Abstract
A phenotypic screen was used to search for drug-like molecules that can interfere with specific steps in membrane traffic. 2-(4-Fluorobenzoylamino)-benzoic acid methyl ester (Exo1), identified in this screen, induces a rapid collapse of the Golgi to the endoplasmic reticulum, thus acutely inhibiting the traffic emanating from the endoplasmic reticulum. Like Brefeldin A (BFA), Exo1 induces the rapid release of ADP-ribosylation factor (ARF) 1 from Golgi membranes but has less effect on the organization of the trans-Golgi network. Our data indicate that Exo1 acts by a different mechanism from BFA. Unlike BFA, Exo1 does not induce the ADP-ribosylation of CtBP/Bars50 and does not interfere with the activity of guanine nucleotide exchange factors specific for Golgi-based ARFs. Thus, Exo1 allows the fatty acid exchange activity of Bars50 to be distinguished from ARF1 activity in the control of Golgi tubulation.
Keywords: Golgi, ADP-ribosylation factor, endoplasmic reticulum (ER), imaging-based screen, Bars50
Eukaryotic cells use internal membrane-bound compartments to spatially segregate metabolic reactions and to create different chemical environments. The distinct identity of these compartments must be established and maintained. This process is presumably particularly difficult for the organelles of the secretory and endocytic pathways, which must exchange membrane components as part of their function. We know that small vesicles and tubules are used to transport the membrane components between organelles so that the parent organelles remain separated (1, 2). We do not yet fully understand, however, the functions of proteins that have been identified as important for secretion and how their functions are integrated. In part this lack of knowledge is because most of the steps in secretion and endocytosis are highly dynamic and highly regulated, and therefore hard to study by using the tools of genetics and biochemistry.
We are interested in the potential of small molecule inhibitors or activators for studying secretion. Because of the dynamic nature of membrane traffic, specific, reversible, fast-acting modulators of secretion would be particularly useful research tools. Unfortunately, very few small molecule modulators of the secretory pathway are known. This may partly be because most small molecule discovery is done in the pharmaceutical industry, and inhibitors of secretion are generally too toxic to be useful as clinical drugs. Brefeldin A (BFA), a particularly useful reagent for study of Golgi function (3, 4) is an example: it was originally discovered at a pharmaceutical company (5) but was essentially ignored for 25 years due to its toxicity.
BFA causes dramatic changes in the morphology and function of the Golgi; the Golgi tubulates, then redistributes its entire contents and membrane to the endoplasmic reticulum (ER). A subset of the endosomal compartment also tubulates and mixes with the trans-Golgi network (TGN; refs. 6 and 7). After much study, the mechanism of BFA has been at least partly elucidated. It stabilizes a transient complex formed between the small GTPase ADP-ribosylation factor (ARF) 1 in its GDP-bound form and some of its exchange factors, preventing GDP/GTP exchange (8, 9). Thus, ARF1-GTP is consumed and not replaced, therefore processes that depend on ARF1-GTP (such as membrane recruitment or activation of various proteins involved in regulation of vesicular traffic) are blocked (10).
BFA has been a powerful tool, and we owe a good deal of our understanding of the Golgi as a dynamic organelle to the use of BFA. However, it has pleiotropic effects in mammalian cells that are confusing and often of unknown mechanism. For example, in addition to inhibiting ARF1-GTP/GDP exchange, BFA is known to cause ADP-ribosylation of CtBP/Bars50 (11, 12). CtBP/Bars50 is an enzyme involved in the transfer of palmitate from palmitoyl CoA to lysophosphatidic acid. This ADP-ribosylation event has been suggested to contribute to tube formation in the Golgi collapse phenomenon, by a mechanism involving changes in membrane curvature due to reduction in CtBP/Bars50 activity (13). An alternative hypothesis is that tubule formation is a consequence of the release of ARF1 from Golgi membranes (14, 15), and these hypotheses have been hard to differentiate.
To find a small molecule tool that would complement BFA in the study of membrane trafficking, we used a phenotypic screen to identify 26 small molecules that perturb exocytosis. Here we show that one of these, 2-(4-fluorobenzoylamino)-benzoic acid methyl ester (Exo1), is a modifier of Golgi ARF1 GTPase activity. Thus far, the observed effects of Exo1 on cells are restricted to the traffic between the ER and Golgi apparatus. Because Exo1 affects a subset of the membrane events that are blocked by BFA, but seems to have a different protein target (and presumably has different side effects), it provides an independent means to interfere with Golgi activity.
Methods
High-Throughput Phenotypic Screen. BSC1 fibroblast cells were mixed with vesicular stomatitis virus fused to GFP (VSVGts-GFP) adenovirus, plated in 384-well clear bottom plates at ≈1,500 cells per 30 μl per well and grown overnight at 40°C in a 5% CO2 incubator. Compounds (10,240 from the DIVERSet E, Chembridge, San Diego) were screened by sampling 100 nl of 10 mg/ml of stocks dissolved in DMSO that were transferred into wells in duplicate plates by using a pin-transfer robot (http://iccb.med.harvard.edu/screening). Cells were incubated further at 40°C for 60 min, transferred to 40°C or 32°C for 2 h, fixed with 4% paraformaldehyde, and imaged by using an automated fluorescence microscope (autoscope, http://iccb.med.harvard.edu/screening) at a rate of about 1 plate per h. Images were visually scored for inhibition of VSVGts-GFP traffic, and positive compounds were retested at different concentrations.
Supporting Information. For additional information, see Appendices I–VIII and Movies 1–6, which are published as supporting information on the PNAS web site, www.pnas.org.
Results
An Image-Based Phenotypic Screen to Identify Small Molecule Inhibitors of the Exocytic Pathway. We developed an automated fluorescence microscopy imaging-based screen to search for small molecules affecting the exocytic traffic of membrane proteins from the ER to the plasma membrane. The screen was based on the behavior of the temperature-sensitive mutant ts045 of the surface glycoprotein of VSVGts-GFP. At the nonpermissive temperature of 40°C, VSVGts-GFP remains in the ER. Shift of the cells to 32°C results in the synchronous export of VSVGts-GFP, first to the Golgi apparatus and then to the plasma membrane (see Appendix I A and B) (16, 17). We screened 10,240 compounds (part of the DIVERSet E, Chembridge library) for effects on the localization of VSVGts-GFP at 40°C and 32°C. Most had no effect, or toxic effects. Twenty-six compounds were considered “hits.” These elicited four distinct phenotypes on the intracellular distribution of VSVGts-GFP (K). These phenotypes can be summarized as ER exit block (C, D), Golgi exit block (E, F), Golgi fragmentation (G, H), and vacuole formation (I, J). We believe these compounds are relatively specific for processes involved in membrane traffic. None of these compounds has been selected as high-priority hits in any of the other ≈100 phenotypic or enzymatic screens performed at Institute of Chemistry and Cell Biology (Harvard Medical School, Boston) with the same set of compounds, and none of them has shown major effects on the appearance of the actin-based cytoskeleton, the rate of transferrin uptake from the plasma membrane to endosome, or the appearance of endosomes and lysosomes (data not shown).
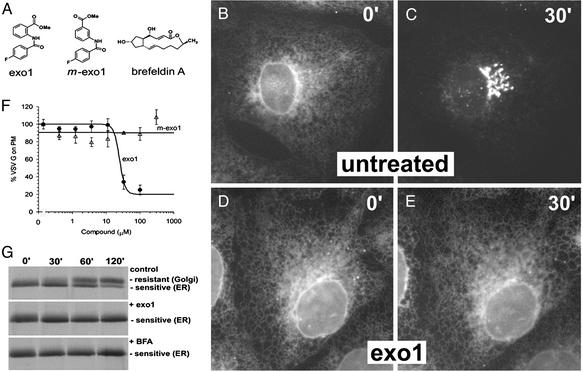
Exo1 Disrupts Vesicular Traffic from the ER to the Golgi Apparatus by Disrupting Golgi Structures. Here we focus on Exo1 (Fig. 1A), one of the two compounds that blocked VSVGts-GFP exit from the ER (Fig. 1 B–E and Movies 1 and 2). We confirmed the identity and purity of the compound by using routine analytical chemistry techniques and by de novo synthesis. The closely related analog 3-(4-fluorobenzoylamino)-benzoic acid methyl ester (m-Exo1; Fig. 1 A) has no apparent effect in this assay. BFA also blocks the same process (3) (see Appendix III) but has a very different structure (Fig. 1 A).
Fig. 1.
Exit of VSVGts-GFP from the ER is inhibited by Exo1. (A) Chemical structures of Exo1, the analog m-Exo1, and BFA. (B–E) VSVGts-GFP accumulates in the ER at 40°C (B) and reaches the Golgi apparatus after a 30-min shift to 32°C (C) but remains in the ER in the presence of 100 μM Exo1 (D and E). Movies 1 and 2 show time-lapse data for these experiments. (F) The effect of different concentrations of Exo1 and m-Exo1 on the relative efficiency of delivery of VSVGts-GFP from the ER to the cell surface. BSC1 cells expressing VSVGts-GFP were incubated at 40°C with different amounts of the compounds, then shifted to 32°C and incubated for 3 h. Under these conditions Exo1 has an IC50 of about 20 μM whereas m-Exo1 has no effect at 300 μM (for dose–response with BFA see Appendix II). (G) Results from a pulse–chase experiment after the acquisition of endoglycosidase H resistance by VSVGts-GFP in the absence (Top) or presence (Middle)of 100 μM Exo1 or 5 μM BFA (Bottom).
Exo1 inhibits exocytosis with an IC50 of ≈20 μM (Fig. 1F). A small SAR study (234 related compounds) was performed but failed to identify more potent analogs (Appendix II). The effects of Exo1 are similar on all cells we tested. It blocks ER exit of VSVGts-GFP in nonpolarized mammalian cells such as Chinese hamster ovary, human HeLa and 293, rat NRK, hamster BHK, and polarized canine MDCK cells (data not shown). Likewise, Exo1 prevents acquisition of endoglycosidase H resistance by newly synthesized proteins such as VSVGts-GFP (Fig. 1G), transferrin, and MHC class I (data not shown).
To understand the mechanism of these effects, we first asked whether Exo1 treatment alters the distribution of a series of markers specific for different organelles along the exocytic and endocytic pathways. Exo1 treatment induces rapid redistribution of Golgi content back to ER [galactosyltransferase-GFP (GalT-GFP); see Fig. 2 A and B and Appendix IV], whereas it has essentially no effect on endocytic organelle structures, demonstrated by staining of transferrin-positive early and recycling endosomes (Fig. 2C) and cathepsin D-positive late endosomes and lysosomes (Appendix IV). The effects of Exo1 on the Golgi apparatus are reversible. Golgi markers (residential enzyme GalT and matrix protein GM130) reappear in the perinuclear area 30 min after Exo1 is removed from the medium (Appendix IV).
Fig. 2.
Exo1 induces tubulation and collapse of the Golgi apparatus but not endosomes and TGN. (A) The intracellular location of GalT-GFP was monitored by time-lapse fluorescence microscopy in BSC1 cells treated with 100 μM Exo1 or 5 μM BFA. Both compounds induced the appearance of tubules emanating from the Golgi apparatus at around 4 min. The tubes eventually reach the ER and this leads to the dramatic redistribution of Golgi contents to the ER at 10 min (Movies 3 and 4). (B) The effect of 100 μM Exo1 or 5 μM BFA on the distribution of ARF1-GFP or GalT-GFP in BSC1 cells was followed as a function of time by time-lapse fluorescence microscopy. The perinuclear Golgi-associated fluorescent signal was monitored for 5 min before addition of the compounds to the media. Each curve represent the average data recorded from 6 movies. Also see Movies 5 and 6. (C) Effects of BFA or Exo1 on the distribution of endosomes (Tfn) and the TGN (GGA3). The early/recycling endosomes were visualized by following the distribution of Texas red-labeled transferrin internalized for 20 min by receptor-mediated endocytosis in the presence of compounds. BFA induced enlargement and tubulation of transferrin-positive endosomes (Inset). Exo1 had no effect. The TGN-associated adaptor protein GGA3 was visualized after a 2-min treatment of BFA or Exo1. BFA induced GGA3 redistribution to cytosol, whereas Exo1 did not.
The Effects of Exo1 Are Similar but Not Identical to Those of BFA. Treatment with Exo1 results in massive tubulation of the Golgi apparatus, formation of contacts between the Golgi tubules, and massive and uncontrolled transfer of Golgi contents primarily to the ER as followed by the movement of GalT-GFP (Fig. 2 A and Movie 3). Similar effects were described with BFA treatment (3) (Movie 4). The primary target of BFA in cells was shown to be ARF1 GTPase (18). BFA treatment causes a rapid decrease of the levels of activated ARF1 on the Golgi membrane and therefore release of many Golgi- and TGN-associated proteins such as coatomer protein I (COPI) coatomers, clathrin coat, and adaptors AP1, AP3, and Golgi-localizing, gamma-adaptin ear homology domain, ARF-binding protein (GGAs) (15). All these events precede the general collapse of the Golgi apparatus. We therefore examined the release of several markers from intracellular membranes in response to Exo1 and BFA.
Kinetic analysis by time-lapse microscopy showed that the average half-life for disappearance of ARF1-GFP from Golgi membranes after treatment with either Exo1 or BFA was less than 1 min (Fig. 2B and Movies 5 and 6). In both cases, the release of COPI is also very rapid after ARF1 dissociation (Appendix V) and this precedes the general collapse of the Golgi apparatus indicated here by GalT-GFP (Fig. 2B and Appendix V). The effects of the two chemicals are not identical, however. Exo1 addition did not induce rapid release of TGN-associated coat proteins such as GGA3, AP-1, AP-3, and clathrin (Fig. 2C and Appendix V), whereas BFA caused release of these proteins to the cytosol within 2 min of treatment. Longer treatments with Exo1 (30 min) led to a dispersal of AP-1, AP-3, and GGA3 signals to small punctate structures, with partial release to the cytosol (data not shown). A second distinction is that Exo1 does not tubulate endosomal membranes labeled with internalized transferrin (Fig. 2C), whereas BFA does. Because of these differences, we conclude that either Exo1 binds to a subset of the protein targets of BFA or the target of Exo1 is different from that of BFA. Because Exo1 is relatively specific for Golgi membranes, we assume that the target of Exo1 is relatively abundant in the Golgi.
ARF-Guanine Nucleotide Exchange Factor (GEF) Is Not the Direct Target of Exo1. The steady-state distribution of ARF1-GTP bound to Golgi membranes depends on the balance between the exchange activity of ARF-GEFs (generating ARF-GTP) and the rate of GTP hydrolysis by ARF. We therefore examined whether Exo1 decreases GTP loading onto ARF1 or increases GTP hydrolysis by ARF1. Either activity would explain the observed release of ARF1 from Golgi membranes. Unlike BFA, Exo1 did not interfere with the guanine nucleotide exchange activity of a number of ARF-GEFs in vitro (Fig. 3A and Appendix VI). Nucleotide exchange in vivo is directly linked to stable binding of ARF1 to the Golgi membrane. We also showed that, in the presence of a sufficient level of Exo1 to rapidly release all wild-type ARF1 from Golgi, ARF1Q71L-GFP could be reloaded to Golgi membrane after it was released from Golgi with BFA pretreatment (Fig. 3B). Thus, Exo1 does not seem to directly inhibit ARF1-GDP/GTP exchange.
Fig. 3.
Exo1 acts on ARF1-GTPase through a different mechanism compared with BFA. (A) Exo1 has no effect on GEF-stimulated GDP/GTP exchange on ARF1. The figure shows the effect of Exo1 and BFA on GTP loading into ARF1 by a recombinant SEC7 domain of GEF. The loading of GTP was followed as a function of time by the increase in relative fluorescence. BFA (10 μM) inhibited the exchange rate by 60% (bar graph, Inset) whereas 100 μM of Exo1 showed no inhibition. (B) Exo1 does not block the loading of ARF1 onto Golgi membrane. Mutant ARF1Q71L-GFP was released from Golgi in cells treated with 5 μM of BFA for 30 min. BFA was then washed out and exchanged for control medium or 100 μM of Exo1. Relocalization of ARF1Q71L-GFP from cytosol to Golgi was measured over the period of 1 h. (C) The effect of Exo1 is blocked by a chemical that masks the activity of the ARF1-GTPase. The extent of association of wild-type ARF1 (ARF1wt-GFP) with Golgi membranes was determined in sequential images recorded by time-lapse fluorescence microscopy. The measurements were made in BSC1 cells preincubated with 50 μM AlCl3 and 30 mM NaF for 30 min, in the absence or presence of 5 μM BFA or 100 μM Exo1. The Golgi association of ARF1 during the preincubation period is shown (Inset). (D) A GTPase-deficient ARF1 mutant is insensitive to Exo1 treatment. The extent of association of a mutant of ARF1 defective in GTP hydrolysis (ARF1Q71L-GFP) with Golgi membranes was determined as in C in the absence or presence of 5 μM BFA or 100 μM Exo1. (E) The expression of ARF1Q71L-GFP in BSC1 cells prevents the dissociation of COPI from the Golgi. The fluorescence images show that 5 μM BFA induces the dissociation of COPI in the presence of ARF1wt-GFP or ARF1Q71L-GFP. In contrast, Exo1 promotes dissociation of COPI only in the presence of ARF1wt-GFP but not when ARF1Q71L-GFP is expressed. (F) Expression of ARF1Q71L-CFP protects cells from the block of ER to Golgi traffic and prevents collapse of the Golgi induced by Exo1. BSC1 cells coexpressing VSVGtso45-yellow fluorescent protein (YFP) with ARF1wt-CFP or with ARF1Q71L-CFP were treated with 5 μM BFA or 100 μM Exo1 for 30 min followed by a temperature shift from 40°C to 32°C. BFA promotes dissociation of ARF1wt-CFP and ARF1Q71L-CFP from Golgi membranes and prevents exit of VSVGtsO45-YFP from the ER. Exo1 had the same effects on cells expressing ARF1wt-CFP, but were blocked after expression of ARF1Q71L-CFP.
It is most complicated to determine whether Exo1 accelerates GTP hydrolysis. In vitro GTPase-activating protein (GAP)-stimulated ARF1-GTP hydrolysis was not sensitive to Exo1 (data not shown). The in vivo ARF1-GTP hydrolysis rate is several thousand-fold higher than the level achievable in vitro, however. We therefore used two indirect approaches to ask whether Exo1 might affect ARF1-GTP hydrolysis in vivo. First, we preincubated cells with AlF4, which has been shown to lock several small GTPases in their GDP.AlF4-binding transition state together with a limiting cofactor GAP (19, 20). It is not yet clear whether AlF4 has the same effect on ARF1. Although an early experiment suggests that AlF4 stabilizes a subset of ARF1 on Golgi membranes (21), recent evidence from Presley et al. (22) indicates that the main effect of AlF4 on membrane is to irreversibly block dissociation of COPI but not the bulk of ARF1. However, we observed that in the presence of BFA, AlF4 slowed the dissociation rate of ARF1 from the Golgi significantly, and AlF4 treatment itself increased the steady-state level of ARF1wt-GFP on the Golgi membrane (Fig. 3C). It is possible that this result indicates that AlF4 has a similar effect on ARF1 as it does on other small GTPases, i.e., tethering the limiting GAP factor in a relatively more stable complex and thus reducing the overall rate of GTP hydrolysis. In this case, the presence of AlF4 should slow (but not entirely prevent) dissociation of ARF1 from the membrane. Because reloading is blocked in the presence of BFA, the dissociation should eventually reach the same extent as in the absence of AlF4. That BFA is able to release all ARF1 from the Golgi membrane and that the rate of release is only slightly reduced, indicate that the AlF4-induced tethering of ARF1, whatever its mechanism, must be short term (Fig. 3C). This finding is in agreement with the observation by Kahn (23). In contrast, AlF4 blocked the ability of Exo1 to induce dissociation of membrane-bound ARF1wt-GFP (Fig. 3C). This result is consistent with the idea that Exo1 might increase the rate of GTP hydrolysis, and that this increase depends on the availability of GAP. It is also possible, however, that Exo1 is having an indirect inhibitory effect on ARF-GEF via another GTPase or via some unknown mechanism. In any case, the effects of Exo1 and BFA are dramatically different.
In the second approach, we used cells transiently expressing a low level of ARF1Q71L-GFP, a mutated form of ARF1 with reduced GTPase activity (24). The dissociation of ARF1Q71L-GFP in response to BFA is slower than the release of wild-type ARF1 (Fig. 3D). COPI, which binds to ARF1-GTP, also dissociates from the membrane (Fig. 3E). Exo1 caused only a transient and incomplete dissociation of the mutated ARF1Q71L-GFP (Fig. 3D) and minimal dissociation of COPI (Fig. 3E). Thus, a mutation that slows the GTPase activity of ARF1 also reduces the sensitivity of the enzyme to Exo1. The presence of biologically active concentrations of Exo1 in these experiments can be confirmed by examining nontransfected cells in the field, which were observed to lose Golgi-associated COPI (Fig. 3E). Consistent with these data, ARF1Q71L rescued ER export of VSVGts-GFP in the presence of Exo1 but not in the presence of BFA (Fig. 3F).
These results strongly support the hypothesis that Exo1 and BFA have different targets, and that the target of Exo1 is likely to be downstream from the ARF1-GTP-loading step. These results are consistent with the notion that Exo1 works by increasing the rate of GTP hydrolysis through the activation of an ARF-GAP-dependent step, although many other models are possible.
Golgi Tubulation Does Not Require ADP-Ribosylation of CtBP/Bars50. As one example of the utility of this tool, we explored the question of whether Golgi tubulation induced by Exo1, like with BFA, also involves ADP-ribosylation of CtBP/Bars50. We found that CtBP/Bars50 is not detectably modified in response to Exo1, whereas it is modified in the presence of BFA (Fig. 4). Therefore, tubulation of the Golgi and migration of Golgi membranes back to the ER do not require ADP-ribosylation of CtBP/Bars50.
Fig. 4.
Exo1 does not induce the ADP-ribosylation of Bars50. The supernatant from a cell lysate of BSC1 cells subjected to high-speed centrifugation was incubated with 10 μM BFA or 100 μM Exo1 in the presence of 32P-ADP-ribose for 30 min at 37°C. The proteins in the lysate were then subjected to denaturing SDS/PAGE, as demonstrated (11). BFA (lane 2) induces the ADP-ribosylation of Bars50. In contrast, Exo1 (lane 3) had no effect. No ADP-ribosylation is detected in the presence of 1% DMSO (lane 1).
Discussion
As a first step toward developing new tools with which to probe events in vesicle trafficking, we describe the identification of Exo1 as an inhibitor of vesicular traffic from the ER to the Golgi. We have not identified the relevant protein target of Exo1 but we have shown that it is distinct from that of BFA.
We present two alternative models to explain the differences between Exo1 and BFA. One possibility is that Exo1 activates a currently unidentified inhibitor of the ARF-GEF. In AlF4–-treated cells, like coatomers, this ARF1 effector would be unable to dissociate from Golgi membranes and recycle back for a new round of GEF activity. Under these circumstances, the target of Exo1 would be sequestered and unavailable for inhibition. Similarly, expression of ARF1Q71L with a slower rate of GTP hydrolysis could also sequester coatomer and the target of Exo1, thus decreasing the effectiveness of Exo1 to prevent GTP-loading of ARF.
A second possibility, which we currently prefer, is that Exo1 might act on a step other than ARF1-GDP/GTP exchange. We consider it likely that Exo1 affects the balance of the futile cycle with the functional cycle of ARF1-GTP hydrolysis (see Appendix VII). ARF-GAP1 has two possible fates when it is recruited to Golgi membranes from the cytosol: either it associates with unloaded COPI coatomers and ARF1-GTP, in which case it is highly active and ARF1 has high GTPase activity (the futile cycle), or it associates with COPI coatomers that are bound to cargo receptors, in which case it gives lower activity and allows ARF1-GTP to persist for longer (functional cycle, Appendix VII) (25, 26, 27). The effects of Exo1 on GAP-dependent ARF1-GTP hydrolysis might result from interference with the futile cycle hydrolysis step, the functional cycle hydrolysis step, or both. Furthermore, Exo1 could affect the functional cycle directly by acting on the GAP, or indirectly by interfering with the formation of the complex of cargo-COPI with ARF1-GTP-GAP that is required to set off the functional cycle. Such interference would cause a decrease in the amount of ARF1-GTP on the membrane because the futile cycle is faster than the functional cycle. Our current favored model is that Exo1 interferes with the formation of the ARF1-GTP-GAP-cargo-COPI complex required to initiate the functional cycle. We do not rule out the possibility that Exo1 directly or indirectly enhances GAP activity in the futile cycle but it seems unlikely because in the futile cycle, GTPase activity is already very high (at least 5 times the rate in the functional cycle) and one would expect that it would be hard to enhance this rate further. It is also possible that Exo1 selectively increases the GAP activity in the ARF1-GTP-GAP-cargo-COPI complex, causing the release of ARF1 without allowing coat assembly. More work will be needed to distinguish between these models.
It is clear from our results that at least one confounding effect of BFA, its ability to cause ADP-ribosylation of CtBP/Bars50, can be avoided by using Exo1. Because Exo1 has no apparent effects on the TGN and endosomes, it will be a valuable reagent to study the consequences of perturbations of ER and Golgi traffic for the movement of ligands from the plasma membrane to the ER.
Supplementary Material
Acknowledgments
We thank H. Vandertol-Vanier for purification of GST-Golgi-associated BFA-resistant GEF 1 (GBF1), Dr. R. Ward for editorial help, and Mr. I. Levesque for administrative assistance. We acknowledge National Institutes of Health Grants R01 GM36548-17 and P01 GM62566-02 (to T.K.), the Alberta Heritage Foundation for Medical Research (P.M.), the Canadian Institutes of Health Research (P.M. and T.K.R.L.), and the Howard Hughes Medical Institute Medical Student Research Fellowship (to A.P.J.).
Abbreviations: BFA, brefeldin A; TGN, trans-Golgi network; VSVGts-GFP, vesicular stomatitis virus fused to GFP; Exo1, 2-(4-fluorobenzoylamino)-benzoic acid methyl ester; m-Exo1, 3-(4-fluorobenzoylamino)-benzoic acid methyl ester; GAP, GTPase-activating protein; ARF, ADP-ribosylation factor; CFP, cyan fluorescent protein; COPI, coatomer protein I; GGA, Golgi-localizing, gamma-adaptin ear homology domain, ARF-binding; GEF, guanine nucleotide exchange factor; GalT, galactosyltransferase; ER, endoplasmic reticulum.
References
- 1.Palade, G. (1975) Science 189, 347–358. [DOI] [PubMed] [Google Scholar]
- 2.Rothman, J. E. (1994) Nature 372, 55–63. [DOI] [PubMed] [Google Scholar]
- 3.Klausner, R. D., Donaldson, J. G. & Lippincott-Schwartz, J. (1992) J. Cell Biol. 116, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson, C. L. (2000) Subcell. Biochem. 34, 233–272. [DOI] [PubMed] [Google Scholar]
- 5.Harri, E., Loeffler, W., Sigg, H. P. & Tamm, H. (1963) Helv. Chim. Acta 46, 1235–1243. [Google Scholar]
- 6.Lippincott-Schwartz, J., Yuan, L. C., Bonifacino, J. S. & Klausner, R. D. (1989) Cell 56, 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippincott-Schwartz, J., Yuan, L., Tipper, C., Amherdt, M., Orci, L. & Klausner, R. D. (1991) Cell 67, 601–616. [DOI] [PubMed] [Google Scholar]
- 8.Peyroche, A., Antonny, B., Robineau, S., Acker, J., Cherfils, J. & Jackson, C. L. (1999) Mol. Cell 3, 275–285. [DOI] [PubMed] [Google Scholar]
- 9.Mansour, S. J., Skaug, J., Zhao, X. H., Giordano, J., Scherer, S. W. & Melancon, P. (1999) Proc. Natl. Acad. Sci. USA 96, 7968–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dascher, C. & Balch, W. E. (1994) J. Biol. Chem. 269, 1437–1448. [PubMed] [Google Scholar]
- 11.Mironov, A., Colanzi, A., Silletta, M. G., Fiucci, G., Flati, S., Fusella, A., Polishchuk, R., Mironov, A., Jr., Di Tullio, G., Weigert, R., et al. (1997) J. Cell Biol. 139, 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spano, S., Silletta, M. G., Colanzi, A., Alberti, S., Fiucci, G., Valente, C., Fusella, A., Salmona, M., Mironov, A., Luini, A., et al. (1999) J. Biol. Chem. 274, 17705–17710. [DOI] [PubMed] [Google Scholar]
- 13.Weigert, R., Silletta, M. G., Spano, S., Turacchio, G., Cericola, C., Colanzi, A., Senatore, S., Mancini, R., Polishchuk, E. V., Salmona, M., et al. (1999) Nature 402, 429–433. [DOI] [PubMed] [Google Scholar]
- 14.Scheel, J., Pepperkok, R., Lowe, M., Griffiths, G. & Kreis, T. E. (1997) J. Cell Biol. 137, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson, J. G., Lippincott-Schwartz, J., Bloom, G. S., Kreis, T. E. & Klausner, R. D. (1990) J. Cell Biol. 111, 2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Presley, J. F., Cole, N. B., Schroer, T. A., Hirschberg, K., Zaal, K. J. & Lippincott-Schwartz, J. (1997) Nature 389, 81–85. [DOI] [PubMed] [Google Scholar]
- 17.Scales, S. J., Pepperkok, R. & Kreis, T. E. (1997) Cell 90, 1137–1148. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson, J. G., Finazzi, D. & Klausner, R. D. (1992) Nature 360, 350–352. [DOI] [PubMed] [Google Scholar]
- 19.Rittinger, K., Walker, P. A., Eccleston, J. F., Smerdon, S. J. & Gamblin, S. J. (1997) Nature 389, 758–762. [DOI] [PubMed] [Google Scholar]
- 20.Scheffzek, K., Ahmadian, M. R., Kabsch, W., Wiesmuller, L., Lautwein, A., Schmitz, F. & Wittinghofer, A. (1997) Science 277, 333–338. [DOI] [PubMed] [Google Scholar]
- 21.Finazzi, D., Cassel, D., Donaldson, J. G. & Klausner, R. D. (1994) J. Biol. Chem. 269, 13325–13330. [PubMed] [Google Scholar]
- 22.Presley, J. F., Ward, T. H., Pfeifer, A. C., Siggia, E. D., Phair, R. D. & Lippincott-Schwartz, J. (2002) Nature 417, 187–193. [DOI] [PubMed] [Google Scholar]
- 23.Kahn, R. A. (1991) J. Biol. Chem. 266, 15595–15597. [PubMed] [Google Scholar]
- 24.Zhang, C. J., Rosenwald, A. G., Willingham, M. C., Skuntz, S., Clark, J. & Kahn, R. A. (1994) J. Cell Biol. 124, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg, J. (1999) Cell 96, 893–902. [DOI] [PubMed] [Google Scholar]
- 26.Szafer, E., Rotman, M. & Cassel, D. (2001) J. Biol. Chem. 276, 47834–47839. [DOI] [PubMed] [Google Scholar]
- 27.Lanoix, J., Ouwendijk, J., Stark, A., Szafer, E., Cassel, D., Dejgaard, K., Weiss, M. & Nilsson, T. (2001) J. Cell Biol. 155, 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.