
Taken together, these experiments indicate that HCMV infection results in the accumulation of PKR in the nucleus. Although we have been unable to make a viable HCMV mutant deleted of both TRS1 and its homologue IRS1 and therefore cannot evaluate whether the effect seen during HCMV infection is due to these genes, the fact that a similar finding was observed with VV expressing pTRS1 (Fig. 1 and 2) makes it likely that pTRS1 (and pIRS1; see below) are responsible for, or at least contribute to, PKR relocalization during HCMV infection.
pTRS1 interacts with PKR.
pTRS1 may act directly on PKR to influence its cellular localization or may act indirectly by regulating other cellular proteins. To begin to address this question, we investigated whether pTRS1 interacts with PKR in uninfected cells and during VVΔE3L infection. HeLa cells were transfected with an empty vector or with plasmids expressing TRS1 or EGFP, both with six-His tags at their carboxy termini, and then mock infected or infected with VVΔE3L 48 h later. Twenty-four hours postinfection, expression of each transgene as well as PKR in cell lysates was determined by immunoblot analysis before (Fig. 5A) and after (Fig. 5B) immunoprecipitation using an anti-His antibody. PKR coimmunoprecipitated with pTRS1 but not with either negative control.
FIG. 5.
pTRS1 interacts with PKR. (A) HeLa cells were transfected with vector alone or with plasmids expressing EGFP or pTRS1, both having carboxy-terminal six-His tags. Forty-eight hours after transfection, cells were mock infected or infected with VVΔE3L at an MOI of 3. At 24 hpi, aliquots of the cell lysates were subjected to immunoblot analyses using anti-His and anti-PKR antibodies. (B) After immunoprecipitation of the lysates (40 times the amount shown in panel A) using an anti-His antibody, the His-tagged proteins and PKR were detected by immunoblot assays. (C) pTRS1 was synthesized in rabbit reticulocyte lysate in the presence of [35S]methionine. Protein binding to empty beads or beads containing GST or GST-PKRK296R was analyzed by SDS-PAGE and autoradiography. Input pTRS1 shown is equal to 10% of that used in the binding assay.
To complement these results and to investigate whether the pTRS1-PKR interaction was direct or mediated by additional cellular proteins, we tested the ability of pTRS1 expressed in a cell-free system to interact with PKR expressed in bacteria as a GST fusion protein. As wild-type PKR is toxic to bacteria (3), we used PKR containing the K296R mutation, which is catalytically inactive yet retains the dimerization and protein-protein interaction characteristics of wt PKR (11, 21, 33, 34, 51, 54, 64). In vitro-translated, radiolabeled pTRS1 was incubated with GST alone or GST-PKRK296R, and protein binding was analyzed by SDS-PAGE and autoradiography. pTRS1 bound to GST-PKRK296R but not to GST alone (Fig. 5C).
These results demonstrate that pTRS1 binds to PKR both in vitro and in mammalian cells, suggesting that the effect of pTRS1 on PKR localization is likely due to a direct protein-protein interaction.
The carboxy terminus of pTRS1 mediates the interaction with PKR.
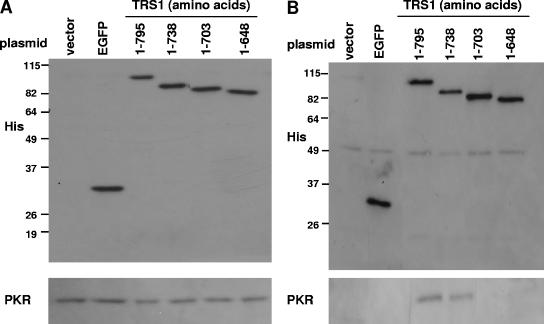
A carboxy-terminal pTRS1 mutant expressing amino acids 1 to 703 is unable to prevent the phosphorylation of eIF2α and the subsequent shutoff of protein synthesis that occur during infection of HeLa cells with VVΔE3L, while a deletion mutant expressing amino acids 1 to 738 behaves similarly to full-length pTRS1 (amino acids 1 to 795) (36). With the finding that pTRS1 interacts with PKR, we investigated whether one role of the carboxy terminus of pTRS1 is to mediate this interaction. HeLa cells were transfected with an empty vector or with plasmids expressing EGFP, full-length pTRS1, or serial carboxy-terminal truncations of pTRS1, all with carboxy-terminal six-His tags. Forty-eight hours posttransfection, the cells were infected with VVΔE3L and, since VVΔE3L contains a lacZ cassette under the control of a late VV promoter, β-Gal was measured 24 hpi as a marker of viral replication (17). Consistent with our previous report (36), full-length pTRS1 and the truncation comprising amino acids 1 to 738 each rescued VVΔE3L replication, while pTRS1 (amino acids 1 to 703) and pTRS1 (amino acids 1 to 648) did not (data not shown). Immunoblot analyses revealed similar levels of expression of the transfected genes (Fig. 6A, top panel) and of PKR (Fig. 6A, bottom panel) in extracts from each sample. After immunoprecipitation using an anti-His antibody, each His-tagged protein was immunoprecipitated to a similar degree (Fig. 6B, top panel [40 times the input shown in A]). PKR coimmunoprecipitated with full-length pTRS1 and the carboxy-terminal truncation of pTRS1 comprising amino acids 1 to 738, but not with the shorter truncations (amino acids 1 to 703 or amino acids 1 to 648) (Fig. 6B, bottom panel). No coimmunoprecipitation of PKR was observed in the mock- or EGFP-transfected lysates. Thus, the interaction of pTRS1 and PKR requires the carboxy terminus of pTRS1, and this interaction correlates with the ability of pTRS1 to inhibit PKR activity during VVΔE3L infection.
FIG. 6.
The carboxy terminus of pTRS1 is required for interaction with PKR. HeLa cells were transfected with vector alone or one of the following six-His-tagged plasmids: EGFP, full-length TRS1 (amino acids 1 to 795), or successive carboxy-terminal truncations of TRS1 comprised of the indicated amino acids. Forty-eight hours later, the cells were infected with VVΔE3L, and lysates were collected 24 hpi. (A) Expression of each six-His-tagged transgene and PKR was assessed by immunoblot assays using an aliquot of each lysate. (B) After immunoprecipitation (of 40 times the amount shown in panel A) with an anti-His antibody, His-tagged proteins and PKR were detected by immunoblot assays.
The carboxy terminus of pIRS1 is necessary for rescue of VVΔE3L host cell range and interaction with PKR.
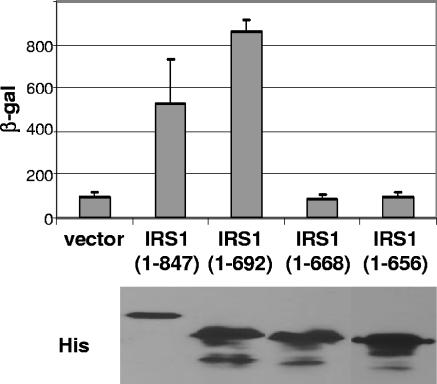
Like pTRS1, IRS1 restores the host cell range of VVΔE3L, inhibits the phosphorylation of eIF2α, and prevents the shutoff of host cell protein synthesis that occur during infection of HeLa cells with VVΔE3L (17). Since the IRS1 and TRS1 genes are 100% homologous over their amino-terminal two-thirds, the dsRBD present near the amino terminus of pTRS1 is identical in pIRS1. The two genes diverge at their carboxy termini yet still retain approximately 50% homology at the amino acid level over this region. Since the divergent region includes sequences of TRS1 required to rescue VVΔE3L host cell range and to bind to PKR, we investigated whether the carboxy terminus of pIRS1 is also required for these functions. 293T cells were transfected with the empty vector or with plasmids expressing full-length pIRS1 (amino acids 1 to 847) or successive carboxy-terminal pIRS1 truncations (amino acids 1 to 692, 1 to 668, or 1 to 656). Twenty-four hours after transfection, the cells were infected with VVΔE3L, and β-Gal was measured 24 hpi. Full-length pIRS1 and the carboxy-terminal truncation comprising amino acids 1 to 692 rescued VVΔE3L replication, but deletions to amino acids 668 and 656 failed to do so (Fig. 7). Expression of each transgene was similar based on immunoblot assay using anti-His antibodies (Fig. 7).
FIG. 7.
The carboxy terminus of pIRS1 is required for rescue of VVΔE3L replication. 293T cells were transfected with vector alone, full-length IRS1 (amino acids 1 to 847), or successive carboxy-terminal truncations of IRS1 comprising amino acids 1 to 692, 1 to 668, and 1 to 656, all of which (except for the vector control) have carboxy-terminal six-His tags. Twenty-four hours after transfection, the cells were infected with VVΔE3L, and 24 hpi β-Gal was measured to assess viral replication. Expression of each transgene was assessed by anti-His immunoblotting (bottom panel).
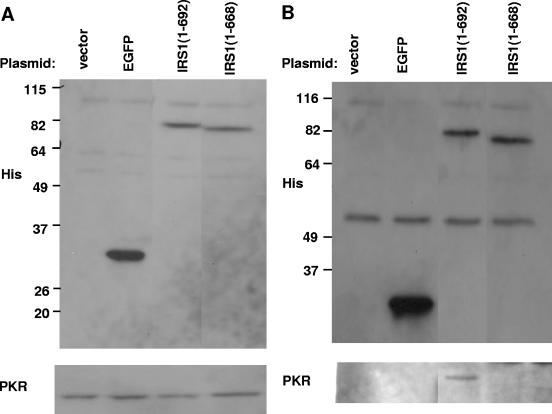
To determine whether the carboxy terminus of pIRS1 mediates an interaction with PKR, HeLa cells were transfected with an empty vector, a plasmid expressing EGFP, or two IRS1 carboxy-terminal truncations, one comprising amino acids 1 to 692 that rescues VVΔE3L replication and one (amino acids 1 to 668) that does not. Infection with VVΔE3L and immunoprecipitation with an anti-His antibody was carried out as was done for pTRS1. Expression levels of each transgene (Fig. 8A, top panel) and PKR (Fig. 8A, bottom panel) were comparable by immunoblot analyses. Each His-tagged construct immunoprecipitated to an equivalent degree (Fig. 8B, top panel). PKR coimmunoprecipitated with the longer pIRS1 protein (amino acids 1 to 692) but not with the shorter one (amino acids 1 to 668) (Fig. 8B, bottom panel). Both negative controls demonstrated the absence of nonspecific coimmunoprecipitation of PKR. Thus, the carboxy terminus of pIRS1, like that of pTRS1, is required for restoration of VVΔE3L host cell range and mediates an interaction with PKR.
FIG. 8.
The carboxy terminus of pIRS1 is required for interaction with PKR. HeLa cells were transfected with the empty vector or with plasmids expressing EGFP or IRS1 carboxy-terminal truncations of amino acids 1 to 692 and 1 to 668. Forty-eight hours later, the cells were infected VVΔE3L and then lysed 24 hpi. (A) Expression of each six-His-tagged transgene and PKR was assessed by immunoblotting using an aliquot of each lysate. (B) After immunoprecipitation (of 40 times the amount shown in panel A) with an anti-His antibody, the His-tagged proteins and PKR were detected by immunoblot assays.
DISCUSSION
The TRS1 gene of HCMV serves several functions during HCMV infection. It has been reported to stimulate the expression of reporter genes driven by various HCMV promoters and to function in oriLyt-dependent DNA replication, both effects being observed in transient-expression assays (52, 53, 61, 63). Additionally, TRS1 has been shown to play a role in viral capsid assembly (1), and deletion of the majority of the TRS1 open-reading frame from HCMV results in a growth defect that is most pronounced at a low MOI (8). IRS1, the TRS1 homologue in HCMV, also stimulates reporter gene expression but is not required for efficient replication of HCMV (8, 31, 42, 61, 63). In addition to these roles, both TRS1 and IRS1 prevent the phosphorylation of eIF2α, the activation of RNase L, and the shutoff of translation that occur upon infection with VVΔE3L and restore the full host cell range of VVΔE3L (17). Attempts to determine the underlying mechanism of action of pTRS1 demonstrated that, like pE3L, it is a dsRNA-binding protein (36). The experiments described in this report were designed to further elucidate the mechanism by which these genes inhibit the phosphorylation of eIF2α.
We were surprised to find that expression of pTRS1 during VVΔE3L infection results in the accumulation of the eIF2α kinase PKR in the nucleus. We observed a similar effect on PKR localization resulting from pIRS1 expressed from VVΔE3L (data not shown). Despite the increase in total PKR in the nucleus during infection by VV expressing TRS1, the amount of PKR phosphorylated at Thr446 remained very low, similar to that of mock-infected cells, suggesting that pTRS1 effectively prevents the activation of PKR by viral dsRNA (24, 28, 59, 73). The cytoplasm is the primary site where PKR is believed to bind to, and become activated by, viral dsRNA (66, 69). Although the exact mechanism by which activated PKR encounters eIF2α is not well defined, it is thought that the dsRBDs of PKR are responsible for localizing PKR to ribosomes, thereby promoting the phosphorylation of ribosome-associated eIF2α that participates in translation initiation (44, 57, 68, 71, 74). Thus, both the activator and the target of PKR with regards to this specific pathway are primarily cytoplasmic. Based on our findings, we propose that pTRS1 inhibits the PKR-mediated phosphorylation of eIF2α in part by sequestering PKR in the nucleus. The observation that the residual cytoplasmic PKR was unactivated as well (Fig. 3) suggests that TRS1 possesses a mechanism to inhibit PKR activation independently of relocalization. However, the relocalization mechanism likely contributes to the overall blockade by preventing PKR accumulation in the cytoplasm, where it would be exposed to and might eventually be activated by dsRNA.
Other viruses block PKR function by interfering with a variety of steps in the pathway. For example, poliovirus infection results in the degradation of PKR (6, 7). Several viruses block activation of PKR by sequestering dsRNA or by interfering with PKR dimerization or enzymatic activity (26, 27, 32, 64, 65, 72). The herpes simplex virus γ34.5 gene product blocks the effects of PKR by cooperating with cellular protein phosphatase 1α to dephosphorylate eIF2α-phosphate (37). To our knowledge, our results represent the first description of a viral gene product that may inhibit PKR activity by affecting its subcellular localization. There are limited data concerning the movement of PKR within the cell in response to viral infection. While adenovirus infection and interferon treatment do not alter PKR localization (40, 41), infection of interferon-treated HeLa cells with encephalomyocarditis virus (EMCV) results in the displacement of PKR from ribosomal subunits and the aggregation of PKR in the perinuclear area (30). However, the PKR that aggregates in the perinuclear area during EMCV infection is activated and is thought to contribute to the inhibition of EMCV replication (30). This effect of EMCV infection on PKR is therefore quite different from that of pTRS1, which causes PKR relocalization to the nucleus and blocks its activation.
We have previously demonstrated that HCMV infection inhibits the eIF2α phosphorylation that normally results from PKR activation during VVΔE3L infection (19). Whether TRS1 and IRS1 are necessary and sufficient for this inhibition during HCMV infection is not yet known. It is possible that HCMV, like herpes simplex virus type 1 and VV, possesses more than one mechanism for inhibiting the PKR pathway (14, 15, 25, 26, 49). Recent studies of murine CMV (MCMV) support the hypothesis that TRS1 and IRS1 are in fact essential for evasion of PKR. The MCMV m142 and m143 genes are similar to TRS1 and IRS1 in that they are members of the betaherpesvirus US22 gene family, and their products in combination bind to dsRNA and rescue VVΔE3L replication in HeLa cells (18). Infection with m142 and m143 deletion mutants results in activation of PKR and a shutoff of protein synthesis and, moreover, TRS1 can rescue the replication deficiency of MCMV mutants lacking m142 or m143 (67). Thus, MCMV homologues of TRS1 and IRS1 are essential for blocking PKR activity, but whether TRS1 and IRS1 are essential for HCMV will require construction and analyses of an HCMV mutant lacking both genes.
While PKR is normally found predominantly in the cytoplasm, it is also present in lower quantities in the nucleolus, where it is thought, although not proven, to play a role in ribosome biogenesis (40, 41). Additionally, there is indirect evidence implicating PKR in the transcriptional up-regulation of select genes in response to viral infection or dsRNA (75), and PKR has been demonstrated to directly interact with several proteins present in the nucleus that may affect regulation of the cell cycle (22, 23, 55). While the functional significance of these interactions is unclear, PKR relocalization to the nucleus during HCMV infection may serve a role distinct from its role in the eIF2α pathway.
Although we do not yet know how TRS1 and IRS1 cause relocalization of PKR, our data demonstrating that they interact directly with PKR suggest this is a direct effect. The factors governing the subcellular distribution of pTRS1 and pIRS1 are not known. They are found in the cytoplasm and nucleus during the immediate-early and early times of HCMV infection but are predominantly cytoplasmic at late times, except for a truncated form of pIRS1 which is nuclear (61). Since the relative abundance of pTRS1, pIRS1, and PKR and the stoichiometry of their interactions are not known, it is possible that there is sufficient pTRS1 and pIRS1 present in the nucleus to sequester PKR by direct binding. Alternatively, the HCMV proteins may shuttle between the nucleus and the cytoplasm while PKR remains trapped in the nucleus once transported there. Since the change in PKR localization was observed during infection with VV recombinants expressing pTRS1 and pIRS1 as well as during HCMV infection, it is likely that if the effect is indirect, it depends on a common cellular rather than viral factor(s).
The PKR-interacting domain of pTRS1 and pIRS1, unlike that of pE3L, is required for maintaining protein synthesis and restoring VVΔE3L host cell range (36). The amino terminus of pE3L mediates a direct interaction with PKR but is dispensable for VV host cell range (62). However, this interaction domain is required for pE3L to inhibit eIF2α phosphorylation by PKR in yeast (60) and to inhibit the activation of PKR and phosphorylation of eIF2α at late times during VV infection (46). It may be that the E3L-PKR interaction enables an additional mechanism by which pE3L inhibits PKR activation as infection proceeds and the dsRNA-binding capacity of pE3L is overwhelmed. Similarly, the interactions of pTRS1 and pIRS1 with PKR may represent a secondary mechanism for blocking PKR. The requirement for the TRS1 and IRS1 PKR interaction domain may reflect a lower dsRNA-binding affinity or capacity of pTRS1 and pIRS1 compared to pE3L and thus the HCMV proteins might become saturated much more readily, necessitating an alternative mechanism to inhibit eIF2α phosphorylation. It is also possible that PKR binding adds to the potency of these dsRNA-binding proteins by localizing them in the vicinity of PKR, where they can be most effective in binding dsRNA that would otherwise activate PKR.
In summary, we found that pTRS1 and pIRS1 cause the nuclear accumulation of PKR during viral infection and at least pTRS1 causes PKR to remain unactivated. Both proteins interact with PKR via their carboxy termini, and this interaction appears to be required for them to inhibit PKR activity and restore the full host cell range of VVΔE3L. Our findings suggest that these HCMV genes sequester PKR away from its activator, cytoplasmic dsRNA generated during the course of viral infection, and also from its target substrate, cytoplasmic ribosome-associated eIF2α that is participating in translation initiation. Since PKR plays a significant role in the life cycle of numerous viruses (9, 12, 20, 29, 48), further studies of how these HCMV genes modulate PKR activity are likely to contribute to our understanding of HCMV disease pathogenesis.
Acknowledgments
We thank Bertram Jacobs (Arizona State University) for VC2 and VVΔE3L, Antonis Koromilas (McGill University) for GST-PKRK296R, Michael Mathews (University of Medicine and Dentistry of New Jersey/New Jersey Medical School) for PKR-EGFP, and Stephanie Child (Fred Hutchinson CRC) and the Fred Hutchinson CRC Genomics Shared Resource for technical assistance.
This work was supported by the Roche Postdoctoral Fellowship Award of the Infectious Disease Society of America (M.H.) and NIH grants K08 AI058089 (M.H.), T32 CA09229 (E.M.), and RO1 AI026672 (A.P.G.).
Footnotes
Published ahead of print on 20 September 2006.
REFERENCES
- 1.Adamo, J. E., J. Schroer, and T. Shenk. 2004. Human cytomegalovirus TRS1 protein is required for efficient assembly of DNA-containing capsids. J. Virol. 78:10221-10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete, J. P., S. J. Child, and A. P. Geballe. 2001. Abundant early expression of gpUL4 from a human cytomegalovirus mutant lacking a repressive upstream open reading frame. J. Virol. 75:7188-7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber, G. N., J. Tomita, A. G. Hovanessian, E. Meurs, and M. G. Katze. 1991. Functional expression and characterization of the interferon-induced double-stranded RNA activated P68 protein kinase from Escherichia coli. Biochemistry 30:10356-10361. [DOI] [PubMed] [Google Scholar]
- 4.Beattie, E., K. L. Denzler, J. Tartaglia, M. E. Perkus, E. Paoletti, and B. L. Jacobs. 1995. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 69:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckett, D., E. Kovaleva, and P. J. Schatz. 1999. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, T. L., G. N. Barber, and M. G. Katze. 1993. Degradation of the interferon-induced 68,000-Mr protein kinase by poliovirus requires RNA. J. Virol. 67:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black, T. L., B. Safer, A. Hovanessian, and M. G. Katze. 1989. The cellular 68,000-Mr protein kinase is highly autophosphorylated and activated yet significantly degraded during poliovirus infection: implications for translational regulation. J. Virol. 63:2244-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blankenship, C. A., and T. Shenk. 2002. Mutant human cytomegalovirus lacking the immediate-early TRS1 coding region exhibits a late defect. J. Virol. 76:12290-12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolovan, C. A., N. M. Sawtell, and R. L. Thompson. 1994. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J. Virol. 68:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouyac-Bertoia, M., J. D. Dvorin, R. A. Fouchier, Y. Jenkins, B. E. Meyer, L. I. Wu, M. Emerman, and M. H. Malim. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025-1035. [DOI] [PubMed] [Google Scholar]
- 11.Brand, S. R., R. Kobayashi, and M. B. Mathews. 1997. The Tat protein of human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J. Biol. Chem. 272:8388-8395. [DOI] [PubMed] [Google Scholar]
- 12.Brandt, T. A., and B. L. Jacobs. 2001. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J. Virol. 75:850-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browne, E. P., B. Wing, D. Coleman, and T. Shenk. 2001. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75:12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassady, K. A., M. Gross, G. Y. Gillespie, and B. Roizman. 2002. Second-site mutation outside of the US10-12 domain of δγ134.5 herpes simplex virus 1 recombinant blocks the shutoff of protein synthesis induced by activated protein kinase R and partially restores neurovirulence. J. Virol. 76:942-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassady, K. A., M. Gross, and B. Roizman. 1998. The second-site mutation in the herpes simplex virus recombinants lacking the γ134.5 genes precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2α. J. Virol. 72:7005-7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Child, S. J., M. Hakki, K. L. De Niro, and A. P. Geballe. 2004. Evasion of cellular antiviral responses by human cytomegalovirus TRS1 and IRS1. J. Virol. 78:197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Child, S. J., L. K. Hanson, C. E. Brown, D. M. Janzen, and A. P. Geballe. 2006. Double-stranded RNA-binding by a heterodimeric complex of murine cytomegalovirus m142 and m143 proteins. J. Virol. 80:10173-10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Child, S. J., S. Jarrahian, V. M. Harper, and A. P. Geballe. 2002. Complementation of vaccinia virus lacking the double-stranded RNA-binding protein gene E3L by human cytomegalovirus. J. Virol. 76:4912-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 21.Cosentino, G. P., S. Venkatesan, F. C. Serluca, S. R. Green, M. B. Mathews, and N. Sonenberg. 1995. Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proc. Natl. Acad. Sci. USA 92:9445-9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuddihy, A. R., S. Li, N. W. Tam, A. H. Wong, Y. Taya, N. Abraham, J. C. Bell, and A. E. Koromilas. 1999. Double-stranded-RNA-activated protein kinase PKR enhances transcriptional activation by tumor suppressor p53. Mol. Cell. Biol. 19:2475-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuddihy, A. R., A. H. Wong, N. W. Tam, S. Li, and A. E. Koromilas. 1999. The double-stranded RNA activated protein kinase PKR physically associates with the tumor suppressor p53 protein and phosphorylates human p53 on serine 392 in vitro. Oncogene 18:2690-2702. [DOI] [PubMed] [Google Scholar]
- 24.Dar, A. C., T. E. Dever, and F. Sicheri. 2005. Higher-order substrate recognition of eIF2α by the RNA-dependent protein kinase PKR. Cell 122:887-900. [DOI] [PubMed] [Google Scholar]
- 25.Davies, M. V., H. W. Chang, B. L. Jacobs, and R. J. Kaufman. 1993. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 67:1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies, M. V., O. Elroy-Stein, R. Jagus, B. Moss, and R. J. Kaufman. 1992. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J. Virol. 66:1943-1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dever, T. E., R. Sripriya, J. R. McLachlin, J. Lu, J. R. Fabian, S. R. Kimball, and L. K. Miller. 1998. Disruption of cellular translational control by a viral truncated eukaryotic translation initiation factor 2α kinase homolog. Proc. Natl. Acad. Sci. USA 95:4164-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dey, M., C. Cao, A. C. Dar, T. Tamura, K. Ozato, F. Sicheri, and T. E. Dever. 2005. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2α substrate recognition. Cell 122:901-913. [DOI] [PubMed] [Google Scholar]
- 29.Donelan, N. R., C. F. Basler, and A. Garcia-Sastre. 2003. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J. Virol. 77:13257-13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois, M. F., and A. G. Hovanessian. 1990. Modified subcellular localization of interferon-induced p68 kinase during encephalomyocarditis virus infection. Virology 179:591-598. [DOI] [PubMed] [Google Scholar]
- 31.Dunn, W., C. Chou, H. Li, R. Hai, D. Patterson, V. Stolc, H. Zhu, and F. Liu. 2003. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 100:14223-14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gale, M., Jr., C. M. Blakely, B. Kwieciszewski, S. L. Tan, M. Dossett, N. M. Tang, M. J. Korth, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1998. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: molecular mechanisms of kinase regulation. Mol. Cell. Biol. 18:5208-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gale, M., Jr., S. L. Tan, M. Wambach, and M. G. Katze. 1996. Interaction of the interferon-induced PKR protein kinase with inhibitory proteins P58IPK and vaccinia virus K3L is mediated by unique domains: implications for kinase regulation. Mol. Cell. Biol. 16:4172-4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 35.Geballe, A. P., and E. S. Mocarski. 1988. Translational control of cytomegalovirus gene expression is mediated by upstream AUG codons. J. Virol. 62:3334-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hakki, M., and A. P. Geballe. 2005. Double-stranded RNA binding by human cytomegalovirus pTRS1. J. Virol. 79:7311-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He, B., M. Gross, and B. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isler, J. A., A. H. Skalet, and J. C. Alwine. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 79:6890-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobs, B. 2000. Translational control in poxvirus-infected cells, p. 951-971. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 40.Jeffrey, I. W., S. Kadereit, E. F. Meurs, T. Metzger, M. Bachmann, M. Schwemmle, A. G. Hovanessian, and M. J. Clemens. 1995. Nuclear localization of the interferon-inducible protein kinase PKR in human cells and transfected mouse cells. Exp. Cell. Res. 218:17-27. [DOI] [PubMed] [Google Scholar]
- 41.Jimenez-Garcia, L. F., S. R. Green, M. B. Mathews, and D. L. Spector. 1993. Organization of the double-stranded RNA-activated protein kinase DAI and virus-associated VA RNAI in adenovirus-2-infected HeLa cells. J. Cell Sci. 106:11-22. [DOI] [PubMed] [Google Scholar]
- 42.Jones, T. R., and V. P. Muzithras. 1992. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J. Virol. 66:2541-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufman, R. J. 2000. The double-stranded RNA-activated protein kinase PKR, p. 503-528. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 44.Kumar, K. U., S. P. Srivastava, and R. J. Kaufman. 1999. Double-stranded RNA-activated protein kinase (PKR) is negatively regulated by 60S ribosomal subunit protein L18. Mol. Cell. Biol. 19:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langland, J., and B. Jacobs. 2002. The role of the PKR-inhibitory genes, E3L and K3L, in determining vaccinia virus host range. Virology 299:133. [DOI] [PubMed] [Google Scholar]
- 46.Langland, J. O., and B. L. Jacobs. 2004. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology 324:419-429. [DOI] [PubMed] [Google Scholar]
- 47.Langland, J. O., S. Pettiford, B. Jiang, and B. L. Jacobs. 1994. Products of the porcine group C rotavirus NSP3 gene bind specifically to double-stranded RNA and inhibit activation of the interferon-induced protein kinase PKR. J. Virol. 68:3821-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leib, D. A., M. A. Machalek, B. R. Williams, R. H. Silverman, and H. W. Virgin. 2000. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc. Natl. Acad. Sci. USA 97:6097-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohr, I., and Y. Gluzman. 1996. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 15:4759-4766. [PMC free article] [PubMed] [Google Scholar]
- 50.Oh, J., and S. S. Broyles. 2005. Host cell nuclear proteins are recruited to cytoplasmic vaccinia virus replication complexes. J. Virol. 79:12852-12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortega, L. G., M. D. McCotter, G. L. Henry, S. J. McCormack, D. C. Thomis, and C. E. Samuel. 1996. Mechanism of interferon action. Biochemical and genetic evidence for the intermolecular association of the RNA-dependent protein kinase PKR from human cells. Virology 215:31-39. [DOI] [PubMed] [Google Scholar]
- 52.Pari, G. S., and D. G. Anders. 1993. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pari, G. S., M. A. Kacica, and D. G. Anders. 1993. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J. Virol. 67:2575-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel, R. C., P. Stanton, N. M. McMillan, B. R. Williams, and G. C. Sen. 1995. The interferon-inducible double-stranded RNA-activated protein kinase self-associates in vitro and in vivo. Proc. Natl. Acad. Sci. USA 92:8283-8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel, R. C., D. J. Vestal, Z. Xu, S. Bandyopadhyay, W. Guo, S. M. Erme, B. R. Williams, and G. C. Sen. 1999. DRBP76, a double-stranded RNA-binding nuclear protein, is phosphorylated by the interferon-induced protein kinase, PKR. J. Biol. Chem. 274:20432-20437. [DOI] [PubMed] [Google Scholar]
- 56.Pe'ery, T., and M. B. Mathews. 2000. Viral translational strategies and host defense mechanisms, p. 371-424. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 57.Ramirez, M., R. C. Wek, and A. G. Hinnebusch. 1991. Ribosome association of GCN2 protein kinase, a translational activator of the GCN4 gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 11:3027-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy, T. R., H. Tang, X. Li, and F. Wong-Staal. 1997. Functional interaction of the HTLV-1 transactivator Tax with activating transcription factor-4 (ATF4). Oncogene 14:2785-2792. [DOI] [PubMed] [Google Scholar]
- 59.Romano, P. R., M. T. Garcia-Barrio, X. Zhang, Q. Wang, D. R. Taylor, F. Zhang, C. Herring, M. B. Mathews, J. Qin, and A. G. Hinnebusch. 1998. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2α kinases PKR and GCN2. Mol. Cell. Biol. 18:2282-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romano, P. R., F. Zhang, S. L. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romanowski, M. J., and T. Shenk. 1997. Characterization of the human cytomegalovirus irs1 and trs1 genes: a second immediate-early transcription unit within irs1 whose product antagonizes transcriptional activation. J. Virol. 71:1485-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shors, T., K. V. Kibler, K. B. Perkins, R. Seidler-Wulff, M. P. Banaszak, and B. L. Jacobs. 1997. Complementation of vaccinia virus deleted of the E3L gene by mutants of E3L. Virology 239:269-276. [DOI] [PubMed] [Google Scholar]
- 63.Stasiak, P. C., and E. S. Mocarski. 1992. Transactivation of the cytomegalovirus ICP36 gene promoter requires the alpha gene product TRS1 in addition to IE1 and IE2. J. Virol. 66:1050-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan, S. L., M. J. Gale, Jr., and M. G. Katze. 1998. Double-stranded RNA-independent dimerization of interferon-induced protein kinase PKR and inhibition of dimerization by the cellular P58IPK inhibitor. Mol. Cell. Biol. 18:2431-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan, S. L., and M. G. Katze. 1998. Biochemical and genetic evidence for complex formation between the influenza A virus NS1 protein and the interferon-induced PKR protein kinase. J. Interferon Cytokine Res. 18:757-766. [DOI] [PubMed] [Google Scholar]
- 66.Tian, B., and M. B. Mathews. 2001. Functional characterization of and cooperation between the double-stranded RNA-binding motifs of the protein kinase PKR. J. Biol. Chem. 276:9936-9944. [DOI] [PubMed] [Google Scholar]
- 67.Valchanova, R. S., M. Picard-Maureau, M. Budt, and W. Brune. 2006. Murine cytomegalovirus m142 and m143 are both required to block protein kinase-mediated shut-down of protein synthesis. J. Virol. 80:10181-10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vattem, K. M., K. A. Staschke, and R. C. Wek. 2001. Mechanism of activation of the double-stranded-RNA-dependent protein kinase, PKR: role of dimerization and cellular localization in the stimulation of PKR phosphorylation of eukaryotic initiation factor-2 (eIF2). Eur. J. Biochem. 268:3674-3684. [DOI] [PubMed] [Google Scholar]
- 69.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weisiger, R. A., and I. Fridovich. 1973. Superoxide dismutase. Organelle specificity. J. Biol. Chem. 248:3582-3592. [PubMed] [Google Scholar]
- 71.Wu, S., K. U. Kumar, and R. J. Kaufman. 1998. Identification and requirement of three ribosome binding domains in dsRNA-dependent protein kinase (PKR). Biochemistry 37:13816-13826. [DOI] [PubMed] [Google Scholar]
- 72.Yue, Z., and A. J. Shatkin. 1997. Double-stranded RNA-dependent protein kinase (PKR) is regulated by reovirus structural proteins. Virology 234:364-371. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, F., P. R. Romano, T. Nagamura-Inoue, B. Tian, T. E. Dever, M. B. Mathews, K. Ozato, and A. G. Hinnebusch. 2001. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J. Biol. Chem. 276:24946-24958. [DOI] [PubMed] [Google Scholar]
- 74.Zhu, S., P. R. Romano, and R. C. Wek. 1997. Ribosome targeting of PKR is mediated by two double-stranded RNA-binding domains and facilitates in vivo phosphorylation of eukaryotic initiation factor-2. J. Biol. Chem. 272:14434-14441. [DOI] [PubMed] [Google Scholar]
- 75.Zinn, K., A. Keller, L. A. Whittemore, and T. Maniatis. 1988. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science 240:210-213. [DOI] [PubMed] [Google Scholar]