Abstract
Ligand activation of the epidermal growth factor receptor (EGFR) causes the binding of Cbls, which leads to EGFR polyubiquitination and internalization through endophilin complexes that contain the adaptor protein SH3-domain encoding, expressed in tumorigenic astrocytes/Cbl-interacting protein of 85 kDa/regulator of ubiquitous kinase (SETA/CIN85/Ruk). In cells grown at high density, high levels of SETA interfered in the recruitment of Casitas B-lineage (Cbl) proteins to the EGFR and reduced its polyubiquitination, suggesting that SETA has a regulatory function in the formation of the EGFR–Cbl–endophilin complex and in EGFR down-regulation. In a situation where there is EGFR signaling but no internalization or down-regulation, as is the case with the EGFR with exons 2–7 deleted (ΔEGFR) oncogene, these proteins were absent altogether. By using mAb 806, which recognizes an EGFR-activation state and preferentially immunoprecipitates ΔEGFR, we show that ΔEGFR did not interact with Cbls, SETA, or endophilin A1, providing a mechanistic explanation for its lack of internalization. As would be expected by the absence of Cbl proteins in the ΔEGFR complex, the mutant receptor was also not polyubiquitinated. The intracellular C terminus and tyrosine autophosphorylation pattern of ΔEGFR are similar to wild-type EGFR, but it signals at a lower intensity as determined by levels of EGFR phosphotyrosine. To test the implication that the lack of interaction with the Cbl–SETA–endophilin complex is because of differences in signal intensity, EGFR-expressing cells were treated with tyrphostin AG1478 EGFR inhibitor. Attenuation of wild-type EGFR signal to levels similar to that found in ΔEGFR resulted in the dissociation of SETA and Cbl proteins and a concomitant attenuation of receptor internalization.
Receptor tyrosine kinase signaling is important in the growth of cells and its dysregulation is a major contributor to cancer. High-level expression of epidermal growth factor receptor (EGFR) can transform NIH 3T3 cells, and is frequently observed in human glioma, because of gene amplification that is also accompanied by rearrangement, and is associated with poorer survival (1–6). The most common of the rearrangements lead to the deletion of exons 2–7 in the EGFR mRNA, causing an in-frame deletion of 801 bp in the extracellular domain (3, 7–9). The resulting protein, EGFR with exons 2–7 deleted (ΔEGFR or EGFRvIII), is phosphorylated in a ligand-independent fashion and confers enhanced tumorigenicity on glioma cells (10). It may also drive clonal selection, as glioma cells expressing ΔEGFR can rapidly outgrow their parental cells in vivo (11). ΔEGFR can also up-regulate molecular effectors of invasion in glioblastoma cells (12), further contributing to their malignancy.
ΔEGFR signaling is constitutive without receptor internalization, in the absence of mutations in the C terminus where this process is regulated (13). ΔEGFR autophosphorylation occurs primarily at tyrosines 1068, 1148, and 1173, with 1173 alone accounting for about half of the total (13). This location is also the preferred phosphorylation site of wild-type EGFR (14), suggesting that the pattern of modification of the active ΔEGFR and wild-type EGFR C terminus may not qualitatively be very different. However, the ΔEGFR shows almost 10-fold lower levels of autophosphorylation than ligand-stimulated wild-type EGFR (13), implying that persistence, rather than intensity of signal, may be a major contributor to ΔEGFR tumorigenicity, and that lack of signal attenuation by internalization may be a key determinant of this phenotype. One consequence of this low level of signaling is that mutation of even a single tyrosine autophosphorylation site in ΔEGFR is sufficient to attenuate its promotion of glioma growth (13), in contrast to the wild-type receptor, where multiple tyrosines need to be ablated to significantly reduce its signaling (15). Together, this finding is consistent with the hypothesis that, although ΔEGFR signals continuously, it does so barely above threshold levels, and that it may be the low level of signaling that allows it to escape from attenuation by internalization.
A new molecular complex has been implicated in the internalization of tyrosine kinase receptors, including EGFR. It includes the Casitas B-lineage (Cbl) proteins, whose ubiquitination of receptors targets them for internalization and potentially degradation by the lysosome (16, 17, 18). c-Cbl, Cbl-b, and Cbl-3 share an N-terminal modified SH2 tyrosine kinase binding domain, a RING finger domain, and, in the case of c-Cbl and Cbl-b, central proline-rich and C-terminal leucine zipper domains (19, 20). Cbl proteins target activated EGFR, and, by recruiting E2 ubiquitin ligases by means of their RING finger, cause them to be polyubiquitinated and down-regulated (21–23). Another component of this complex is the adaptor molecule SETA/CIN85/Ruk, identified variously as associated with malignant transformation of astrocytes SH3-domain encoding, expressed in tumorigenic astrocytes (SETA) (24), a binding partner for c-Cbl, Cbl-interacting protein of 85 kDa (CIN85) (25, 26), or as a binding partner of p85α and a negative regulator of phosphatidylinositol 3-kinase (PI3K), and regulator of ubiquitous kinase (Ruk) (27). SETA/CIN85/Ruk proteins exist in several isoforms (27–29), the longest of which encodes three SH3 domains in the N-terminal half, a central proline-rich region, and a C-terminal coiled–coil domain involved in multimerization (29, 30).
Ligand activation of EGFR leads to the binding and phosphorylation of c-Cbl or Cbl-b proteins, and the recruitment of SETA/CIN85 by binding of its SH3 domains to the C terminus of the Cbls (25, 26, 31). SETA/CIN85 is monoubiquitinated at lysines in its C terminus, whereas the EGFR is polyubiquitinated (26, 31, 32). Endophilins are recruited by virtue of constitutive interaction with SETA/CIN85, which is mediated by the endophilin SH3 domain and the SETA/CIN85 C terminus (26, 31). The internalization and polyubiquitination of the EGFR can be mechanistically separated, and the interaction of the SETA/CIN85-Cbl complex with the receptor is primarily involved in internalization into clathrin-coated vesicles, whereas the ubiquitination state may regulate subsequent sorting into recycling or degradation pathways (26, 31). SETA also has a growing list of interactions with signaling molecules, regulators of the cytoskeleton, and modulators of apoptosis, including Crk-I, Crk-II, p130(Cas), Grb2, Sos1, and apoptosis-linked gene-2-interacting protein 1 (AIP1) (29–33), suggesting that it represents a point of crosstalk between different signaling pathways, which prompted an investigation of whether cellular state could alter the functioning of the SETA–Cbl–EGFR complex. That SETA/CIN85-Cbl complexes were constitutively associated with tyrosine kinase receptors that were continuously active, including autocrine stimulation of platelet-derived growth factor receptor (PDGFR), activation by overexpression of EGFR in A431 cells and activating mutations in c-Kit (31) prompted the investigation as to whether these proteins interacted with ΔEGFR.
Materials and Methods
Transfections were performed with SETA123cc and lacZ in pcDNA 6 (Invitrogen; refs. 29 and 33); EGFR and ΔEGFR (13) in 1726zeoG retrovirus, a derivative of 1726zeo (33) with a Gateway (Invitrogen) cassette in the unique EcoRI site; Cbl-b with a C-terminal hemagglutinin (HA)-tag, c-Cbl, and Cbl-3 with an N-terminal HA-tag in pCEFL (Cbl constructs provided by Stan Lipkowitz, National Cancer Institute, National Institutes of Health, Bethesda; refs. 22 and 34); HA-tagged polyubiquitin in pMT123 (provided by Dirk Bohmann, University of Rochester, Rochester, NY; ref. 35); and HA-tagged endophilin A1 in pcDNA3 (from Ivan Dikic, Ludwig Institute for Cancer Research, Uppsala).
For Western blot analysis, we used the following: goat anti-Cbl-3 (N-19); rabbit anti-c-Cbl (C15); anti-Cbl-b (H-121); anti-HA (F-7) (Santa Cruz Biotechnology); monoclonal anti-EGFR against the extracellular domain (Ab-1, Oncogene Science) and (Ab-11, Labvision NeoMarkers, Fremont, CA); goat and rabbit anti-EGF receptor against the intracellular domain (Santa Cruz Biotechnology, catalog no. 1005); monoclonal anti-ΔEGFR 806 (36); rabbit antiubiquitin (DAKO); mouse (4G10) and rabbit antiphosphotyrosine (Upstate Biotechnology, Lake Placid, NY); and anti-SETA Abs (33).
Human embryonic kidney (HEK)293 and Chinese hamster ovary (CHO)-K1 cells were cultured under standard conditions in DMEM or F12K medium (Kaighn's modification), respectively, with antibiotics and 10% FCS, and were transfected by a calcium-phosphate procedure. The day before transfection, either 2 million CHO cells or 2 or 5 million HEK293 cells were plated per 10-cm dish to examine nonconfluent or confluent cells, respectively, after 2 days. For EGF-induction experiments, 5 million HEK293 cells were transfected and serum-deprived for 20 h, beginning with the next day, and were then incubated with 100 ng/ml recombinant human EGF (Sigma) in serum-free medium. HEK293 cells were used in most experiments because they express endogenous EGFR, and so are likely to contain all of the proteins relevant to this receptor's signaling complex. CHO cells were used in receptor-internalization studies because HEK293 cells are poorly adherent, and were transfected with EGFR, and after 24 h, were incubated for 1 h with AG1478 EGFR kinase inhibitor, and were then serum-deprived for 30 min at 4°C in F12K medium plus 0.1% BSA and 10 mM Hepes. Cells were then incubated in medium containing 5 nM EGF and 1 nM 125I-EGF for 1 h on ice. After washing, internalization was initiated at 37°C and stopped by transfer to wet ice. Cells were washed with PBS plus 0.1% BSA or stripped of surface EGF with PBS (pH 3.4) plus 0.1% BSA on ice for 5 min, and were washed, lysed, and analyzed in a γ-counter. The percentage of signal from stripped cells vs. nonstripped cells was calculated from triplicate data points and expressed as the percentage of internalized EGFR. Alternatively, EGFR-transfected CHO cells were incubated for 1 h with AG1478, serum-starved, and were either left nonstimulated or were incubated with 50 ng/ml EGF at 37°C for the indicated times. Cells underwent an acid wash with PBS plus 0.1% BSA (pH 3.4) to remove surface EGF, and were incubated for 1.5 h with 125I-EGF at 4°C. The amount of surface-bound 125I-EGF was determined by a γ-counter and expressed as the proportion of EGFR remaining at the cell surface.
For immunoprecipitations (IPs) Cells were washed twice with ice-cold PBS and lysed on ice for 30 min in a modified radio-immunoprecipitation assay buffer [50 mM Hepes pH 7.5, 150 mM NaCl/1% Igepal CA-630/0.5% deoxycholate/0.1% SDS/5 mM EDTA/1 mM EGTA/1 mM DTT/4 mM sodium azide/1 mM PMSF/5 mM benzamidine with protease inhibitors (2 μg/ml aprotinin and leupeptin, 10 μg/ml E-64 and trypsin inhibitor, 1 μg/ml pepstatin A) and phosphatase inhibitors (2 mM sodium vanadat, 2 nM sodium fluoride, 5 mM sodium molybdate, and 15 mM p-nitrophenylphosphate)]. To measure polyubiquitination, 50 μM MG132 was added to inhibit proteasomal degradation. After lysis, the cell suspension was sheared through 18G1[1/2] and IM1 needles, incubated on ice for 30 min, cleared by centrifugation at 12,000 × g at 4°C, and used for IPs. Primary Ab was added and the sample was rotated at 4°C for at least 1 h. In case of ΔEGFR immunoprecipitations, wild-type EGFR was removed by EGFR Ab (Ab-1, Oncogene) IP before IP with mAb806. Ab–protein complexes were precipitated with 50 μl of Protein A-Agarose solution (Roche) by rotation at 4°C overnight, collected by centrifugation at 12,000 × g for 5 min at 4°C, washed seven times with precipitation buffer on ice, and analyzed by Western blotting.
For Western blotting, samples were separated by SDS/PAGE using NuPAGE 1-mm 4% to 12% or 10% Bis-Tris gels (Invitrogen), blotted to PVDF and incubated for 1 h in blocking buffer (5% BSA and 1% Tween 20 in TBS) followed by primary Ab, washed and incubated for 1 h with alkaline-phosphatase conjugated secondary Ab in blocking buffer (Sigma; dilutions: antimouse Ab 1:3,000, anti-rabbit Ab 1:5,000, and anti-goat Ab 1:15,000). After additional washing steps, Ab was visualized by Immun-Star AP substrate (Bio-Rad).
Results
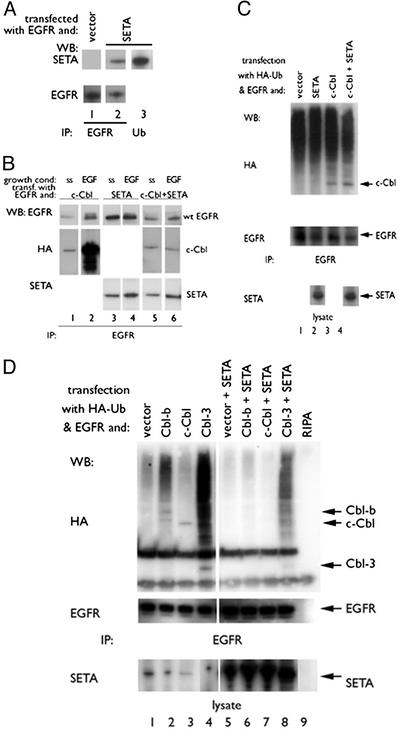
SETA Can Modulate the Polyubiquitination of EGFR by Cbls. Cotransfection of SETA and EGFR into HEK293 cells followed by IP showed that SETA was associated with EGFR (Fig. 1, lane 2). SETA was also present in an IP prepared with antiubiquitin Abs (Fig. 1 A, lane 3). However, neither IP of SETA and antiubiquitin Western blot, nor cotransfection of an HA-tagged polyubiquitin provided evidence that SETA itself is polyubiquitinated (data not shown). We did not investigate the monoubiquitination of SETA, because it has been established that the protein is modified in this way (32, 37).
Fig. 1.
SETA regulates the Cbl–EGFR interaction. (A) In HEK293 cells, EGFR IP (lanes 1 and 2) identified SETA as part of the EGFR complex (lane 2). Antipolyubiquitin IPs contained SETA (lane 3). (B) In HEK293, cells were allowed to reach confluency, serum-starved (ss), and were then exposed to 100 ng/ml EGF for 5 min (EGF). c-Cbl proteins and SETA could be seen to move to the EGFR on EGF stimulation (lanes 1–4). Cotransfection of SETA abrogated the increased association of c-Cbl with the EGFR after stimulation (lanes 5 and 6). (C) In subconfluent HEK 293 cells, analysis of EGFR IP showed that the presence of c-Cbl and the levels of EGFR polyubiquitination, detected as a high-molecular-weight smear, were unaffected by the presence of SETA. (D). In contrast, in confluent HEK293 cells, analysis of EGFR IPs showed that SETA and Cbl proteins were present, and that SETA reduced the amount of EGFR-associated Cbls (compare lanes 2–4 with lanes 6–8), as well as EGFR polyubiquitination.
Previous reports had shown that EGF stimulation resulted in an increased association of SETA with the EGFR (26) and c-Cbl with SETA (25, 26). To test whether EGFR stimulation leads to an increase in a complex of all three proteins, EGFR, c-Cbl, and SETA were transfected into HEK293 cells, which were allowed to reach confluency (Fig. 1B). EGF stimulation led to a dramatic increase in c-Cbl associated with the EGFR when c-Cbl was transfected without SETA (Fig. 1B, lanes 1 and 2). Transfection of SETA without c-Cbl led to a more moderate but detectable increase in SETA associated with the active EGFR (Fig. 1B, lanes 3 and 4). Interestingly, when SETA and c-Cbl were cotransfected, the increase in EGFR-associated c-Cbl in response to EGF was abrogated, whereas the modest increase in SETA remained (Fig. 1B, lanes 5 and 6). Importantly, this effect was not observed when cells were subconfluent (not shown), suggesting that cell density directly influences this complex.
The possibility that SETA also modulates the polyubiquitination of the EGFR by Cbls was tested by cotransfecting HA-tagged polyubiquitin. In nonconfluent HEK293 cells, Cbls and EGFR coexisted in a complex, and EGFR was polyubiquitinated by Cbl proteins independent of transfected SETA (Fig. 1C; ref. 26). Interestingly, in HEK293 cells that reached confluency, a marked effect of SETA on Cbl-mediated EGFR polyubiquitination was observed. These cells had a lower level of background polyubiquitination than nonconfluent cells, and so a greater increase in polyubiquitination was obtained when Cbls were transfected. Cbl-b, Cbl-3, and to a much smaller degree, c-Cbl, which were HA-tagged and so appear on the HA Western blot, increased EGFR polyubiquitination (Fig. 1D, lanes 2–4) as expected (26). This polyubiquitination was detected as a HA-positive smear created by the stepwise addition of ubiquitin, and showed that Cbl-3 had the greatest effect and c-Cbl the had weakest effect. Cotransfection of SETA reduced the level of c-Cbl and Cbl-b proteins found in the EGFR immunoprecipitates (Fig. 1D, lanes 6 and 7), suggesting that SETA interfered in Cbl binding to EGFR in confluent HEK293 cells, in support of Fig. 1B. SETA also reduced c-Cbl- or Cbl-b-mediated EGFR polyubiquitination (Fig. 1D, lanes 6 and 7). In the case of Cbl-3, which does not interact with SETA/CIN85 (31), detectable levels of protein and persistent levels of polyubiquitination were detected when SETA was cotransfected (Fig. 1D, lane 8). Therefore, SETA may primarily be affecting the recruitment of c-Cbl and Cbl-b proteins to the EGFR in confluent cells, rather than Cbl activity. Cellular growth status has a significant impact on the interaction of these proteins in that increased levels of SETA in confluent cells reduces EGFR-associated Cbl proteins and, consequently, the polyubiquitination of EGFR.
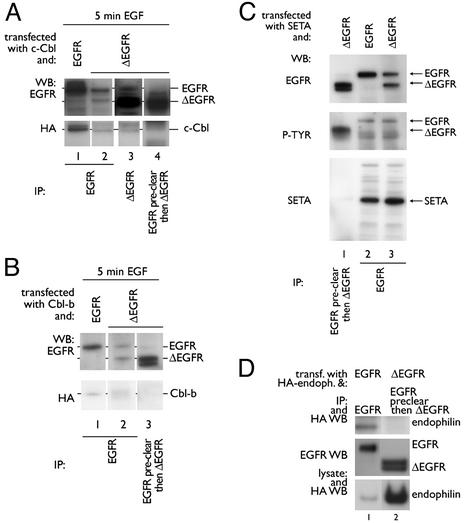
ΔEGFR Does Not Associate with c-Cbl, SETA, or Endophilin and Is Not Polyubiquitinated by Cbl Proteins. To analyze the proteins associated with the ΔEGFR in cells that also express endogenous EGFR, and so are likely to have the relevant intracellular signaling proteins, and, as is usually the case in gliomas, we chose to work with the HEK293 cells, using the mAb 806 (36). This Ab preferentially recognizes the ΔEGFR, but crossreactivity with EGFR has been reported, particularly when EGFR is highly expressed, because it recognizes the activity-dependent conformation, rather than the neoepitope of ΔEGFR (36, 38, 39). Accordingly, EGFR was observed in mAb 806 IPs (Fig. 2A, lane 3). Furthermore, when HEK293 cells were transfected with ΔEGFR, both ΔEGFR and EGFR were recovered in IPs made with an EGFR Ab against the extracellular region (Ab-1, Oncogene Science; lane 2) even though the two receptors differ in their ligand-binding domain. Ab crossreactivity is the best explanation for this result, because no detectable crossphosphorylation and, so, presumably no crossdimerization between ΔEGFR and wild-type EGFR has been observed (13). To reduce the level of EGFR, lysates were precleared by IP with an EGFR Ab (Ab-1 Oncogene Science), followed by IP with mAb806, which resulted in predominant recovery of ΔEGFR (lane 4), and this product will be referred to as ΔEGFR-specific IP. By using this approach, no interaction between c-Cbl and ΔEGFR was observed in that no c-Cbl was present when only ΔEGFR was recovered (lane 4), whereas c-Cbl was clearly present in any IP that contained EGFR, and to a degree that reflected the amount of EGFR, but not ΔEGFR, recovered (lanes 1–3). Similarly, Cbl-b was found to be associated with the EGFR, but not with ΔEGFR (Fig. 2B). These experiments were performed after serum-starvation and triggering of the transfected cells for 5 min with EGF because this resulted in the highest level of EGFR-c-Cbl interaction (Fig. 1B; ref. 26). SETA was also not found in ΔEGFR-specific IP (Fig. 2C, lane 1), whereas it was recovered in EGFR complexes (Fig. 2C, lanes 2 and 3). Antiphosphotyrosine Western blotting showed that it was not the absence of activation of the ΔEGFR that prevented interaction with SETA. To test whether the lack of interaction of ΔEGFR with SETA and Cbl proteins also extended to endophilins, HA-tagged endophilin A1 was cotransfected and EGFR or ΔEGFR-specific immunoprecipitates were prepared. Although endophilin A1 was readily detected in EGFR complexes, it did not associate with ΔEGFR (Fig. 2D). Taken together, these data suggest that although active, ΔEGFR does not interact with SETA, Cbl, or endophilin A1.
Fig. 2.
ΔEGFR does not interact with c-Cbl, Cbl-b, SETA, or endophilin. (A) HEK293 cells were transfected with c-Cbl and EGFR (lane 1) or ΔEGFR (lanes 2–4), serum-starved overnight, and incubated with 100 ng/ml EGF for 5 min. EGFR IP (Ab-1, Oncogene Science) revealed one band corresponding to wild-type EGFR (lane 1), or two bands corresponding to EGFR and ΔEGFR when cells were transfected with ΔEGFR (lane 2). When a mAb 806 IP was prepared from cells transfected with ΔEGFR, two bands were obtained, with the higher, less-intense band corresponding to wild-type EGFR (lane 3). When anti-EGFR preclearing preceded mAb806 IP, only one band corresponding to ΔEGFR was obtained (lane 4). c-Cbl was found in IPs when EGFR, but not ΔEGFR, was present (lanes 1–4). (B) Similar experiments with HEK293 cells transfected with Cbl-b showed Cbl-b in wild-type but not ΔEGFR-specific IPs (lanes 1–3). (C) Similar studies in cells grown in the continuous presence of serum demonstrated that SETA was found in IPs from HEK293 cells that contained wild-type EGFR (lanes 2 and 3), but not ΔEGFR alone (lane 1). A phosphotyrosine blot verified that receptors were activated. Please note that IPs in lanes 1 and 3 were from the same lysates, and so SETA protein was expressed in the sample from which lane 1 was prepared. (D) Although expression levels of endophilin A1 were higher when it was cotransfected with ΔEGFR than with EGFR (compare lanes 1 and 2), it was not present in ΔEGFR-specific IP (lane 2), but was present in EGFR IP (lane 1) as expected. Differences in endophilin A1 levels may relate to its higher turnover when EGFR is cotransfected.
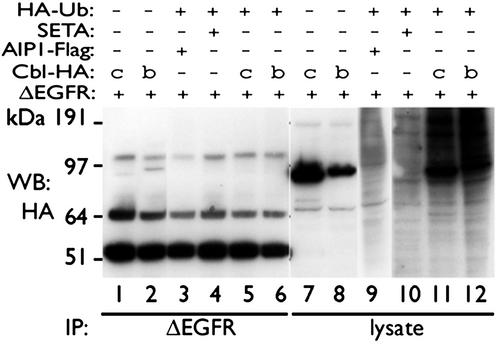
To test whether this lack of interaction between ΔEGFR and the Cbl–SETA–endophilin complex had functional consequences, the level of polyubiquitination of ΔEGFR was examined in HEK293 cells. Cbls (Fig. 3, lanes 7, 8, 11, and 12), but not SETA or the SETA-binding protein AIP1 (lanes 9 and 10), elevated total protein polyubiquitination (indicated by the black smear in the cell lysates in lanes 11 and 12). However, polyubiquitination of ΔEGFR could not be detected in any condition in ΔEGFR-specific IP (lanes 1–6), suggesting that it is also uncoupled from the Cbl protein-mediated polyubiquitination pathway and that this may contribute to its lack of internalization and degradation by the proteasome.
Fig. 3.
ΔEGFR is not polyubiquitinated. ΔEGFR and c-Cbl (lanes 1, 5, 7, and 11), Cbl-b (lanes 2, 6, 8, and 12), AIP1 (lanes 3 and 9), and SETA (lanes 4 and 10), with or without HA-tagged polyubiquitin (HA-Ub), were cotransfected into HEK293 cells. ΔEGFR-specific IPs as described in Fig. 2, and the lysates from which they were derived, were subjected to HA Western blotting. c-Cbl or Cbl-b increased polyubiquitination of proteins in the lysates, which was detected as an HA-reactive high-molecular-weight smear when ub-HA was cotransfected (compare lanes 11 and 12 with lanes 9 and 10 with lanes 7 and 8). No smear could be detected in the corresponding ΔEGFR immunoprecipitates (lanes 5 and 6). SETA or its binding partner AIP1, which is Flag-tagged, did not mediate polyubiquitination of any proteins on their own and serve as controls.
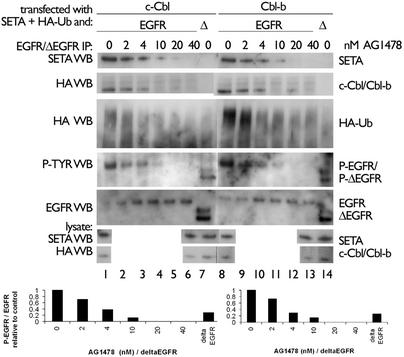
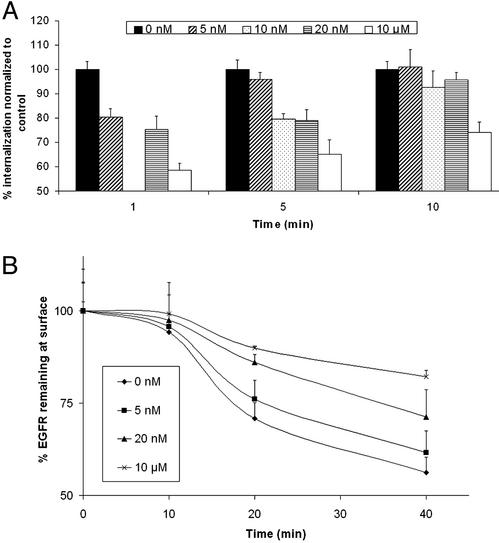
ΔEGFR signals persistently, but at lower levels of autophosphorylation than EGFR (13), a possible reason that it does not interact with Cbls, SETA, or endophilin. To test whether reducing EGFR activity would also lead to changes in internalization and protein associations, we transfected CHO cells with the EGFR, then treated them with the kinase inhibitor AG1478, and examined the complexes that were formed as well as internalization of radiolabeled EGF. These experiments could not be carried out in HEK293 cells because they are not adherent enough to allow for the washing steps required to measure receptor internalization. AG1478 caused a stepwise reduction in the level of EGFR tyrosine phosphorylation, with 4 nM AG1478 reducing EGFR to levels similar to what was observed for the untreated ΔEGFR, measured ≈20% of untreated EGFR (Fig. 4). Concomitant with this reduction was a loss of association with SETA and c-Cbl or Cbl-b, suggesting that a reduction in EGFR kinase activity resulted in a reduction in interaction with these proteins (Fig. 4). This outcome had functional consequences in that the level of EGFR polyubiquitination was also reduced. Examination of Fig. 4 shows that each of these activities appears to have a threshold between 4 and 10 nM AG1478, suggesting that they are coregulated. Furthermore, the activity level of the ΔEGFR lies very close to this threshold of activity, suggesting that its lack of association with SETA and Cbls, and lack of polyubiquitination, is related to its lower level of tyrosine phosphorylation. Treatment with AG1478 also caused a step-wise, dose-dependent reduction in EGFR internalization, demonstrating that the changes in activity and complex formation observed in Fig. 4 had functional consequences (Fig. 5). This reduction in receptor internalization was detectable both in the amount of radiolabeled EGF ligand that was internalized at a given point after stimulation with EGF (Fig. 5A), and in the proportion of EGF-binding sites that remained available on the cell surface at different times after stimulation with EGF (Fig. 5B).
Fig. 4.
Inhibition of EGFR reduces phosphorylation, association with SETA and Cbl, and polyubiquitination. CHO cells were treated with AG1478, and, if transfected with EGFR, were also stimulated with EGF for 5 min. EGFR or ΔEGFR-specific IPs were prepared as appropriate and showed that, with increased dosage of AG1478, the level of phosphotyrosine on EGFR was reduced, as was its binding to SETA and Cbls. In addition, polyubiquitination of the EGFR, visible as an HA-positive smear, was suppressed by AG1478. (Lower) Quantification of the phosphotyrosine signal relative to the EGFR signal is shown, with the values for untreated EGFR being set arbitrarily to 1 in each case. The phosphorylation level of ΔEGFR is similar to that of EGFR from cells treated with 4 nM AG1478. This concentration is the start of the threshold at which levels of SETA and Cbl proteins in EGFR complexes declined.
Fig. 5.
Inhibition of EGFR by AG1478 reduces its internalization. (A) The percentage EGFR internalization was measured in CHO cells, transfected with EGFR, and treated with AG1478, using radiolabeled EGF. Treatment with 5–10 nM AG1478 results in a decline in the amount of EGFR internalization. (B) The relative remaining EGFR on the surface of CHO cells, transfected with EGFR and treated with the indicated concentration of AG1478, was measured after exposure to EGF for the times indicated, using radiolabeled EGF. The values are expressed as a percentage of control cells that were not exposed to EGF. Treatment with AG1478 inhibited the decline in EGFR that was present at the cell surface over time.
Discussion
SETA is an adaptor that interacts with signaling molecules, including positive regulators such as PI3K (27) and negative regulators such as the Cbls (25, 26, 29). The implication of SETA/CIN85 in the internalization and down-regulation of tyrosine kinase receptors, including EGFR (26), and the observation that it is associated with constitutively activated receptors in transformed cells (31), prompted this investigation. EGFR signaling, in particular through the mutated ΔEGFR, plays an important role in glioma formation and progression (10, 11, 13).
EGFR stimulation leads to the association between c-Cbl or Cbl-b and SETA/CIN85, and this association depends on the phosphorylation of the Cbls (25, 26, 31). We investigated the association of Cbl and SETA in combination with EGFR. In confluent HEK293 cells, EGF mediated a dramatic increase of EGFR-associated c-Cbl, and a more modest increase in SETA, when these proteins were tested individually (Fig. 1), which was in line with previous studies (26). However, rather than a c-Cbl-mediated stimulation of SETA association with EGFR (26), we observed that SETA could prevent the increase in c-Cbl binding to the activated EGFR when the two proteins were coexpressed, but only in confluent cells. SETA could be shortening the association between the SETA/CIN85-Cbl complex and EGFR, which otherwise can persist for at least 30 min (31), or competing with EGFR for binding to Cbls. The impact of SETA on EGFR-Cbl association had functional consequences, in that it suppressed EGFR polyubiquitination (Fig. 1D), which was not previously observed (26, 31). It should be emphasized that this phenomenon was not observed in nonconfluent cells, suggesting density, perhaps related to the disposition of the cytoskeleton with which SETA interacts (33, 40), may play an important role in modulating these protein interactions, and also why other studies did not make similar findings.
The association of SETA/CIN85 and Cbl proteins with tyrosine kinase receptors depends on receptor activation (25, 26, 31). In normal cells this process requires exogenous ligand, whereas in many transformed cells, tyrosine kinase receptors are aberrantly active on a continuous basis. In the case of cells with an autocrine platelet-derived growth factor (PDGF)–PDGFR loop, A431 cells with very high levels of EGFR and cells harboring activating mutations in c-Kit this combination leads to constitutive association with SETA/CIN85-Cbl complexes (31). Here we examined whether this was also the case for the ΔEGFR, a common ligand-independent and constitutively active deletion mutant in brain cancer. Immunoprecipitation of ΔEGFR with mAb 806, after preclearing of lysates with an anti-EGFR Ab, showed no association of ΔEGFR with c-Cbl, Cbl-b, SETA, or endophilin, despite the fact that it showed tyrosine phosphorylation (Figs. 2 and 4). In support of this observation, and in agreement with the observation that ΔEGFR is not internalized (13), we also did not observe any polyubiquitination of ΔEGFR (Fig. 4). It should be noted that our transfection experiments were not able to model the extreme levels of ΔEGFR overexpression encountered in glioblastomas with genomic amplification of this mutant, and so leave open the possibility that interaction between this receptor, SETA/CIN85, and Cbls occurs under such circumstances.
There is evidence that the signal emanating from ΔEGFR is lower in intensity but similar in nature to that of wild-type EGFR (13, 14, 15). To test whether EGFR signal intensity, measured by autophosphorylation levels, was related to association with SETA and Cbls and internalization, we attenuated its signaling with the inhibitor AG1478. When EGFR tyrosine phosphorylation levels were reduced to ≈20% of untreated control values, similar to what was observed for the uninhibited ΔEGFR, binding of SETA and Cbl proteins was lost and EGFR polyubiquitination was reduced to background levels (Fig. 4). Treatment of cells with the same concentrations of AG1478 that caused a reduction in interactions (4–10 nM) also resulted in a measurable decrease in EGFR internalization, measured both in terms of internalized EGF and in terms of remaining EGF-binding sites (Fig. 5). Taken together, this finding suggests that there is an EGFR-activity threshold below which association with Cbl proteins and SETA is weak and receptor internalization is inefficient. Furthermore, the constitutive signal emanating from the ΔEGFR appears to fall below this threshold. Therefore, it appears that in some cases, constitutive association with SETA and Cbl proteins does not accompany sustained signaling through tyrosine kinase receptors. Together, these data support the hypothesis that receptors need to exceed a threshold in signal intensity before they attract the proteins that cause their internalization, explaining why ΔEGFR can be constitutively active but not internalized, and suggest that its low level of signal intensity may contribute to its promotion of tumorigenicity by permitting it to evade negative feedback regulation.
Acknowledgments
We thank Drs. Ivan Dikic, Dirk Bohmann, and Stan Lipkowitz for constructs. This work was supported by National Cancer Institute Grant CA-R01-84109 (to O.B.), James S. McDonnell Foundation Grant 98-62 BC-GLO.05 (to O.B.), and by the Hermelin Brain Tumor Center donors, with particular thanks to William and Karen Davidson.
Abbreviations: Cbl, Casitas B-lineage; EGF, epidermal growth factor; EGFR, EGF receptor; ΔEGFR, EGFR with exons 2–7 deleted; SETA, SH3-domain encoding, expressed in tumorigenic astrocytes; CIN85, Cbl-interacting protein of 85 kDa; HEK, human embryonic kidney; CHO, Chinese hamster ovary; IP, immunoprecipitation; HA, hemagglutinin.
References
- 1.Wong, A. J., Bigner, S. H., Bigner, D. D., Kinzler, K. W., Hamilton, S. R. & Vogelstein, B. (1987) Proc. Natl. Acad. Sci. USA 84, 6899–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libermann, T. A., Nusbaum, H. R., Razon, N., Kris, R., Lax, I., Soreq, H., Whittle, N., Waterfield, M. D., Ullrich, A. & Schlessinger, J. (1985) Nature 313, 144–147. [DOI] [PubMed] [Google Scholar]
- 3.Ekstrand, A. J., Sugawa, N., James, C. D. & Collins, V. P. (1992) Proc. Natl. Acad. Sci. USA 89, 4309–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlegel, J., Merdes, A., Stumm, G., Albert, F. K., Forsting, M., Hynes, N. & Kiessling, M. (1994) Int. J. Cancer 56, 72–77. [DOI] [PubMed] [Google Scholar]
- 5.Jaros, E., Perry, R. H., Adam, L., Kelly, P. J., Crawford, P. J., Kalbag, R. M., Mendelow, A. D., Sengupta, R. P. & Pearson, A. D. (1992) Br. J. Cancer 66, 373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurtt, M. R., Moossy, J., Donovan-Peluso, M. & Locker, J. (1992) J. Neuropathol. Exp. Neurol. 51, 84–90. [DOI] [PubMed] [Google Scholar]
- 7.Sugawa, N., Ekstrand, A. J., James, C. D. & Collins, V. P. (1990) Proc. Natl. Acad. Sci. USA 87, 8602–8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphrey, P. A., Wong, A. J., Vogelstein, B., Zalutsky, M. R., Fuller, G. N., Archer, G. E., Friedman, H. S., Kwatra, M. M., Bigner, S. H. & Bigner, D. D. (1990) Proc. Natl. Acad. Sci. USA 87, 4207–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong, A. J., Ruppert, J. M., Bigner, S. H., Grzeschik, C. H., Humphrey, P. A., Bigner, D. S. & Vogelstein, B. (1992) Proc. Natl. Acad. Sci. USA 89, 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishikawa, R., Ji, X. D., Harmon, R. C., Lazar, C. S., Gill, G. N., Cavenee, W. K. & Huang, H. J. (1994) Proc. Natl. Acad. Sci. USA 91, 7727–7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagane, M., Coufal, F., Lin, H., Bogler, O., Cavenee, W. K. & Huang, H. J. (1996) Cancer Res. 56, 5079–5086. [PubMed] [Google Scholar]
- 12.Lal, A., Glazer, C. A., Martinson, H. M., Friedman, H. S., Archer, G. E., Sampson, J. H. & Riggins, G. J. (2002) Cancer Res. 62, 3335–3339. [PubMed] [Google Scholar]
- 13.Huang, H. S., Nagane, M., Klingbeil, C. K., Lin, H., Nishikawa, R., Ji, X. D., Huang, C. M., Gill, G. N., Wiley, H. S. & Cavenee, W. K. (1997) J. Biol. Chem. 272, 2927–2935. [DOI] [PubMed] [Google Scholar]
- 14.Downward, J., Parker, P. & Waterfield, M. D. (1984) Nature 311, 483–485. [DOI] [PubMed] [Google Scholar]
- 15.Helin, K., Velu, T., Martin, P., Vass, W. C., Allevato, G., Lowy, D. R. & Beguinot, L. (1991) Oncogene 6, 825–832. [PubMed] [Google Scholar]
- 16.Levkowitz, G., Waterman, H., Zamir, E., Kam, Z., Oved, S., Langdon, W. Y., Beguinot, L., Geiger, B. & Yarden, Y. (1998) Genes Dev. 12, 3663–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyake, S., Mullane-Robinson, K. P., Lill, N. L., Douillard, P. & Band, H. (1999) J. Biol. Chem. 274, 16619–16628. [DOI] [PubMed] [Google Scholar]
- 18.Lee, P. S., Wang, Y., Dominguez, M. G., Yeung, Y. G., Murphy, M. A., Bowtell, D. D. & Stanley, E. R. (1999) EMBO J. 18, 3616–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thien, C. B. & Langdon, W. Y. (1998) Immunol. Cell Biol. 76, 473–482. [DOI] [PubMed] [Google Scholar]
- 20.Thien, C. B. & Langdon, W. Y. (2001) Nat. Rev. Mol. Cell Biol. 2, 294–307. [DOI] [PubMed] [Google Scholar]
- 21.Levkowitz, G., Waterman, H., Ettenberg, S. A., Katz, M., Tsygankov, A. Y., Alroy, I., Lavi, S., Iwai, K., Reiss, Y., Ciechanover, A., et al. (1999) Mol. Cell 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- 22.Ettenberg, S. A., Keane, M. M., Nau, M. M., Frankel, M., Wang, L. M., Pierce, J. H. & Lipkowitz, S. (1999) Oncogene 18, 1855–1866. [DOI] [PubMed] [Google Scholar]
- 23.Ettenberg, S. A., Rubinstein, Y. R., Banerjee, P., Nau, M. M., Keane, M. M. & Lipkowitz, S. (1999) Mol. Cell. Biol. Res. Commun. 2, 111–118. [DOI] [PubMed] [Google Scholar]
- 24.Bogler, O., Furnari, F. B., Kindler-Roehrborn, A., Sykes, V. W., Yung, R., Huang, H.-J. S. & Cavenee, W. K. (2000) Neuro-Oncol. 2, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Take, H., Watanabe, S., Takeda, K., Yu, Z. X., Iwata, N. & Kajigaya, S. (2000) Biochem. Biophys. Res. Commun. 268, 321–328. [DOI] [PubMed] [Google Scholar]
- 26.Soubeyran, P., Kowanetz, K., Szymkiewicz, I., Langdon, W. Y. & Dikic, I. (2002) Nature 416, 183–187. [DOI] [PubMed] [Google Scholar]
- 27.Gout, I., Middleton, G., Adu, J., Ninkina, N. N., Drobot, L. B., Filonenko, V., Matsuka, G., Davies, A. M., Waterfield, M. & Buchman, V. L. (2000) EMBO J. 19, 4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchman, V., Luke, C., Borthwick, E., Gout, I. & Ninkina, N. (2002) Gene 295, 13–17. [DOI] [PubMed] [Google Scholar]
- 29.Borinstein, S. C., Hyatt, M. A., Sykes, V. W., Straub, R. E., Lipkowitz, S., Boulter, J. & Bogler, O. (2000) Cell. Signalling 12, 769–779. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe, S., Take, H., Takeda, K., Yu, Z. X., Iwata, N. & Kajigaya, S. (2000) Biochem. Biophys. Res. Commun. 278, 167–174. [DOI] [PubMed] [Google Scholar]
- 31.Szymkiewicz, I., Kowanetz, K., Soubeyran, P., Dinarina, A., Lipkowitz, S. & Dikic, I. (2002) J. Biol. Chem. 277, 39666–39672. [DOI] [PubMed] [Google Scholar]
- 32.Haglund, K., Shimokawa, N., Szymkiewicz, I. & Dikic, I. (2002) Proc. Natl. Acad. Sci. USA 99, 12191–12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen, B., Borinstein, S. C., Gillis, J., Sykes, V. W. & Bogler, O. (2000) J. Biol. Chem. 275, 19275–19281. [DOI] [PubMed] [Google Scholar]
- 34.Keane, M. M., Ettenberg, S. A., Nau, M. M., Banerjee, P., Cuello, M., Penninger, J. & Lipkowitz, S. (1999) Oncogene 18, 3365–3375. [DOI] [PubMed] [Google Scholar]
- 35.Treier, M., Staszewski, L. M. & Bohmann, D. (1994) Cell 78, 787–798. [DOI] [PubMed] [Google Scholar]
- 36.Johns, T. G., Stockert, E., Ritter, G., Jungbluth, A. A., Huang, H. J., Cavenee, W. K., Smyth, F. E., Hall, C. M., Watson, N., Nice, E. C., et al. (2002) Int. J. Cancer 98, 398–408. [DOI] [PubMed] [Google Scholar]
- 37.Verdier, F., Valovka, T., Zhyvoloup, A., Drobot, L. B., Buchman, V., Waterfield, M. & Gout, I. (2002) Eur. J. Biochem. 269, 3402–3408. [DOI] [PubMed] [Google Scholar]
- 38.Mishima, K., Johns, T. G., Luwor, R. B., Scott, A. M., Stockert, E., Jungbluth, A. A., Ji, X. D., Suvarna, P., Voland, J. R., Old, L. J., et al. (2001) Cancer Res. 61, 5349–5354. [PubMed] [Google Scholar]
- 39.Luwor, R. B., Johns, T. G., Murone, C., Huang, H. J., Cavenee, W. K., Ritter, G., Old, L. J., Burgess, A. W. & Scott, A. M. (2001) Cancer Res. 61, 5355–5361. [PubMed] [Google Scholar]
- 40.Schmidt, M. H. H., Chen, B., Randazzo, L. & Bogler, O. (2003) J. Cell Sci., in press. [DOI] [PubMed]