Abstract
We have established and studied a colony of mice with a unique trait of host resistance to both ascites and solid cancers induced by transplantable cells. One dramatic manifestation of this trait is age-dependent spontaneous regression of advanced cancers. This powerful resistance segregates as a single-locus dominant trait, is independent of tumor burden, and is effective against cell lines from multiple types of cancer. During spontaneous regression or immediately after exposure, cancer cells provoke a massive infiltration of host leukocytes, which form aggregates and rosettes with tumor cells. The cytolytic destruction of cancer cells by innate leukocytes is rapid and specific without apparent damage to normal cells. The mice are healthy and cancer-free and have a normal life span. These observations suggest a previously unrecognized mechanism of immune surveillance, which may have potential for therapy or prevention of cancer.
Regression of human cancers without treatment (spontaneous regression, SR) is well documented for many types of cancer, but occurs infrequently (1–5). The most intriguing implication of SR is that there might be a rare, but extremely effective, mechanism engaged to eradicate cancer cells after the development of advanced malignancy. Despite efforts over many decades, the mechanism(s) of SR in humans and animals has remained elusive.
Because of the absence of MHC, mouse S180 cells form highly aggressive cancers in all strains of laboratory mice (6, 7) and rats (8, 9). When injected into the peritoneal cavity, S180 cells grow exponentially with a generation time of 12–18 h (10). Growing primarily in suspension in the peritoneal cavity, S180 cells gradually plug peritoneal lymphatic drainage, leading to accumulation of ascites fluid within 2 weeks. S180 cells can also metastasize into major organs near the peritoneal cavity, such as liver, kidney, pancreas, lung, stomach, and intestine (Z.C. and M.C.W., unpublished data). Mice that develop ascites normally die in 3–4 weeks (10). S180-induced ascites represents one of the most aggressive transplantable cancers in experimental mouse models. Resistance to S180-induced ascites has never been reported to our knowledge.
Because of their consistent response to transplanted S180 cells, BALB/c mice have become a standard strain for ascites production. In our laboratory, one male BALB/c mouse unexpectedly remained ascites-free after repeated injections of S180 cells. We show here that this resistance [SR/complete resistance (CR)] is germ-line transmissible, and we describe the properties of this unique trait.
Materials and Methods
Cell Lines and Mouse Strains. Mouse cells were from the American Type Culture Collection. Meth A sarcoma was a generous gift from Lloyd Old (Ludwig Institute for Cancer Research, New York). Mouse cancer cells were propagated in culture according to the supplier's recommendations. BALB/c and C57BL/6 mice were from Charles River Breeding Laboratories, and CAST/Ei and athymic C57BL/6foxn1/foxn1 nude mice were from The Jackson Laboratory. Mice were housed in plastic cages covered with air filter tops, containing hardwood shavings as bedding, allowed free access to water and regular chow, and exposed to a 12-h fluorescent light/dark cycle.
Cytoprep and Histology. Hematoxylin and 4′,6-diamidino-2-phenylindole staining of peritoneal cells washed from mice were standard procedures. For immunocytochemistry of surface markers, fixed cells in cytopreps were probed with anti-CD4, anti-CD8, anti-CD11c, or anti-CD45 and F4/80, Ly6G, and NK1.1. These cells were followed by rhodamine-conjugated secondary antibodies and counted with a Zeiss Axioplan 2 fluorescence microscope.
Flow Cytometry. Cells from peritoneal washes were stained with specific antibodies to cell surface markers according to standard procedures recommended by the manufacturer and analyzed on a FACStar (BD Biosciences, Mountain View, CA). Forward scatter and side scatter gain settings were tuned to sort live cells from cell fragments.
Generation of S180 Cells with GFP Expression. The GFP-expressing vector was purchased from Invitrogen. S180 cells were transfected with the plasmid by using calcium phosphate precipitation and selected with 500 μg/ml G418. A strong GFP-expressing clone was selected by using fluorescence microscopy and maintained with culture medium containing 500 μg/ml G418.
In Vitro Assay of Tumor Cell Lysis by Infiltrating Leukocytes. To induce peritoneal infiltration of tumor-killing leukocytes, the SR/CR mice were challenged with an i.p. injection of 2 × 10e7 S180 cells 12 h before peritoneal washes. Under anesthesia, the peritoneal cavity was infused with PBS or culture medium (DMEM) via an 18-G needle attached to a syringe. The wash solution was then thoroughly retrieved with the same needle and syringe, resulting in at least 95% of volume recovery. Because the WT mice had only insignificant numbers of tumor-infiltrating leukocytes in response to the S180 challenge, splenocytes of WT mice were isolated according to standard procedures as control effector cells; these showed no killing of S180 cells under the conditions used. To detect killing by leukocytes, we initially tested assays using 51Cr release. However, the cytolytic killing by SR/CR leukocytes often required a latent period of >12 h, and the rate of 51Cr leakage out of cells was too high to be useful. To circumvent this problem, we labeled target cells with fluorescent CellTracker Orange or DiO (Molecular Probes) at 39°C for 1 h in culture medium before mixing with effector cells. Effector cells were mixed with target cells at a ratio of 50:1 and incubated for 24 h at 39°C to allow killing to occur. After incubation, the cell mixtures were also stained with Trypan blue to distinguish dead cells from live cells. Dead cells were both positive for Trypan blue staining and negative for fluorescence labeling (CellTracker or DiO). Live target cells were identified by their size, morphology, and absence of Trypan blue staining. Target cell killing was interpreted as positive if >50% of target cells were destroyed in 24 h, when compared with a control consisting of target cells without effector cells. For generation of MethA tumors, 2 × 10e6 cells were injected i.p. per mouse; these injections generated lethal ascites in both BALB/c and C57BL/6 control mice within 3 weeks.
Results
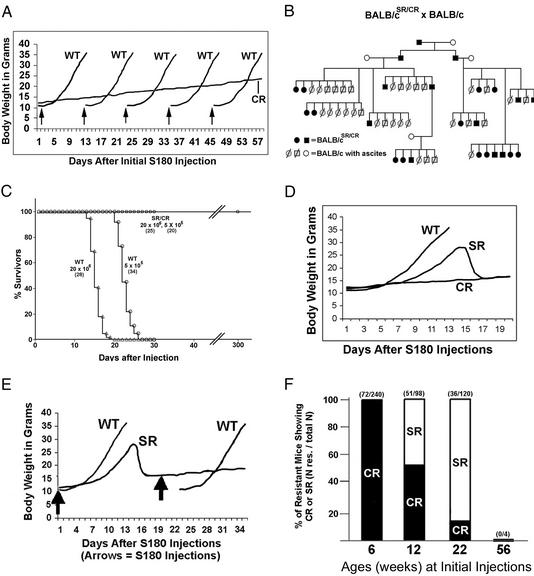
SR/CR Cancer Resistance Is Genetically Defined and Dose Independent. An S180-resistant founder mouse was initially identified within a group of BALB/c mice as a result of its failure to develop ascites upon an injection of 5 × 10e5 S180 cells. To verify that this failure was true resistance, the founder mouse was given two more injections of 2 × 10e6 S180 cells, as were control BALB/c mice, followed by two further injections of 2 × 10e7 S180 cells. No ascites developed in the founder mouse. This unique mouse remained healthy and cancer free and eventually died of old age at 26 months of age. This resistance was independent of tumor burden in the range tested (5 × 10e5 to 2 × 10e9 or up to 10% of total body weight) and independent of whether the S180 cells had been passaged in vivo or through tissue culture. Fig. 1A shows typical changes of body weights in resistant and control mice that received similar injections of S180 cells. Additional injections of cells were given at ages 6, 12, and 18 months.
Fig. 1.
Properties of the SR/CR phenotype. (A) A representative SR/CR BALB/c mouse (CR) was weighed daily for monitoring the presence of ascites after repeated injections (arrows) of S180 cells (2 × 10e6 per injection) along with control BALB/c mice (WT) for each injection. All mice were 6 weeks old at the time of the first injection. The development of ascites in control mice was determined by a rapid increase of body weight and an enlarged abdomen. (B) Pedigree analysis was performed by crossing S180-resistant BALB/c mice with S180-sensitive normal BALB/c mice. The progeny were weaned at 3 weeks and injected with 2 × 10e6 S180 cells. Males are represented by squares and females by circles. Filled squares and circles are resistant mice. Empty circles represent control mice that are sensitive to S180 cells. Slashed squares and circles are S180-sensitive progeny. (C) Survival analysis was performed by injecting either 5 × 10e5 or 2 × 10e6 S180 cells in the progeny from the cross of a CR BALB/c and an S180-sensitive C57BL/6. Fifteen litters consisting of a total of 107 mice were divided into two dosage groups. The injections were given at 6 weeks of age. The mice that developed ascites were marked as WT and ascites-free mice were marked as SR/CR. This demonstrates the lethality of S180 cells in the sensitive mice and uniform survival of the SR/CR mice. The number of mice in each group is shown in parentheses. (D) The daily body weight graph representative of an S180-sensitive mouse (WT), a mouse with CR to ascites (CR), and a mouse that underwent SR of ascites (SR) is shown. Tumor regression in the SR phenotype occurred after day 14 and was complete at day 15. (E) SR protected mice from developing ascites again upon repeated injection (second arrow) of S180 cells. Immediately after regression, one SR mouse and one control WT mouse were injected with 2 × 10e6 S180 cells. The SR mouse failed to develop ascites again. (F) The display of either the CR or the SR phenotypes depends on the age (in weeks) when the first injection of tumor cells was given. When the first injection was given at 6 weeks, 72 of 240 progeny (cross between SR/CR BALB/c and WT C57BL/6) show the CR phenotype. When the first injection was given at 12 weeks, 51 of 98 progeny were resistant, with 26 displaying the SR phenotype and 25 displaying the CR phenotype. When the first injection was given at 22 weeks, 31 of 120 progeny showed the SR phenotype and 5 displayed the CR phenotype. Four mice that had passed the resistant trait to their offspring developed ascites and died when the first injection of S180 cells was delayed until ≈56 weeks.
A breeding experiment was performed to determine whether the cancer-resistant trait was germ-line transmissible in the BALB/c background (Fig. 1B). Table 1 summarizes the results and genetic analysis from this breeding. The resistance phenotype was inherited in the F1 generation directly from crossing between resistant mice and S180-sensitive BALB/c mice, indicating that the phenotype was dominant to its WT counterpart. The overall frequency of the SR/CR phenotype from outcrossing was ≈38%. This rate suggests strongly that only one locus is involved.
Table 1. Results and genetic analysis of breeding.
| Crosses | SR/CR | Total | % |
|---|---|---|---|
| BALB/c (WT) | 0 | >50 | 0 |
| C57BL/6 (WT) | 0 | >50 | 0 |
| BALB/cSR/CR × BALB/c | 24 | 63 | 38 |
| BALB/cSR/CR × C57BL/6 | 7 | 24 | 29 |
| (BALB/cSR/CR × C57BL/6)SR/CR × C57BL/6 | 43 | 122 | 35 |
| [(BALB/cSR/CR × C57BL/6)SR/CR × C57BL/6]SR/CR × C57BL/6 | 38 | 64 | 59 |
| BALB/cSR/CR × CAST/EI | 13 | 39 | 33 |
| Total | 125 | 312 | 40 |
| Male | 54 | ||
| Female | 71 |
The resistance trait was transmitted independently of gender of either parent or progeny in F1 and subsequent generations. Thus, the trait is likely to be linked to one of the 19 mouse autosomes, but not to the X or Y chromosome. To determine whether the resistance trait was also effective in different genetic backgrounds, the SR/CR BALB/c mice were crossed to sensitive C57BL/6 inbred mice. The trait was transmitted with a similar frequency into N1, N2, and N3 progeny in the C57BL/6 background (Table 1). A similar transmission frequency was also observed in breeding the trait into a wild inbred CAST/Ei background (Table 1). These results argue strongly that the trait was truly a dominant gain-of-function mutation. Fig. 1C summarizes survival data from resistant progeny compared with sensitive progeny by using two different doses of S180 cells. Fig. 1C demonstrates that this trait represents a powerful phenotype of resistance to transplanted cancer cells in a dose-independent manner.
The SR/CR Phenotype Is Age Dependent and Involves Priming. In contrast to CR to S180-induced ascites, a portion of the S180-resistant mice displayed SR, dependent on the age at the first injection of S180 cells. After injection of S180 cells, SR mice developed ascites for the first 2 weeks, which rapidly disappeared in <24 h (Fig. 1D). The mice then became healthy and immediately resumed normal activities, including mating. S180 cells in the regressed ascites were equivalent to 3 g of solid tumor mass or 3 × 10e9 of cells. The mice that underwent regression remained ascites-free thereafter. We then determined whether a repeated injection of S180 cells would induce a repeated ascites/regression in the mice that had shown ascites/regression once. However, the mice that had once undergone regression became completely protected from S180 cells and never developed ascites again in response to subsequent i.p. injections of S180 cells (Fig. 1E). The initial development of ascites suggested that the anticancer mechanism might not be engaged immediately in response to the implantation of cancer cells in older animals. After an initial period of latency, an anticancer mechanism was rapidly engaged in these mice, leading to destruction of S180 cells, clearance of peritoneal lymphatic drainage, and regression of ascites. The lasting protection against S180-induced ascites after initial regression suggests that the anticancer mechanism, after being engaged once, is primed for later engagement in response to subsequent exposures to S180 cells.
The manifestation of the CR or SR phenotypes was related to the age of mice at the time of the first injection with S180 cells. When the first injection was given at the age of 6 weeks, essentially all of the resistant mice display the CR phenotype. When the first injection of S180 cells was given at the age of 12 weeks, ≈50% of the S180-resistant mice showed the SR phenotype and the other 50% showed the CR phenotype. When the first injection of S180 cells was given at the age of 22 weeks, the majority of the resistant mice showed SR. In a small number of mice tested at 56 weeks, however, the first injection of S180 cells resulted in ascites and death even in mice whose offspring were cancer resistant (Fig. 1F). Genetic analysis also shows that CR and SR are derived from the same mutation locus, because the SR phenotype was inherited from CR parents and vice versa (Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org).
SR/CR Resistance Is Not Restricted to S180-Induced Ascites. To examine whether the SR/CR mice would resist the formation of solid tumors from s.c.-injected S180 cells, we injected a total of 2 × 10e6 S180 cells in each of two sites (one left and one right) in the shoulder regions of SR/CR mice that had been demonstrated previously to be resistant to S180-induced ascites. Four weeks after injection, visible solid tumors developed in all control mice, but not in the SR/CR mice (results not shown). Evidence for regression of solid tumor masses was also found in the SR/CR mice. Two solid tumor nodules ≈0.3 cm in diameter were found on the wall of the peritoneal cavity immediately after regression of ascites in an older mouse injected i.p. with 2 × 10e7 S180 cells 16 days previously. The tumors were removed, fixed, and examined histologically. Significantly different from S180-derived solid tumors in the control mice, these two tumors contained isolated groups of S180 cells surrounded by extensive desmoplasia, consistent with regression of solid tumors (see Fig. 6, which is published as supporting information on the PNAS web site).
To address the question of whether the SR/CR mice could resist other types of transplantable cancers, we tested the ability of the mice to eradicate transplanted cell lines and/or the ability of leukocytes from the SR/CR mice to kill different cell lines in cell culture. The results are summarized in Table 2. It appears that the resistance extends to a broad array of cancer cells.
Table 2. Ability of leukocytes from SR/CR mice to kill cell lines.
| SR/CR cell killing
|
||||||
|---|---|---|---|---|---|---|
| Cell line | Cancer type | MHC-I | Source | Haplotype | In vivo | In vitro |
| S180 | Sarcoma | - | Swiss | H2q | Yes | Yes |
| L5178Y | Lymphoma | - | DBA/2 | H2d | Yes | nd |
| MethA | Sarcoma | + | BALB/c | H2d | Yes | Yes |
| P815 | Mastocytoma | - | DBA/2 | H2d | nd | Yes |
| LL/2 | Lung carcinoma | - | C57BL | H2b | nd | Yes |
| BW5147.3 | T cell lymphoma | - | AKR/J | H2k | nd | Yes |
| Hepa 1-6 | Hepatoma | - | C57BL | H2b | nd | Yes |
| KLN 205 | Squamous cell Ca | - | DBA/2 | H2d | nd | Yes |
| EL-4 | B cell lymphoma | + | C57BL | H2b | nd | Yes |
Cell killing assays (in vitro and in vivo) were performed as described in Materials and Methods. nd, not done.
Randomly selected SR/CR mice were examined histologically for signs of autoimmune diseases. No signs of pathology were detected (results not shown), and all of the SR/CR mice showed normal behavior, normal body weight, and a normal life span.
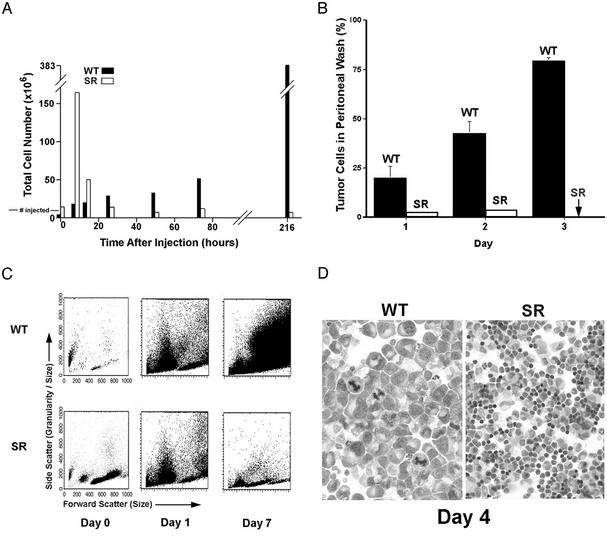
Infiltration of Host Immune Cells and Rapid Destruction of Cancer Cells via Cytolysis. To study the cell death events in the peritoneal cavity, GFP-transfected S180 cells were used for injection. At specific time points after injection of S180 cells, the peritoneal cavity of each anesthetized or killed mouse was washed with either PBS or culture medium. The S180/GFP cells were readily distinguishable from leukocytes by their difference in size, morphology, and GFP fluorescence. We found that an SR/CR mouse was capable of destroying up to 20 million S180 cells in the first 12 h. After the majority of S180 cells were destroyed, residual S180 cells could be occasionally detected in the first 48 h, but were completely absent thereafter (Fig. 2 A and B). The total cells in the peritoneal washes from both control and SR/CR mice were also analyzed by flow cytometry using forward scatter (cell size) on the x axis and side scatter (granularity and size) on the y axis. At day 7, S180 cells became the dominant cell population in the peritoneal cavity of control mice, but were not detected in the SR/CR mice (Fig. 2C). A day 4 cytoprep also showed that cancer cells were completely eliminated in the SR/CR mice (Fig. 2D). In contrast, some leukocytes in the control mice showed apoptosis. Interestingly, 6–12 h after injection, as many as 1.6 × 10e8 leukocytes migrated into the peritoneal cavity in SR/CR mice in response to the presence of S180 cells, yet disappeared after cancer cells were destroyed (Fig. 2 A).
Fig. 2.
Analysis of cells derived from peritoneal washes. (A) The total number of cells of all types recovered from SR/CR or WT mice at different times after injection of S180 cells. Note the rapid influx of leukocytes at 6 h in the SR mice, followed by a rapid decline. Total cells gradually increased in the WT mice, and these were mostly cancer cells as shown (B). In the SR/CR mice, no cancer cells remained after 3 days (arrow). (C) A similar analysis using flow cytometry. By day 7, large cancer cells were dominant in the WT mice, but were absent in the SR/CR mice. (D) Cytospins of cells from the washes (shown at equal magnifications, ×100) show large cancer cells in the WT mice, but mixed leukocytes in the SR/CR mice. All data are representative of at least three analyses with similar results.
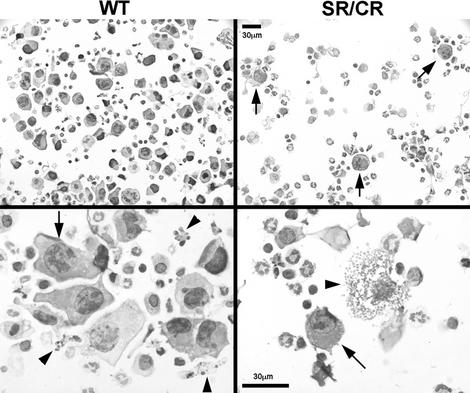
In the peritoneal washes from control mice, S180 cells were scattered evenly throughout the cytoprep fields. No significant aggregation of cells was observed. In sharp contrast, S180 cells from the SR/CR mice were surrounded by immune cells forming rosettes and larger cellular aggregates (Fig. 3). Additionally, many S180 cells in rosettes were ruptured, suggesting a primary cytolytic event. Apoptotic morphology was not observed in the injected S180 cells.
Fig. 3.
Formation of rosettes between cancer cells and leukocytes in day 1 peritoneal washes of the SR/CR mice. Cytopreps of peritoneal washes from WT or SR/CR mice injected with S180 cells on the previous day show prominent rosette formation around cancer cells (arrows) in the SR mouse, but not in the WT mice. Cancer cells from the WT mice (arrow) appeared intact and were often adjacent to apoptotic leukocytes (arrowheads), whereas the few remaining cancer cells in the SR/CR mice (arrow) often appear to have undergone cytolysis (arrowhead).
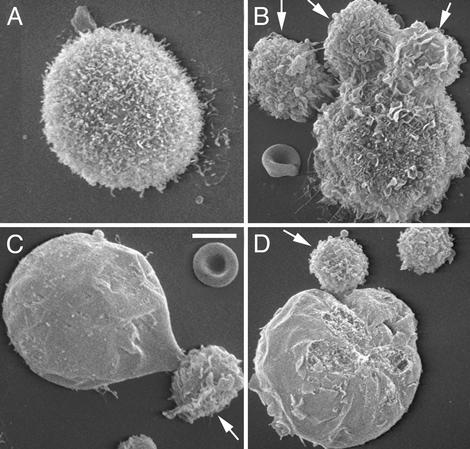
The cells in the peritoneal washes were also examined by scanning electron microscopy. S180 cells in the peritoneal washes of control mice displayed larger diameters than leukocytes and had numerous surface microvilli (Fig. 4A). In the peritoneal washes of the SR/CR mice challenged with 2 × 10e7 S180 cells for 24 h, S180 cells displayed a variety of morphological changes, including swelling, flattening and simplification of microvilli, tight contact with leukocytes, and surface erosions consistent with membrane damage (Fig. 4 B–D).
Fig. 4.
Scanning electron microscopic images of S180 cell–leukocyte interactions. Cells recovered from the peritoneum of a WT mouse show the normal surface morphology of S180 cells with extensive microvilli (A). Cells recovered from an SR/CR mouse after S180 injection show rosettes of leukocytes (arrows) surrounding a tumor cell (B) and ballooning, loss of microvilli, and membrane defects in tumor cells undergoing lysis (C and D). (Bar = 7 μm.)
To verify that the destruction of cancer cells was via cell rupture, the culture peritoneal cells were recorded by using time-lapse video phase-contrast microscopy. In addition to formation of cell–cell aggregates, cytolytic rupture of cancer cells was also evident (see video presentations at www.wfubmc.edu/pathology/research/srmouse.htm).
T Lymphocytes Are Not Involved in the Destruction of Cancer Cells. T cells have long been believed to be the primary effector cells in host immunity against cancer. We undertook a genetic approach to determine whether the resistance to S180 cells in the SR/CR mice required mature T cells. The experimental design was to determine whether resistance to S180 cells occurred in an athymic nude background in which the maturation of T cells is blocked by the absence of a thymus. The phenotype of T cell absence in the nude mice is sometimes thought to be “leaky.” However, the fact that nude mice accept transplants from different species argues that this leakiness of T cells does not impair successful heterotransplantation. Female mice of the N3 progeny of the C57BL/6 SR/CR congeneic line were crossed to homozygous nude (foxn1—/foxn1—) males in the C57BL/6 background (S180-sensitive). All progeny from this cross carried a single copy of the recessive nude gene (foxn1—) and grew hair. All progeny were injected with S180 cells. Approximately 40% of these mice were ascites free. The SR/CR females from this cross were then crossed again with homozygous nude (foxn1—/foxn1—) males. Sixteen of 31 progeny were nude mice (foxn1—/foxn1—). Upon i.p. injection of S180 cells, 10 of 16 nude mice (foxn1—/foxn1—) developed and succumbed to ascites, and six were ascites free. Similar to parental nude mice, SR/CR nude mice also completely lacked thymus, consistent with impairment of T cell development (results not shown). This finding indicates that lack of T cells did not impair the SR/CR phenotype that, thus, may require other immune components.
Leukocytes of the Innate Immune System Appear to Mediate Tumor Cell Killing. By analysis of fluorescence-labeled surface markers and cell morphology in SR/CR mice, the infiltrating leukocytes found enriched in the peritoneal washes and associated with dying tumor cells were mainly leukocytes of the innate immune system, including neutrophils, macrophages, and natural killer cells (results not shown). In preliminary experiments, washed peritoneal infiltrating cells were harvested from SR/CR mice and adoptively transferred into control mice before challenge with S180 tumor cells. Such recipient mice showed resistance to subsequently injected S180 cells, indicating that the mechanism of tumor cell killing was mediated by these infiltrating immune cells.
Discussion
The SR/CR mouse model represents a unique opportunity to examine cancer/host interactions. The killing of tumor cells primarily by cytolysis in SR/CR mice was extremely rapid and effective, yet was achieved with profound selectivity, with most normal cells being unharmed. The efficiency of this cell killing has a number of striking features. Once primed by the initial challenge of S180 cells, the SR/CR mice could withstand repeated daily challenge of >2 × 10e7 S180 cells and could also remain ascites free after a single challenge of up to 3 × 10e9 S180 cells (10% of body weight). Tumor cell killing was accompanied by a dramatic migration of leukocytes that form rosettes and aggregates with cancer cells. After cell contact, tumor cells undergo lysis. This cellular debris was then engulfed by peritoneal macrophages. The mice were then subsequently tumor free. The resistance mechanism appears to involve cells of the innate immune system and does not depend on T cell function. Histological examination of tissues in SR/CR mice showed normal morphology. Although life span studies have not been completed, there was no sign of a shortened life span in SR/CR mice.
Several intriguing implications derive from the properties of the SR/CR mouse. First, this model demonstrates the existence of a host resistance gene that can prevent the growth of advanced, MHC-negative cancers. The existence of host cancer-resistance genes has been postulated to be one explanation for the existence of individuals in the human population who fail to develop cancers, despite prolonged and intense exposure to carcinogens (11). The gene(s) responsible for the SR/CR phenotype may well be an example of such a resistance gene that might have a direct human ortholog. Second, the concept of immune surveillance has been debated for decades and has been difficult to prove, although recent studies have lent support to this concept (12). The SR/CR mouse may also provide a potential example of such a surveillance mechanism. Third, the alteration in the type of response seen with age in these mice suggests an intriguing possibility. The appearance of cancer in older individuals at a much higher frequency may not solely be caused by the accumulation of mutations in individual preneo-plastic cells. This mouse model suggests that there may also be host resistance mechanisms that decline with age. Fourth, the rare phenomenon of SR of cancers has been documented in humans, but has been difficult to study because of a lack of an appropriate animal model. The SR/CR mouse may provide such a model and allow identification of the cellular and genetic machinery necessary to reject a fully developed malignancy.
Mouse models of immune-mediated rejection of transplanted tumors through T cell-mediated recognition or through abrogation of immune suppressive cytokines have been clearly demonstrated (e.g., ref. 13). The SR/CR mouse, however, provides an example of a unique genetically determined mechanism of resistance independent of T cells. Further studies of the underlying genetic, cellular, and biochemical mechanisms in the SR/CR mouse should yield a deeper understanding of how tumor cells evade host immune rejection. Further, the ability of adoptively transferred infiltrating leukocytes from SR/CR mice to protect control mice from S180 cells (seen in preliminary studies) may suggest a potentially feasible strategy for treatment of advanced cancers that could be translatable into human patients.
Supplementary Material
Acknowledgments
We thank Katherine Barrett, Ken Grant, and Liya Qin for technical assistance and Dr. Dennis K. Watson for helpful advice. This work has been supported by intramural grants from the Office of Research and Comprehensive Cancer Center of Wake Forest University and extramural grants from the Charlotte Geyer Foundation and the National Cancer Institute (Grant R55CA93868 to Z.C.). C.J.D. and A.M.H. were supported by Signal Transduction and Cellular Function Training Grant CA-09422 from the National Institutes of Health. H.M.W. has been supported by a graduate scholarship from the Department of Biochemistry at Wake Forest University.
Abbreviations: SR, spontaneous regression; CR, complete resistance.
References
- 1.Everson, T. C. (1967) Prog. Clin. Cancer 3, 79–95. [PubMed] [Google Scholar]
- 2.Cole, W. H. (1981) J. Surg. Oncol. 17, 201–209. [DOI] [PubMed] [Google Scholar]
- 3.Challis, G. B. & Stam, H. J. (1990) Acta Oncol. 29, 545–550. [DOI] [PubMed] [Google Scholar]
- 4.Bodey, B., Bodey, B., Siegel, S. E. & Kaiser, H. E. (1998) In Vivo 12, 107–122. [PubMed] [Google Scholar]
- 5.Papac, R. J. (1998) In Vivo 12, 571–578. [PubMed] [Google Scholar]
- 6.Tarnowski, G. S., Mountain, I. M. & Stock, C. C. (1973) Cancer Res. 33, 1885–1888. [PubMed] [Google Scholar]
- 7.Alfaro, G., Lomeli, C., Ocadiz, R., Ortega, V., Barrera, R., Ramirez, M. & Nava, G. (1992) Vet. Immunol. Immunopathol. 30, 385–398. [DOI] [PubMed] [Google Scholar]
- 8.Coffey, J. W. & Hansen, H. J. (1966) J. Immunol. 96, 1021–1026. [PubMed] [Google Scholar]
- 9.Salaün, J. (1968) Eur. J. Cancer 4, 413–424. [DOI] [PubMed] [Google Scholar]
- 10.Schiffer, L. M., Nelson, J. S., Dilettuso, B., Migliorato, D. & Randolph, W. (1973) Cell Tissue Kinet. 6, 165–172. [DOI] [PubMed] [Google Scholar]
- 11.Balmain, A. & Nagase, H. (1998) Trends Genet. 14, 139–144. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J. & Schreiber, R. D. (2002) Nat. Immunol. 3, 991–998. [DOI] [PubMed] [Google Scholar]
- 13.Gorelik, L. & Flavell, R. A. (2001) Nat. Med. 7, 1118–1122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.