Abstract
We have recently demonstrated that immunization with hepatitis C virus-like particles (HCV-LPs) generated in insect cells can elicit both humoral and cellular immune responses in BALB/c mice. Here, we evaluate the immunogenicity of HCV-LPs in HLA2.1 transgenic (AAD) mice in comparison to DNA immunization. HCV-LP immunization elicited a significantly stronger humoral immune response than DNA immunization. HCV-LP-immunized mice also developed stronger HCV-specific cellular immune responses than DNA-immunized mice as determined by using quantitative enzyme-linked immunospot (ELISpot) assay and intracellular cytokine staining. In BALB/c mice, immunization with HCV-LPs resulted in a >5 log10 reduction in vaccinia titer when challenged with a recombinant vaccinia expressing the HCV structural proteins (vvHCV.S), as compared to 1 log10 decrease in DNA immunization. In HLA2.1 transgenic mice, a 1–2 log10 reduction resulted from HCV-LP immunization, whereas no reduction was seen from DNA immunization. Adoptive transfer of lymphocytes from HCV-LP-immunized mice to naive mice provided protection against vvHCV.S challenge, and this transferred immunity can be abrogated by either CD4 or CD8 depletion. Our results suggest that HCV-LPs can induce humoral and cellular immune responses that are protective in a surrogate HCV challenge model and that a strong cellular immunity provided by both CD4 and CD8 effector lymphocytes may be important for protection from HCV infection.
Hepatitis C virus (HCV) is a major causative agent of acute and chronic hepatitis worldwide. Prospective studies have shown that 75% of persons infected with HCV can progress to chronic infection, and of the chronically infected 10–20% will develop complications of chronic liver disease such as liver cirrhosis and 1–5% will develop hepatocellular carcinoma (1). Therapy has exclusively used IFN-based regimens, which despite recent improvement in treatment response with ribavirin combination therapy is effective in only ≈50% of infected persons (2, 3). To date, there is no effective vaccine against HCV infection. Ab response, which has been the linchpin of protective immunity in many viral infections, does not appear to play a major role in viral clearance during acute HCV infection or treatment of chronic hepatitis C (4, 5). However, the role of neutralizing Abs in protective immunity against HCV infection is still uncertain (6), because appropriate neutralization assays are lacking. Studies in humans and chimpanzees have indicated that failure to generate multispecific cellular immune responses against HCV in the acute phase of infection is associated with chronicity (7–9). Conversely, patients with strong HCV-specific cytotoxic T lymphocyte (CTL) responses have lower levels of viremia, and these responses correlate with recovery (10–13). Therefore, an ideal HCV vaccine may need to induce strong humoral responses against the envelope proteins and to prime broad HCV-specific T helper and CTL responses.
Virus-like particles are attractive as a recombinant protein vaccine because they mimic closely the properties of native virion. We have reported the synthesis of HCV-like particles (HCV-LPs) by using a recombinant baculovirus that contains the cDNA of the HCV structural proteins (core/E1/E2). These HCV-LPs have biophysical, ultrastructural, and antigenic properties similar to those of the putative virions (14, 15). We recently reported that BALB/c mice immunized with HCV-LPs generated strong and broad humoral and cellular immune responses against HCV structural proteins (15, 16). Furthermore, HCV-LPs without p7 elicited a Th1-biased cellular immunity in BALB/c mice (16). In this study, we evaluated the immunogenicity of p7-HCV-LP in HLA2.1 transgenic (AAD) mice (17) and compared the immune responses to those induced by DNA immunization. Because of the lack of a convenient animal model for HCV infection, we tested the protective immunity induced by HCV-LP in a surrogate challenge model with recombinant HCV-vaccinia. Here we present evidence that immunization with HCV-LP protects against HCV-vaccinia challenge in mice, and this immunity is probably mediated by both CD4 and CD8 effector lymphocytes.
Materials and Methods
Production of HCV-LPs and HCV Structural Proteins. Construction of recombinant baculovirus bvHCV.Sp7-, bvGUS, and recombinant vaccinia virus (Western Reserve strain) containing the same complementary DNA for the HCV structural proteins (vvHCV.S) (genotype 1b J strain) as bvHCV.S have been described (14). HCV-LPs were partially purified by sucrose gradient centrifugation as described (14). Purification of E1/E2 from vvHCV.S-infected BHK-21 cells and of core from a bacterial expression system has been described (14, 15).
Plasmids for DNA Immunization. P7020.S expressing the core/E1/E2 polyprotein (amino acids 1–830) under the control of the cytomegalovirus promoter was constructed by cloning the same HCV cDNA from the bvHCV.S as described (18). Plasmid DNA was purified by using an endotoxin-free plasmid extraction kit (Qiagen, Valencia, CA) and dissolved in PBS at a concentration of 2 mg/ml.
Immunization and Challenge of Mice. Female BALB/c (H-2d) mice 6 to 8 wk old were purchased from Charles River Breeding Laboratories. Female AAD mice expressing the transgene with the α1 and α2 domains from the human HLA-A2.1 and the α3 domain of murine H-2Dd in the C57BL/6 background (17) were obtained from Victor Engelhard (University of Virginia, Charlottesville). For HCV-LP immunization, AAD mice were immunized three times at 3-wk intervals with 20 μg of p7-HCV-LP dissolved in 100 μl of PBS injected into the quadriceps muscles. For DNA immunization, AAD mice were injected three times with 100 μg of the plasmid DNA (p7020 control vector or p7020.S) into the quadriceps muscles, which had been injected 3 days earlier with 0.25% bupivacaine. For the challenge experiment, 1 × 107 plaque-forming units (pfu) of vvHCV.S or vaccinia expressing β-galactosidase (vvLacZ) was inoculated i.p. into immunized BALB/c or AAD mice 2 wk after the last immunization. In both challenge experiments, the mice were killed 5 days after challenge (peak of vaccinia titer in ovaries), and their ovaries were harvested. After freeze-thawing and homogenization procedure, vaccinia titer was determined on BSC-1 cells by plaque assay.
Anti-HCV Envelope Abs and Lymphoproliferative Assay. Blood samples obtained by retro-orbital puncture before and 2 wk after the last immunization were analyzed for anti-HCV E1/E2 Abs by ELISA as described (16). Unimmunized and preimmunized serum samples were analyzed for background determination, and a signal-to-noise ratio of 2 was used as the cut-off. Spleen cells from immunized mice were cultured in triplicate by using 96-well round-bottom plates at 2 × 105 cells per well in 200 μl of RPMI medium 1640 containing 10% heat-inactivated FCS, 2 mmol/liter l-glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. Cells were stimulated with 5 μg/ml recombinant HCV core or E1/E2 proteins in triplicate. As negative controls, effector cells were stimulated with medium alone. After stimulation for 5 days, [3H]thymidine was added (1 μCi per well; 1 μCi = 37 kBq). Cells were incubated for an additional 18 h and [3H]thymidine incorporation into DNA was measured after harvesting the plates. Lymphocyte stimulation index (SI) was calculated as the mean cpm measured after antigen stimulation divided by the mean cpm of cells incubated with medium alone, and a SI of >3 was considered as positive. Splenocytes from unimmunized mice were analyzed as controls, and they all showed a SI of <1.5.
Quantitative Enzyme-Linked Immunospot (ELISpot) Assay for IFN-γ and IL-4. The 96-well nitrocellulose-bottom plates (Millititer, Millipore) were coated with 100 μl of anti-IFN-γ (R4–6A2) or anti-IL-4 (BVD4–1D11) Abs (PharMingen) at 2 μg/ml in PBS and incubated overnight at 4°C. After removal of unbound Abs by four washings with PBS, plates were blocked for 1 h with 100 μl of 1% enzyme-grade BSA in PBS. After washing with PBS, plates were blocked for 1 h with 100 μl of complete T cell culture medium (CTM) containing 5% FCS. Splenocytes (3 × 105 per well) were incubated with antigen (core or E1/E2 protein at 1 μg/ml) in a volume of 100 μl at 37°C, under a 5% CO2/95% air atmosphere. All determinations were run in duplicate. After 30 h of incubation, cells were removed by washing thoroughly with PBS containing 0.05% Tween 20. Biotinylated anti-IFN-γ (XMG1.2) or anti-IL-4 (BVD6–24G2) Abs at 0.25 μg/ml (PharMingen) were added and incubated overnight at 4°C. The wells were washed extensively in PBS containing 0.05% Tween 20 and incubated for 2 h with 100 μl of peroxidase-labeled streptavidin (Kirkegaard & Perry Laboratories). Unbound Abs were removed by washing thoroughly with PBS containing 0.05% Tween 20. Finally, 100 μl of a substrate solution containing 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Bio-Rad) was added and incubated until blue spots appear (≈30 min). The reaction was stopped by rinsing with distilled water. Spots as counted by KS Elispot-Axioplan 2I (Zeiss) are expressed as spot-forming units (SFUs) per 3 × 105 cells, and ≥10 SFU was considered positive. Splenocytes from unimmunized mice typically showed <5 SFU in the ELISpot assay.
CTL Assay and Intracellular Cytokine Staining (ICS). Splenocytes were resuspended in CTM (RPMI medium 1640 containing 10% heat-inactivated FCS, 2 mmol/liter l-glutamine, 50 units/ml penicillin, 50 μg/ml streptomycin) and 10% rat T-stim (Becton Dickinson). Responder cells (3 × 107) in 5 ml of CTM with 10% rat T-stim were cocultured with 10 μg/ml HCV core (amino acids 131–140, ADLMGYIPLV) or E2 (amino acids 614–622, RLWHYPCTI) peptide (18) in a six-well plate in a humidified incubator at 37°C, 5% CO2. Five days after stimulation, cytolytic activity of in vitro-stimulated CTLs was measured by using a 6-h assay with 51Cr-labeled target cells. The cell line C1R-AAD cells expressing the chimeric HLA-A2.1/H-2Dd molecules (17) were pulsed overnight with 10 μg/ml core or E2 peptide and used as target cells. The percentage of specific lysis was calculated as [(experiment release - spontaneous release) × 100]/(maximum release - spontaneous release). The maximum release was determined from supernatant of target cells lysed by addition of 1% SDS. Percentage of specific lysis was calculated by subtracting the background lysis against CIR-AAD cells without peptide, and a specific lysis of >10% was considered positive based on the results of splenocytes from unimmunized mice, which showed specific lysis of <5%. For intracellular cytokine staining, 5 ml of CTM containing 4 × 107 responder cells was cocultured with 10 μg/ml HCV core or E2 peptide in six-well plate in a humidified incubator at 37°C, 5% CO2. Five days after incubation, cells were harvested and 2 × 106 cells were cocultured with or without peptides (10 μg/ml HCV core or E2 peptide) in a 96-well round-bottom plate in 200 μl of CTM for 2 h before addition of 10 μg/ml brefeldin A (Sigma). After an additional 4-h incubation, cells were washed and stained with FITC-anti-CD8 (PharMingen) at 4°C for 30 min. After washing, the cells were fixed with 1% paraformaldehyde for 20 min at room temperature. After washing, cells were stained with phycoerythrin (PE)-conjugated anti-mouse IFN-γ (PharMingen) in PBS containing 0.3% saponin (Calbiochem) overnight at 4°C. Cells were washed and analyzed by fluorescence-activated cell sorter. The background from lymphocytes cultured for 5 days with HCV core or E2 peptide but no peptide during the ICS step was subtracted from each data. Splenocytes from unimmunized mice typically showed <0.1% IFN-γ+CD8+ cells in the ICS assay.
Adoptive Transfer and Vaccinia Challenge. BALB/c mice were immunized three times at 3-wk intervals with HCV-LP as described above. Suspensions of splenocytes from 10 animals were pooled. After washing with PBS, CD4+ or CD8+ cells were depleted by using Dynabeads (Dynal, Oslo). CD4- or CD8-depleted splenocytes or whole splenocytes without depletion (3 × 107 cells) were resuspended in 100 μl of PBS and injected into syngenic BALB/c mice through the tail vein. Twenty-four hours after adoptive transfer, the mice were challenged with 1 × 106 pfu of vvHCV.S by i.p. injection. Mice were killed 4 days after challenge, and the ovaries were harvested. For this experiment, a lower-dose inoculation of vvHCV.S was used because adoptive transferred lymphocytes were expected to be less potent than those in direct vaccination. Ovaries were harvested on day 4 because vaccinia titer reaches its peak 4 days after inoculation with 106 pfu of vvHCV.S.
Statistical Analysis. Comparison of the proliferative and CTL assays, ICS, and ELISpot results between groups of mice was conducted by using Student's t test. The anti-E1/E2 titers and vaccinia titers were log10 transformed and compared by using the Mann–Whitney U test. All tests were two-tailed, and differences were considered significant for P < 0.05.
Results
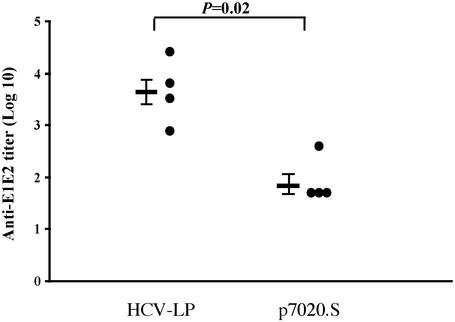
Humoral Immune Response. We previously reported that anti-envelope Abs could be detected after the third immunization with HCV-LP and steadily increased after each boost (16). Here, we assessed the E1/E2-specific humoral immune responses by ELISA in sera of HCV-LP-immunized AAD mice (n = 4), compared to those in DNA-immunized mice (n = 4). Endpoint titration of sera collected 2 wk after the third immunization indicated that all mice developed anti-E1/E2 Abs with titers ranging from 800 to 25,600 in HCV-LP-immunized AAD mice. In contrast, the titers of anti-E1/E2 in all DNA-immunized AAD mice were below 400 (Fig. 1).
Fig. 1.
Anti-E1/E2 titers in HCV-LP- or DNA-immunized AAD mice. The AAD mice were immunized, and the serum was harvested and assayed 2 wk after the last immunization for anti-E1/E2 as described in Materials and Methods. The anti-E1/E2 titer by endpoint dilution in ELISA of each mouse is shown. Mean values are expressed as geometric mean titers ± SEM and shown as bars on the left. Sera tested negative by ELISA were assigned an arbitrary titer of one-half of the initial dilution of 100. The P value is shown above. These data are representative of three similar experiments.
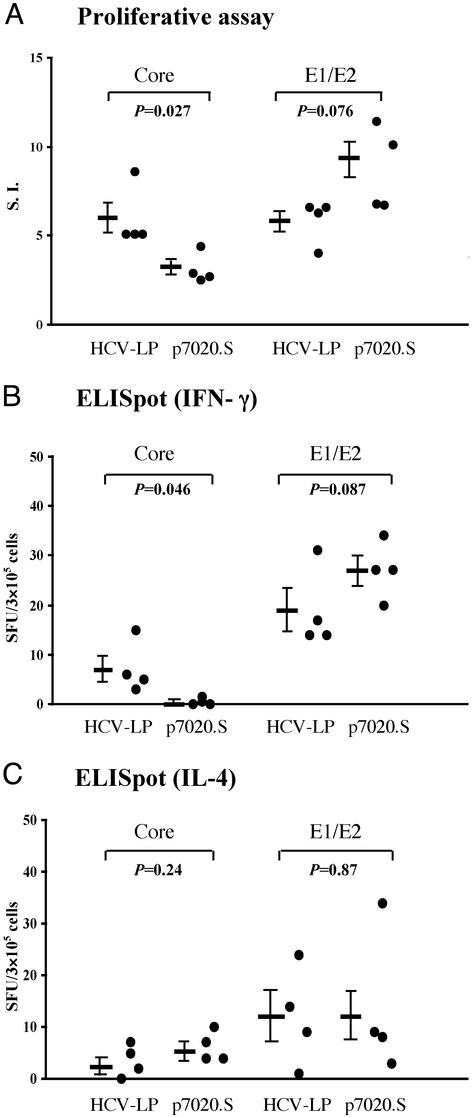
CD4+ Proliferative Response. To investigate the induction of T-helper responses against the HCV structural proteins in AAD mice immunized with either HCV-LP or DNA, splenocytes were harvested after the third immunization and stimulated with recombinant HCV core or E1/E2 proteins. As shown in Fig. 2A using the cutoff SI of 3, HCV-LP immunization induced proliferative response against both the core and E1/E2 proteins in all animals, whereas DNA immunization induced proliferative response in four/four animals against the E1/E2 but only in one/four animals against the core protein. The mean SI was higher against the core protein and lower against the E1/E2 in the HCV-LP-immunized group than that of DNA-immunized group. To better quantitate the above findings, we performed ELISpot assay for IFN-γ and IL-4. As shown in Fig. 2B, a weak response (seven IFN-γ SFU/3 × 105 cells as the mean) against the core protein was detected in HCV-LP-immunized mice, but not in DNA-immunized mice. In contrast, IFN-γ-positive spots against E1/E2 protein were detected in all mice immunized with HCV-LP or DNA. The numbers of SFU were not significantly different between HCV-LP- and DNA-immunized mice. On the other hand, both immunized groups exhibited IL-4-positive SFU against core or E1/E2 but there were no significant differences in the number of IL-4-positive SFU between the two groups (Fig. 2C).
Fig. 2.
CD4 T helper responses in HCV-LP- or DNA-immunized AAD mice. AAD mice were immunized, and splenocytes were harvested 2 wk after the last immunization as described in Materials and Methods.(A) Proliferative response. Splenocytes were stimulated in vitro for 5 days with 5 μg/ml recombinant HCV core or E1/E2 proteins. Data from individual mice are shown, and the mean SI ± SEM is indicated as bar on the left. SI of >3 (dotted line) is considered positive. IFN-γ (B) and IL-4 (C) ELISpot assays of splenocytes after stimulation with recombinant HCV core or E1/E2 protein are shown. Spots from individual mice are shown, and the mean ± SEM is indicated as bar on the left. The P values are shown. These data are representative of three similar experiments.
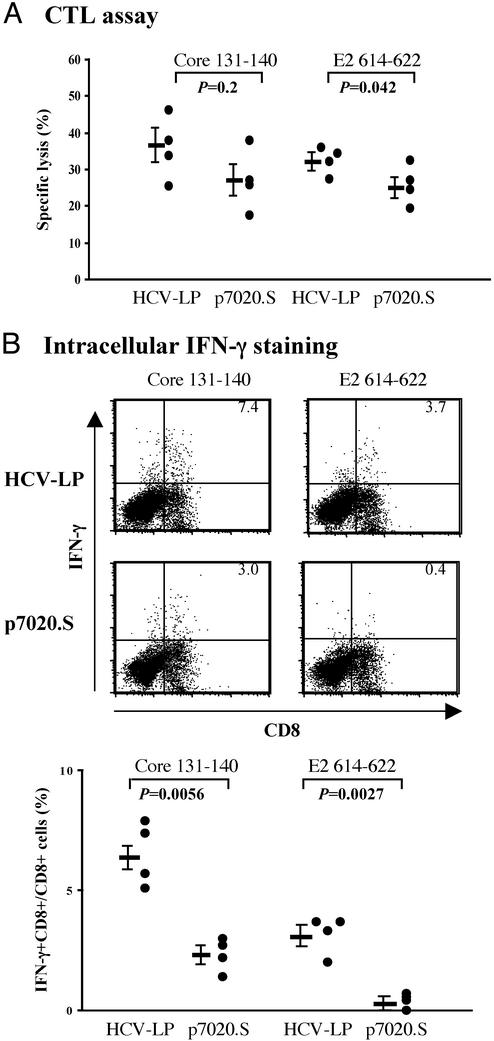
CD8+ CTL Response. To compare the CD8+ CTL responses between HCV-LP- and DNA-immunized AAD mice, we analyzed the spleen cells for CTL activities after in vitro stimulation with HLA-A2.1-restricted core or E2 peptide. All HCV-LP-immunized mice exhibited cytotoxic activities against both core and E2 peptide-pulsed target cells (Fig. 3A). In our previous studies in BALB/c mice (16), CTL responses against E2 were much stronger than those against core. However in AAD mice, CTL response against core and E2 peptides were similar. DNA immunization also elicited CTL responses against both peptides, although their responses tended to be lower than those of HCV-LP-immunized mice. To examine the CTL response more quantitatively, we performed ICS. As shown in Fig. 3B, the percentage of HCV core-specific IFN-γ+CD8+ cells in HCV-LP-immunized mice was higher than that in DNA-immunized mice. Similarly, the percentage of HCV-E2-specific IFN-γ+CD8+ cells was higher in HCV-LP-immunized mice than that in DNA-immunized mice, although the E2-specific response was in general lower than that of HCV-core-specific response.
Fig. 3.
CD8 CTL responses in HCV-LP- or DNA-immunized AAD mice. AAD mice were immunized and splenocytes were harvested 2 wk after the last immunization as described in Materials and Methods.(A) The cytotoxic activities (percentage specific lysis) of splenocytes against core or E2 peptide target are indicated at an effector-to-target cell ratio of 30:1. Data from individual mice are shown, and the mean percentage specific lysis ± SEM is indicated as the bar on the left. (B) Intracellular cytokine staining. Splenic lymphocytes were stimulated in vitro with HCV CTL peptide (core or E2) and then analyzed for intracellular IFN-γ staining. Representative fluorescence-activated cell sorter flowgrams (10,000 events were collected for counting) are shown in Upper (percentages of IFN-γ+CD8+ cells are shown in the right upper quadrants) and summary of percentages of IFN-γ-producing CD8+ cells with means ± SEM are shown in Lower. For background determination, lymphocytes cultured for 5 days with HCV CTL core or E2 peptide were stimulated without any peptide and assayed for ICS. Individual data are shown after subtraction of the background. For both CTL and ICS assays, splenocytes were stimulated with an irrelevant peptide (HBV core 18–27 peptide) as control, and the results showed background activities (<5% specific lysis for CTL and <0.2% for ICS). The P values are shown. These data are representative of three similar experiments.
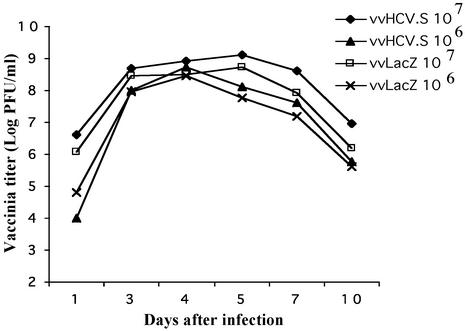
Protective Immunity in a Surrogate Challenge Model. To assess the biological relevance of the immunity induced by either vaccination (HCV-LP or DNA), we performed challenge experiments in which a recombinant vaccinia virus expressing HCV structural proteins was inoculated into immunized BALB/c and AAD mice; vvLacZ was used as a control challenge. To determine the growth curve of vaccinia in vivo for the challenge experiment, we inoculated unimmunized BALB/c mice with vvHCV.S and vvLacZ at 106 or 107 pfu per animal and harvested the ovary at various times for viral titering (Fig. 4). The results showed similar growth curves for vvHCV.S and vvLacZ with slightly higher titers in the vvHCV.S group. Mice inoculated with 107 pfu had higher viral load, which peaked at about day 5 after inoculation as compared to 106-pfu inoculation, which seemed to peak slightly earlier at day 4. Similar growth curves were seen in the AAD mice (not shown).
Fig. 4.
Vaccinia growth curve in BALB/c mice. BALB/c mice were inoculated i.p. with 106 or 107 pfu of vvHCV.S or vvLacZ in triplicate. The ovaries were harvested at various times, and the mean vaccinia titers were determined and plotted.
In HCV-LP-immunized BALB/c mice challenged with vvHCV.S, vaccinia viral titers were significantly reduced as compared with those with vvLacZ challenge and control-immunized mice with vvHCV.S challenge (Fig. 5). The reduction in mean viral titers between HCV-LP and control mice was greater than log10 = 5. Furthermore, five of the seven HCV-LP-immunized animals had no detectable virus after challenge with vvHCV.S. Two animals in this group also had several logs of reduction in viral titer as compared with the control group (Fig. 5A). Interestingly, analysis of the CTL activities in the HCV-LP-immunized mice challenged with vvHCV.S demonstrated high activities (20–50% of specific lysis) in mice with undetectable viral level but low activities (≈10% of specific lysis) in mice with detectable viral level, supporting the importance of CTL-mediated immunity in this model. In comparison, DNA immunization resulted in only 1 log reduction in mean viral titer (Fig. 5B). We performed the same challenge experiment in AAD mice. HCV-LP-immunized mice demonstrated a 1- to 2-log reduction in the mean viral titer, whereas no reduction was seen in DNA-immunized mice after challenge with vvHCV.S (Fig. 5C).
Fig. 5.
Vaccinia titers in immunized BALB/c mice challenged with vvHCV.S or vvLacZ. Mice were killed 5 days after challenge. Vaccinia titers in ovaries were determined. Data are shown for each mouse, and the mean vaccinia titer ± SEM is indicated. (A) Mice (six or seven per group) were immunized with HCV-LP or control preparation and then challenged with vvHCV.S or vvLacZ 2 wk after the third immunization. (B) Mice (three to five per group) were immunized with p7020.S or p7020 (control vector) and then challenged with vvHCV.S or vvLacZ. (C) Vaccinia titers in immunized AAD mice challenged with vvHCV.S or vvLacZ. Six mice per group were immunized with HCV-LP, p7020.S, or control. Mice were killed, and vaccinia titers in ovaries were determined. Data are shown for each mouse, and the mean vaccinia titer ± SEM is indicated. The P values are shown. These data are representative of two similar experiments.
To determine the effector mechanisms of this induced immunity, we performed an adoptive transfer experiment (Fig. 6). In this experiment, BALB/c mice were used because of the strong protection induced by HCV-LP immunization against the vvHCV.S challenge. After three immunizations with HCV-LP, splenocytes were harvested, depleted of either CD4 or CD8 lymphocytes, and adoptively transferred to syngenic naive BALB/c mice. Nondepleted splenocytes were also transferred for comparison. The mice were then challenged with vvHCV.S. The reduction of viral titer after challenge was seen in the whole lymphocytes-transferred mice (without CD4 or CD8 depletion), whereas none of the mice adoptively transferred with CD4- or CD8-depleted lymphocytes showed any significant protection from vaccinia virus challenge. These results suggest that both HCV-specific CD4 and CD8 T lymphocytes may be important for viral protection in this model. It is possible that the CD4 or CD8 T cells from immunized mice may provide partial protection, but given the modest protection by the whole splenocytes (1- to 2-log reduction in vaccinia titer), one may not be able to detect a partial effect. Therefore, the relative importance of the CD4 and CD8 populations in the observed protective immunity remains to be established.
Fig. 6.
Vaccinia titer in adoptive transferred BALB/c mice. Ten BALB/c mice were immunized three times with HCV-LP. Two weeks after the third immunization, splenic lymphocytes were harvested and pooled. After washing with PBS extensively, CD4 or CD8 cells were depleted. Cells (3 × 107) with or without depletion were injected into syngenic naive BALB/c mice through the tail vein. After adoptive transfer (24 h), the mice were challenged with 106 pfu of vvHCV.S i.p. Mice were killed 4 days after challenge, and then vaccinia titers in ovaries were determined. Data are shown for each mouse, and the mean vaccinia titer ± SEM is indicated. The P values are shown. These data are representative of three similar experiments.
Discussion
We previously reported the production of HCV-LP containing HCV structural proteins (core/E1/E2) in insect cells by using a baculoviral expression system (14). These noninfectious 40- to 50-nm HCV-LPs were able to elicit a strong humoral and cellular immune response against HCV structural proteins (core/E1/E2) in BALB/c mice (15, 16). We have also shown that HCV-LPs generated from constructs with or without p7 are indistinguishable with respect to morphologic and biophysical properties, suggesting that p7 is not required for viral assembly (15). Interestingly, the HCV-LP without p7 (p7-HCV-LP) was shown to be more potent in inducing cellular immune responses with a Th1 bias than HCV-LP with p7 (16). In this study, we showed that the p7-HCV-LP can also induce both humoral and cellular immunities in AAD mice. We also demonstrated that immunization with HCV-LP protects against recombinant HCV-vaccinia challenge in mice and this immunity may be mediated by both CD4+ and CD8+ T lymphocytes. However additional experiments, such as in knockout mouse models, are needed to precisely define the role of CD4 and CD8 responses in this observed protective immunity.
Several human studies have presented evidence that a strong HCV-specific CD4+ T cell response is associated with viral clearance in acute hepatitis C (19–21) or chronic HCV infection successfully treated with IFN (22–24). The importance of HCV-specific CD4+ T cells in viral control has also been demonstrated in patients with HCV recurrence after loss of virus-specific CD4+ T cell response (24). In addition, HCV/HIV coinfection with low numbers of CD4+ T cells leads to high HCV RNA level. Therefore, our findings that HCV-LP can induce proliferative responses against core and E1/E2 proteins with robust production of IFN-γ are important. Similarly, CD8+ T cells are also crucial in viral clearance. Several clinical studies have shown an inverse correlation between the strength of HCV-specific CTL activities and viral load or clearance (10, 11, 25). In a chimpanzee study, viral clearance during acute hepatitis C was more closely dependent on CD8+ CTL activities than Abs (12). Therefore, cellular immunity is likely crucial for viral clearance and disease control in HCV infection.
One of the major advantages of virus-like particles (VLPs) is their ability to induce CTL response, as has been shown for papillomavirus, HIV, and hepatitis B virus VLPs (26–28). In contrast to recombinant proteins that do not assemble into particles, VLPs can be processed by the major histocompatibility complex class I pathway. In our study, we confirmed that HCV-LP immunization elicited strong CTL responses and presented evidence that the strength of HCV-specific CTLs may be important for viral protection. Quantitative assays such as ICS revealed that HCV-LP immunization elicited a more vigorous CD8+ T cell response against both core and E2 targets than did DNA immunization. In addition, HCV-LP immunization induced a more potent CD4+ response toward the core protein (but not the envelope protein) than did DNA immunization. These differences may be the reason why immunization with HCV-LP conferred a stronger protection against vvHCV.S challenge than did DNA immunization. The observation that the protected mice demonstrated stronger CTL activities than the less protected mice lends further credence to this explanation. This study supports our previous results and highlights the importance of considering not only the magnitude but also the type of T cell response in designing effective vaccines. Analyses of the cytokine profiles of HCV-specific T cells revealed that persons displaying a Th1 profile (antigen-dependent production of IL-2 and IFN-γ) are more likely to experience viral clearance (29–31). Our previous study showed the induction of an IFN-γ-producing Th1 response by p7-HCV-LP immunization in BALB/c mice (16). Similarly in AAD mice, we found by using the ELISpot assay that HCV-LP immunization elicited predominantly IFN-γ-producing lymphocytes against both HCV recombinant protein and CTL peptides.
Finally, adoptive transfer experiments of lymphocytes from HCV-LP-immunized mice supported that both CD4+ and CD8+ T cells may be indispensable for viral protection in this surrogate challenge model. It is understandable that the adoptively transferred mice exhibited less protection than the directly immunized mice because of the quantity and experimental manipulation of the adoptively transferred lymphocytes. In our HCV-LP immunization studies, major differences in the quantity and quality of cellular immune responses appeared to exist between BALB/c and AAD mice. In addition, the degree of protection against vvHCV.S challenge after HCV-LP immunization are rather disparate between the two strains. This disparity is most likely due to the different genetic backgrounds, for the two strains have been shown to exhibit divergent cytokine responses and susceptibility to various infections (32–34).
Our studies have shown that immunization with HCV-LP is superior to immunization with DNA and can induce broad and vigorous humoral and cellular immune responses in a mouse model expressing human HLA background. The HCV-vaccinia challenge model, although valuable in demonstrating induction of certain protective immunity, is a surrogate model and does not represent natural infection. In addition, the mechanism for viral clearance of vaccinia in mice is likely quite different from that of HCV in humans. Immunization and challenge studies in chimpanzees should allow us to address this issue. Regardless, based on our studies, HCV-LP represents a promising vaccine candidate against HCV infection.
Acknowledgments
We thank Barbara Rehermann, Marion Major, Stephen Feinstone, and Jay Berzofsky for helpful advice, John Vergalla for excellent technical assistance, and Victor Engelhard for the AAD mice.
Abbreviations: HCV, hepatitis C virus; HCV-LP, HCV-like particle; CTL, cytotoxic T lymphocyte; SI, stimulation index; pfu, plaque-forming unit; SFU, spot-forming unit; ICS, intracellular cytokine staining.
References
- 1.Liang, T. J., Rehermann, B., Seeff, L. B. & Hoofnagle, J. H. (2000) Ann. Intern. Med. 132, 296-305. [DOI] [PubMed] [Google Scholar]
- 2.Manns, M. P., McHutchison, J. G., Gordon, S. C., Rustgi, V. K., Shiffman, M., Reindollar, R., Goodman, Z. D., Koury, K., Ling, M. & Albrecht, J. K. (2001) Lancet 358, 958-965. [DOI] [PubMed] [Google Scholar]
- 3.Fried, M. W., Shiffman, M. L., Reddy, K. R., Smith, C., Marinos, G., Goncales, F. L., Jr., Haussinger, D., Diago, M., Carosi, G., Dhumeaux, D., et al. (2002) N. Engl. J. Med. 347, 975-982. [DOI] [PubMed] [Google Scholar]
- 4.Baumert, T. F., Wellnitz, S., Aono, S., Satoi, J., Herion, D., Tilman Gerlach, J., Pape, G. R., Lau, J. Y., Hoofnagle, J. H., Blum, H. E. & Liang, T. J. (2000) Hepatology 32, 610-617. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi, M., Tanaka, E., Matsumoto, A., Ichijo, T. & Kiyosawa, K. (1997) J. Gastroenterol. Hepatol. 12, 73-76. [DOI] [PubMed] [Google Scholar]
- 6.Choo, Q. L., Kuo, G., Ralston, R., Weiner, A., Chien, D., Van Nest, G., Han, J., Berger, K., Thudium, K., Kuo, C., et al. (1994) Proc. Natl. Acad. Sci. USA 91, 1294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassett, S. E., Guerra, B., Brasky, K., Miskovsky, E., Houghton, M., Klimpel, G. R. & Lanford, R. E. (2001) Hepatology 33, 1479-1487. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari, C., Valli, A., Galati, L., Penna, A., Scaccaglia, P., Giuberti, T., Schianchi, C., Missale, G., Marin, M. G. & Fiaccadori, F. (1994) Hepatology 19, 286-295. [PubMed] [Google Scholar]
- 9.Tsai, S. L., Liaw, Y. F., Chen, M. H., Huang, C. Y. & Kuo, G. C. (1997) Hepatology 25, 449-458. [DOI] [PubMed] [Google Scholar]
- 10.Hiroishi, K., Kita, H., Kojima, M., Okamoto, H., Moriyama, T., Kaneko, T., Ishikawa, T., Ohnishi, S., Aikawa, T., Tanaka, N., et al. (1997) Hepatology 25, 705-712. [DOI] [PubMed] [Google Scholar]
- 11.Rehermann, B., Chang, K. M., McHutchison, J. G., Kokka, R., Houghton, M. & Chisari, F. V. (1996) J. Clin. Invest. 98, 1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper, S., Erickson, A. L., Adams, E. J., Kansopon, J., Weiner, A. J., Chien, D. Y., Houghton, M., Parham, P. & Walker, C. M. (1999) Immunity 10, 439-449. [DOI] [PubMed] [Google Scholar]
- 13.Thimme, R., Oldach, D., Chang, K. M., Steiger, C., Ray, S. C. & Chisari, F. V. (2001) J. Exp. Med. 194, 1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumert, T. F., Ito, S., Wong, D. T. & Liang, T. J. (1998) J. Virol. 72, 3827-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumert, T. F., Vergalla, J., Satoi, J., Thomson, M., Lechmann, M., Herion, D., Greenberg, H. B., Ito, S. & Liang, T. J. (1999) Gastroenterology 117, 1397-1407. [DOI] [PubMed] [Google Scholar]
- 16.Lechmann, M., Satoi, J., Vergalla, J., Murata, K., Baumert, T. F. & Liang, T. J. (2001) Hepatology 34, 417-423. [DOI] [PubMed] [Google Scholar]
- 17.Shirai, M., Arichi, T., Nishioka, M., Nomura, T., Ikeda, K., Kawanishi, K., Engelhard, V. H., Feinstone, S. M. & Berzofsky, J. A. (1995) J. Immunol. 154, 2733-2742. [PubMed] [Google Scholar]
- 18.Satoi, J., Murata, K., Lechmann, M., Manickan, E., Zhang, Z., Wedemeyer, H., Rehermann, B. & Liang, T. J. (2001) J. Virol. 75, 12121-12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botarelli, P., Brunetto, M. R., Minutello, M. A., Calvo, P., Unutmaz, D., Weiner, A. J., Choo, Q. L., Shuster, J. R., Kuo, G., Bonino, F., et al. (1993) Gastroenterology 104, 580-587. [DOI] [PubMed] [Google Scholar]
- 20.Missale, G., Bertoni, R., Lamonaca, V., Valli, A., Massari, M., Mori, C., Rumi, M. G., Houghton, M., Fiaccadori, F. & Ferrari, C. (1996) J. Clin. Invest. 98, 706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen, H. R., Miner, C., Sasaki, A. W., Lewinsohn, D. M., Conrad, A. J., Bakke, A., Bouwer, H. G. & Hinrichs, D. J. (2002) Hepatology 35, 190-198. [DOI] [PubMed] [Google Scholar]
- 22.Cramp, M. E., Rossol, S., Chokshi, S., Carucci, P., Williams, R. & Naoumov, N. V. (2000) Gastroenterology 118, 346-355. [DOI] [PubMed] [Google Scholar]
- 23.Barnes, E., Harcourt, G., Brown, D., Lucas, M., Phillips, R., Dusheiko, G. & Klenerman, P. (2002) Hepatology 36, 743-754. [DOI] [PubMed] [Google Scholar]
- 24.Gerlach, J. T., Diepolder, H. M., Jung, M. C., Gruener, N. H., Schraut, W. W., Zachoval, R., Hoffmann, R., Schirren, C. A., Santantonio, T. & Pape, G. R. (1999) Gastroenterology 117, 933-941. [DOI] [PubMed] [Google Scholar]
- 25.Lechner, F., Wong, D. K., Dunbar, P. R., Chapman, R., Chung, R. T., Dohrenwend, P., Robbins, G., Phillips, R., Klenerman, P. & Walker, B. D. (2000) J. Exp. Med. 191, 1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenstone, H. L., Nieland, J. D., de Visser, K. E., De Bruijn, M. L., Kirnbauer, R., Roden, R. B., Lowy, D. R., Kast, W. M. & Schiller, J. T. (1998) Proc. Natl. Acad. Sci. USA 95, 1800-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo, L., Li, Y., Chang, J. S., Cho, S. Y., Kim, T. Y., Choi, M. J., Cheong, H. S., Kim, H. J., Ahn, H. J., Min, M. K., et al. (1998) Virology 240, 316-325. [DOI] [PubMed] [Google Scholar]
- 28.Schirmbeck, R., Melber, K., Kuhroeber, A., Janowicz, Z. A. & Reimann, J. (1994) J. Immunol. 52, 1110-1119. [PubMed] [Google Scholar]
- 29.Diepolder, H. M., Zachoval, R., Hoffmann, R. M., Wierenga, E. A., Santantonio, T., Jung, M. C., Eichenlaub, D. & Pape, G. R. (1995) Lancet 346, 1006-1007. [DOI] [PubMed] [Google Scholar]
- 30.Kamal, S. M., Rasenack, J. W., Bianchi, L., Al Tawil, A., El Sayed Khalifa, K., Peter, T., Mansour, H., Ezzat, W. & Koziel, M. (2001) Gastroenterology 121, 646-656. [DOI] [PubMed] [Google Scholar]
- 31.Woitas, R. P., Lechmann, M., Jung, G., Kaiser, R., Sauerbruch, T. & Spengler, U. (1997) J. Immunol. 159, 1012-1018. [PubMed] [Google Scholar]
- 32.Mullbacher, A. & Lobigs, M. (2001) J. Virol. 75, 8353-8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smiley, S. T., Kaplan, M. H. & Grusby, M. J. (1997) Science 275, 977-979. [DOI] [PubMed] [Google Scholar]
- 34.Webb, J. R., Lee, S. H. & Vidal, S. M. (2002) Genes Immun. 3, 250-262. [DOI] [PubMed] [Google Scholar]