Abstract
Mediated by guanylate cyclase-activating proteins (GCAPs), cytoplasmic Ca2+ levels regulate the activity of photoreceptor guanylate cyclase (GC) and the synthesis of cGMP, the internal transmitter of phototransduction. When GCAP1 is expressed in transgenic mice on a GCAP null background, it restores the wild-type flash responses in rod photoreceptors. In this communication, we explored the role of GCAP1 in cone photoreceptors by using electroretinograms (ERGs). Under cone isolation conditions, ERGs recorded from mice lacking both GCAP1 and GCAP2 had normal amplitudes of the saturated a-wave and b-wave. However, recordings from these mice demonstrated a widened b-wave and increased sensitivity of both M- and UV-cone systems. Paired-flash ERGs revealed a delayed recovery of both the cone driven b-wave and a-wave and suggest that the delay originated from the photoreceptors. To test whether GCAP1 could restore normal cone response recovery, mice that expressed only transgenic GCAP1 in the absence of wild-type GCAP expression were tested. Immunohistochemical analysis demonstrated that cones of these mice expressed high levels of GCAP1. Paired-flash ERGs showed that the recovery of the cone-driven a-wave was restored to normal, whereas recovery of the cone-driven b-wave was slightly faster than that observed in wild-type mice. These studies reveal that, similar to rods, deletion of GCAP1 and GCAP2 delays the recovery of light responses in cones and GCAP1 restores the recovery of cone responses in the absence of GCAP2.
The retina is capable of detecting and processing light over an enormous range of intensities. This difficult task is partly accomplished by dividing the wide range of sensitivity between two classes of photoreceptors: rods and cones. Rods are optimized for dim light conditions, sacrificing temporal resolution for high sensitivity (responses to single photons). Cones are 30 times less sensitive but exhibit faster response kinetics, and adapt over a wider range of intensities (1, 2). The differences in rod and cone light responses are unlikely to be caused by the different outer segment structure or quantum efficiencies of photopigments (3). Distinct components of rod and cone phototransduction cascades are probably responsible for the different response characteristics (4). Like the rod cascade, the cone cascade relies on cGMP as an internal transmitter, but is composed of proteins encoded by distinct genes. The difference in response kinetics may arise from the enzymatic properties or different expression levels of proteins regulating the recovery, either at the cascade shutoff level, or mediated by Ca2+, an important second messenger in light adaptation and recovery. Many proteins involved in response recovery are shared between rods and cones, such as G-protein receptor kinase 1 (GRK-1), an enzyme phosphorylating rod and cone pigments (5), the Ca2+ binding protein recoverin (6), GTPase-activating (GAP) proteins (RGS9-1/Gβ5L; ref. 7), guanylate cyclases (GC1/GC2; refs. 8 and 9), and their Ca2+-dependent activators (GCAPs; ref. 10).
In murine rod photoreceptors, two different GC-activating proteins (GCAP1 and GCAP2) are thought to be important in modulating GC activity and light response recovery (11, 12). In low Ca2+ (corresponding to the light-activated state), GCAP1 stimulates GC1 whereas GCAP2 stimulates both GC1 and GC2, events that accelerate synthesis of cGMP (13). Both GCAPs are inactive when free Ca2+ is high (corresponding to the dark-adapted state). Immunohistochemistry and in situ hybridization in mouse and human retina have shown that cone photoreceptors primarily express GC1 and GCAP1 at relatively high levels (14). The presence of GCAP2 in cone outer segments has not been ruled out (15), but the relative contribution of GCAP2 in cone response recovery has not been determined (16). In this communication, we sought to investigate the recovery of cone responses in mice in which GCAP1/GCAP2 expression was disabled by genetic manipulation. We used single-flash electroretinograms (ERGs) with a rod-suppressing background to measure cone sensitivity and paired-flash ERGs to measure the recovery of the cone-driven a- and b-waves. We further investigated the response in cones of transgenic animals that expressed only GCAP1 by reintroducing the native GCAP1 gene on a GCAP-/- null background.
Methods
GCAP1 Transgene and GCAP1/GCAP2 Knockout Constructs. Generation and characterization of the GCAP1 transgenic line G1T3 and GCAP1/GCAP2 knockouts (GCAP-/-) are described elsewhere (11, 12). Animals were raised in 12-hr light/dark cycle. All animals were handled in accordance with the University of Utah and Baylor College of Medicine's policies on the treatment of laboratory animals.
Immunohistochemistry. Eyes from G1T3+GCAP-/-, GCAP-/-, and wild-type (GCAP+/+) mice were fixed in 4% paraformaldehyde/0.1 M phosphate buffer overnight at 4°C. Twelve-micrometer-thick cryosections were used for immunocytochemical analysis of GCAP1 and cone pigment colocalization studies. The pAB UW14 (specific for GCAP1, diluted 1:2,000), and the COS-1 (recognizes M cones, diluted 1:100), and OS-2 (recognizes UV-cones, diluted 1:1,000) mAbs (generously provided by P. Röhlich, Semmelweis University, Budapest) have been previously characterized (14, 16, 17). Sections were incubated with FITC-conjugated goat anti-rabbit and rhodamine-conjugated goat anti-mouse secondary antibodies (Vector Laboratories; diluted 1:100) and analyzed on a Zeiss LSM 510 confocal microscope. Captured images were subsequently processed by using PHOTOSHOP (Adobe Systems, Mountain View, CA). ERGs. Before testing, mice were allowed to dark adapt overnight. Under dim red light, mice were anesthetized with a solution of ketamine (100 mg/ml) and xylazine (10 mg/ml). Pupils were dilated with a single drop of 0.5% mydryacil and 2.5% phenylephrine. Mice were placed on a heating pad maintained at 39°C, inside a Ganzfeld dome coated with reflective white paint. A small amount of 2.5% methylcellulose gel was applied to the eye and a platinum electrode was placed in contact with the center of the cornea. Similar platinum reference and ground electrodes were placed in the forehead and tail respectively. After placement in the dome, mice were allowed to remain in complete darkness for several minutes. Signals were amplified with a Grass Instruments (Quincy, MA) P122 amplifier (band pass 0.1—1,000 Hz). Data were acquired at a sampling rate of 10,000 HZ with Lab-PC DAQ board (National Instruments, Austin, TX). Traces were averaged and analyzed with custom software written in MATLAB (Mathworks, Natick, MA).
Light Stimulation. Light stimulation was provided by two 1,500-watt xenon flash lamps (Novatron, Dallas, TX) and an incandescent steady lamp (LKC Systems, Gaithersburg, MD) positioned at the top of the dome. Baffles were positioned directly in front of the lamps to ensure even distribution of light in the Ganzfeld dome and to prevent hot spots of illumination on the retina. The spectral intensity of the white flashes was measured from 320 to 620 nm in 10-nm increments by using an IL-790 monochromator attached to an IL-1700 radiometer (International Light, Newburyport, MA). The number of photoisomerizations (φ) per cone was calculated by using values adapted from previous studies, as were the protocols for paired-flash stimulation (18–20). For cone sensitivity tests, rods were suppressed by exposure to a 540-nm steady background light (estimated to produce 6,000 φ/rod/s) for at least 15 min. Flashes were filtered with 365-nm or 500-nm interference filters (Edmund Optics, Barrington, NJ) to elicit UV- or M-cone-driven responses. These flashes were estimated to produce between 3.8 × 102 and 3.5 × 104 photons per μm2. A white flash (5.2 × 106 photons per μm2) was used to the measure the saturated response, to which the amplitudes of other flashes were normalized.
For paired flashes, a conditioning (test) flash was first used to suppress the circulating current of both the rod and cone photoreceptors. A second flash, termed the probe flash, was delivered at different interstimulus intervals (ISIs) and was used to monitor the recovery of the photocurrent. The conditioning flash produced 4.6 × 106 φ per M-cone and 35,000 φ per UV-cone, and the probe flash produced 1.8 × 106 φ per M-cone and 14,000 φ per UV-cone. These calculations are estimates and do not account for coexpression of the cone pigments within the same cell type (21, 22). The ISI was varied from 300 to 5,000 ms. Xenon flashes have been shown to produce an artifact in ERG recordings with two parts: a rapid electromagnetic component and a slower photoelectric component (23). Our flashes produced an artifact with a slow negative component that measured ≈10 μv. Because the amplitude of this artifact was of similar magnitude as the cone-driven a-wave, we found it necessary to quantify and subtract it from a-wave recordings.
Analysis of a- and b-Wave Recovery. Previous work has shown that the amplitude of cone-driven responses increases during adaptation with steady light (24). We found that the repeated flashes from our protocol could elicit a similar increase in the b-wave of the cone-driven ERG. To prevent any differences that might result from adaptation, we limited the number of trials averaged for b-wave recovery to three, and we were careful to ensure that the same cumulative amount of light was delivered to each mouse. The cone-driven a-wave, because of its small amplitude, was difficult to assess with this limited number of trials. Therefore, to measure the recovery of the cone a-wave, we conducted a separate set of experiments averaging ten trials for each ISI. The b-wave is traditionally measured from the trough of the a-wave to the peak of the b-wave. However, the cone-driven oscillatory potentials can skew these measurements. It is possible to filter out the oscillatory potentials by using a low-pass filter (fc = 30 Hz), but this cutoff frequency will also filter the small cone a-wave. Thus, we measured the minimum of the b-wave at 12.5 ms from unfiltered data and the maximum at 40 ms from filtered data. The data were then normalized to b-wave amplitude measured for an ISI of 5,000 ms, an interval for which the cone b-wave has fully recovered and the rod contribution is minimal. In processing the data, we experimented with many different techniques of indexing and normalizing the b-wave. Whereas different methods shifted the curves somewhat, they did not change the relationships found between the different genotypes of mice.
Exponential Fits. Recovery has customarily been fit by using a single exponential. Recent work has demonstrated that the recovery of rods has two phases that are better fit by a double exponential (∥, 25). However, we found little improvement by using a double exponential for fitting cone recovery. Perhaps the late phases of cone recovery are contaminated by rod recovery. We used a single exponential of the form: 1 - e(-(t - T0)/τ). T0 is the critical time before which there is no recovery, and τ is the time constant of recovery.
Results
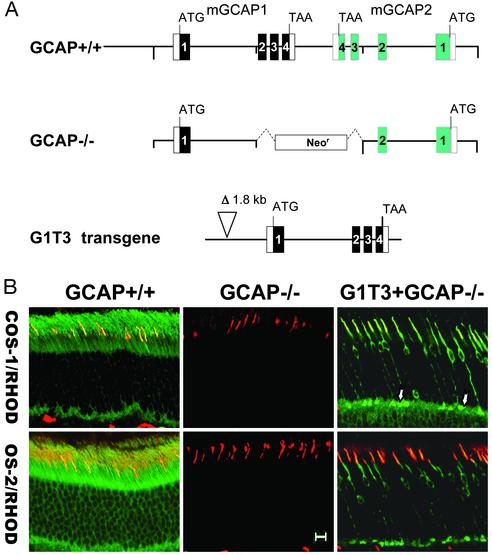
Expression of Transgenic GCAP1 in Cones. The distribution of GCAPs in photoreceptors seems often species-specific, and their relative roles have been controversial. For example, in situ hybridization with GCAP subclass-specific RNA in human indicated that GCAP1 and GCAP2 are present in rod and cone inner segments (14). Immunocytochemistry with GCAP2-monospecific antibodies detected GCAP2 mostly in human cone inner segments (26). However, immunocytochemistry did not detect significant levels of GCAP2 in mouse cones, whereas mouse rods expressed GCAP1 and GCAP2 at nearly the same levels (16). To clearly define the role of GCAP1 in cone response recovery, we took advantage of a mouse line (G1T3+) that expresses transgenic GCAP1 (identical to native GCAP1) under the control of the mouse GCAP1 promoter containing a deletion in the upstream region (ref. 12; Fig. 1A). The deletion, removing a 1.8-kb fragment 1.4 kb upstream of the translation start codon, affected GCAP expression primarily in rods. Immunocytochemistry showed that G1T3+GCAP-/- mouse cones expressed GCAP1 at high levels whereas very little or no GCAP1 expression was seen in rods (Fig. 1B). GCAP1 colocalized with cone pigments when frozen sections were double stained with COS-1 (specific for M-cone pigment) and OS-2 (UV-cone pigment) (27). The staining of G1T3+GCAP-/- cones with UW-14 (GCAP1) seems uniform. The results are consistent with high levels of GCAP1 expression in both UV- and M-cones.
Fig. 1.
Immunolocalization of GCAP1 to cones. (A) Gene constructs of animal models used in this study. GCAP+/+, wild-type GCAP gene array in tail-to-tail orientation. GCAP-/-, knockout construct disabling both GCAP genes. G1T3 transgene, transgenic mouse line expressing native GCAP1 under the control of the endogenous promoter. A portion of the upstream region (1.8 kb) was deleted, presumably during integration of the transgene into the mouse genome (12). (B) Coimmunolocalization of GCAP1 (FITC) with cone pigments (rhodamine) in various mouse lines. COS-1, M-cones; OS-2, UV (S)-cones. (Left) Wild-type retina. (Center) GCAP-/- mice. Note absence of GCAP1 fluorescence and exclusive labeling of cone outer segments. (Right) G1T3+GCAP-/- mice. Note absence of GCAP1 in rods in this animal model and high levels of GCAP1 in cone somata and pedicles (arrows). Scale bar = 10 μm.
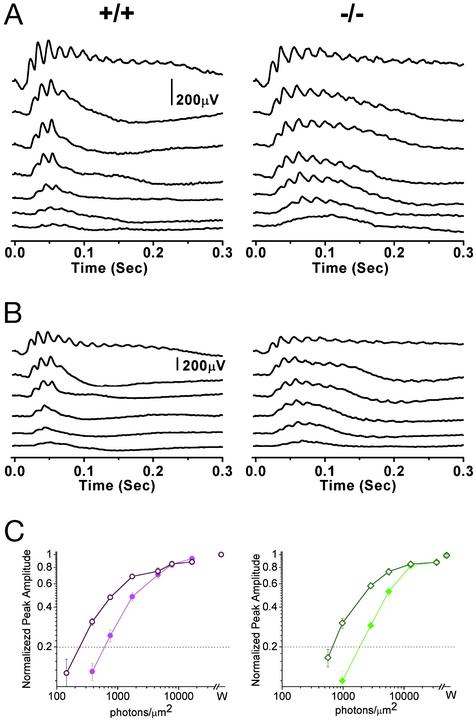
Cone Sensitivity in GCAP-/- Mice. Single-flash ERGs under cone isolation conditions reveal that there is no significant difference in the saturated b-wave or a-wave amplitude of cone-driven signals (Fig. 2 A and B; Table 1). There is a notable increase in the width of the b-wave in null mice compared with wild-type mice. This prolongation of the b-wave in GCAP-/- mice most likely reflects the delayed recovery of the cone photoreceptors because, in the absence of GCAPs, synthesis of cGMP is not accelerated. At lower intensities, there is an increase in the peak b-wave amplitude, indicating an increase in the sensitivity of both UV- and M-cone-driven signals in GCAP-/- mice (Table 1). This change results in a leftward shift of the response-intensity curve (Fig. 2C). There was not a significant change in the ratio of M-cone sensitivity to UV-cone sensitivity between wild-type and null mice (Table 1). This finding indicates that the sensitivity of the M- and UV-cone systems is equally affected by the loss of GCAPs.
Fig. 2.
Cone-isolated ERGs and sensitivity. (A) Cone-driven ERGs in response to a 365-nm flash from GCAP+/+ and GCAP-/- mice in the presence of a rod suppressing 540-nm background. Each trace represents the average of 3–10 trials. Intensities range from 3.8 × 102 to 1.5 × 104 photons per μm2 at the cornea (bottom to top). The top flash is the response to white light (5.2 × 106 photons per μm2 at the cornea). (B) Similar to A, but using 500-nm flashes. Intensities range from 9.7 × 102 to 3.4 × 104 photons per μm2 at the cornea (bottom to top). The top flash is the response to white light (5.2 × 106 photons per μm2 at the cornea). (C) Peak b-wave amplitude (filtered and normalized to the saturating response) vs. intensity for 365-nm flashes (Left) and 500-nm flashes (Right). W represents a white flash, which produces 5.2 × 106 photons per μm2 at the cornea. Sensitivity was estimated by fitting a line to the results bracketing a threshold criterion response of 20%. GCAP-/- mice (○) demonstrate 2.5-fold higher sensitivity for 365-nm stimuli and 3.7-fold higher sensitivity for 500-nm stimuli than wild-type mice (•).
Table 1. Cone ERG parameters.
| Genotype | bmax,phot (μV) | amax,phot (μV) | SM (photons·μm-2)-1 | SUV (photons·μm-2)-1 | SUV/SM |
|---|---|---|---|---|---|
| +/+ (n = 7) | 200 ± 25 | 40 ± 5 | (1.11 ± 0.1) × 10-4 | (4.12 ± 0.4) × 10-4 | 4.0 ± 0.5 |
| -/- (n = 6) | 235 ± 25 | 35 ± 2 | (4.67 ± 1.2) × 10-4 | (11.72 ± 2.2) × 10-4 | 3.1 ± 0.8 |
The first column indicates the genotype and number of mice tested. The parameters bmax,phot and amax,phot represent the saturating amplitude of the photopic b-wave and a-waves, respectively. The first value represents the mean and the second value represents the SE. The parameters SM and SUV represent the sensitivity of the M- and UV-cone driven systems, measured by determining the amount of light needed to reach a criterion threshold response (20% of the normalized saturated amplitude) and dividing 0.2 by the flash strengths. SUV/SM is the ratio of the UV- and M-cone sensitivities. Compared with UV-cones, M-cones require three to four times the amount of light to reach the threshold response.
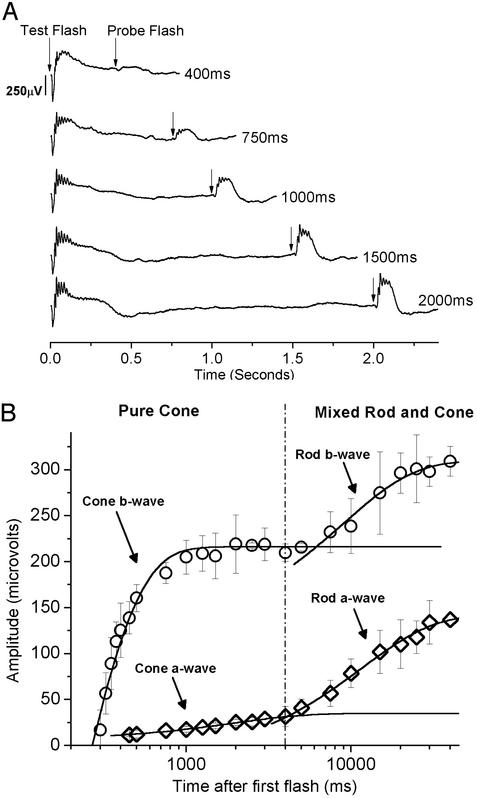
Probing Cone Function by Paired-Flash ERG Analysis. The paired-flash technique has been used effectively to measure the recovery of both rod and cone photocurrents (9, 18–20, 28, 29). The first flash, which isomerizes ≈1.4% of the M-cone pigment and ≈0.1% of the UV-cone pigment, leads to closure of cyclic nucleotide-gated (CNG) channels in both rods and cones and thus suppresses the response to the second flash. Reopening of the CNG channels depends in part on the rate of cGMP synthesis, which is mediated by GCAPs. As the ISI is increased, the CNG channels reopen as cGMP levels rise, and the response to the second flash increases (Fig. 3A). When the amplitude of the a- and b-wave are plotted vs. the ISI, there are two limbs of recovery present, the first occurring between 300 and 4,000 ms and the second between 5,000 and 45,000 ms (Fig. 3B). Single cell recordings from other species have demonstrated that, after a bright flash, cones emerge from saturation sooner than rods (30). The shape of the ERG response for ISIs <5,000 ms is comparable to cone-isolated ERGs recorded by using a rod-suppressing background. For ISIs <4,000 ms, we conclude that cones are the primary contributor to the response of the second flash.
Fig. 3.
Using paired-flash ERGs to measure cone recovery. (A) ERG traces from wild-type mice demonstrating the paired-flash technique. First, a white test flash, which isomerizes ≈1.4% of the M-cone pigment and ≈0.1% of the UV-cone pigment, is delivered to suppress the dark current of both rod and cone photoreceptors. A second probe flash, isomerizing 0.5% of the M-cone pigment and 0.04% of the UV-cone pigment, is delivered at various times after the first flash. For short ISI, the response to the second flash is suppressed because the cones have not been able to recover. As the ISI is increased, the response to the second flash recovers. (B) Amplitude of the b-wave (○) and a-wave (⋄) from the second flash vs. the time between the flashes. Error bars are SEM. Note the two branches of recovery: cones recover faster, followed by rods. For intervals <4,000 ms, rod recovery is minimal and cone recovery can be analyzed.
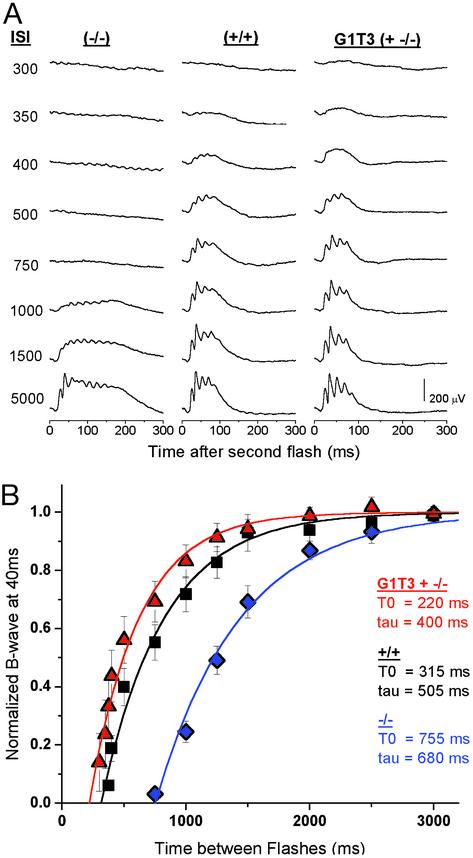
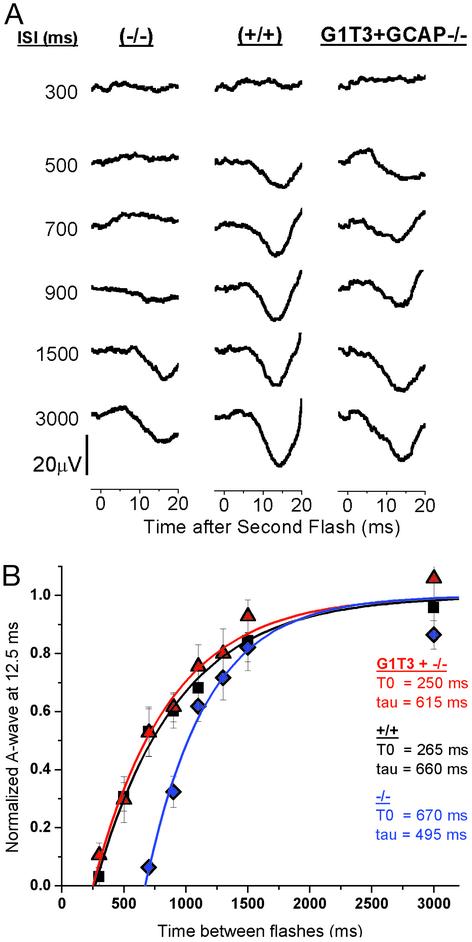
Delay of Recovery of Cone-Driven b-Waves in GCAP-/- Mice. We first analyzed the recovery of the cone-driven b-wave in wild-type, GCAP-/-, and G1T3+GCAP-/- mice (Fig. 4A). After normalization and exponential fitting (see Methods), it is apparent that cones from wild-type mice are >95% recovered after two seconds (T0 = 315 ms, τ = 505 ms; Fig. 4B). In comparison, cones from GCAP-/- mice demonstrate a significantly slower recovery (T0 = 755 ms, τ = 680 ms). The white flashes used in these experiments stimulated both UV- and M-cones. To test whether there was a relative difference in the recovery of UV- vs. M-cones in the absence of GCAPs, we used paired flashes, with the second flash filtered to 365 nm or 500 nm (data not shown). In GCAP-/- mice, recoveries for UV-cone- vs. M-cone-isolated responses were delayed to similar degrees compared with corresponding UV- and M-cone responses from wild-type mice. These results are consistent with immunohistochemical staining, which shows that GCAP1 is expressed in similar levels between the two classes of cones (Fig. 1B).
Fig. 4.
Cone b-wave recovery in GCAP1 transgenic mice. (A) Raw traces from paired-flash ERGs at different ISIs. Only the responses to the second flash are shown. Wild-type mice (+/+) demonstrate a steady recovery starting around 350 ms. Responses in GCAP-/- are suppressed for intervals <750 ms. The delay is due to the inability to accelerate cGMP synthesis as a response to decreased cytoplasmic Ca2+. G1T3+GCAP-/- mice, which express a transgenic GCAP1 on a GCAP null background, show recovery times very similar to wild-type mice, indicating full replenishment of GCAP1 in cones. (B) Normalized amplitudes of cone-driven b-waves for GCAP-/- (♦, n = 6), GCAP+/+ (▪, n = 5), and G1T3+GCAP-/- (▴, n = 5) mice plotted vs. the ISI between the test flash and probe flash. Data points were fit by using a single exponential, 1 - e(-(t - T0)/τ) (solid lines). T0 is the critical time before which there is no recovery, and τ is the time constant of recovery.
GCAP1 Rescues the GCAP-/- b-Wave Phenotype. G1T3+GCAP-/- mice, which express GCAP1 but not GCAP2 in the cones, exhibit recovery times similar to but slightly faster than wild-type mice (t0 = 220 ms, τ = 400 ms; Fig. 4B). This result demonstrates that transgenic GCAP1 expression in GCAP-/- mice is capable of restoring the cone recovery. Further, it is evident from the traces in Fig. 4A that some G1T3+GCAP-/- mice had a b-wave that terminated slightly earlier than the wild-type. Because the b-wave is the measurement of the activity of the second order bipolar cells and GCAP1 levels seem higher, based on immunohistochemistry, in synaptic pedicles of cones (Fig. 1B, arrows), these changes might also reflect an alteration in synaptic transmission.
Recovery of the Cone-Driven a-Wave. To elucidate the source of delayed cone recovery, we measured the recovery of the cone-driven a-wave. The cone-driven a-wave is thought to arise primarily from the suppression of circulating current in the cones. However, some care must be taken with this interpretation because, in primates, it has been shown that some of the signal may reflect contributions from off-bipolar cells (31, 32). The relative contribution of cones vs. off-bipolar cells in the murine cone-driven a-wave has yet to be determined. Measurements of the cone-driven a-wave recovery reveal that cones from wild-type mice began to recover as early as 300 ms, whereas cones from GCAP-/- mice did not start to recover until 700 ms (Fig. 5A). Interestingly, unlike the cone-driven b-wave, which recovered slightly faster than wild-type, the cone driven a-wave from G1T3+GCAP-/- mice had recovery times indistinguishable from wild-type mice (Fig. 5B). These results show that GCAP1 expression in the cones is sufficient for recovery of the cone-driven a-wave.
Fig. 5.
Measurement of cone a-wave recovery. (A) Raw traces from paired-flash ERGs at different ISIs scaled to show the cone-driven a-wave. Only the responses to the second flash are shown. Recovery of the cone a-wave in GCAP-/-seems delayed. G1T3+GCAP-/-, which express a transgene for GCAP1, show cone a-wave recovery similar to wild-type mice, consistent with levels of GCAP1 in the transgenic G1T3 cones equal to wild-type cones. (B) Normalized amplitudes of cone-driven a-waves for GCAP-/- (♦, n = 9), GCAP+/+ (▪, n = 6), and G1T3+GCAP-/- (▴, n = 8) mice plotted vs. the ISI between the test flash and probe flash. Compared with wild-type mice, GCAP-/- mice displayed a 300-ms shift in the ISI required to reach 50% of normalized a-wave amplitude.
Discussion
We used genetically altered mice and ERGs to test cone function first in the absence of GCAP1 and GCAP2, and then in the presence of transgenic GCAP1 on a GCAP null background. In GCAP-/- mice, recoveries of the cone driven a- and b-waves were significantly delayed, consistent with the function of GCAPs and a delay in cGMP synthesis, an event necessary for reopening of the cyclic nucleotide-gated channels. We showed furthermore that the expression of GCAP1 on a null background restores the recovery kinetics of the response to a bright flash in cones, as judged by recovery of a- and b-wave characteristics in paired-flash experiments, indicating that GCAP1, in the absence of GCAP2, is capable of mediating normal recovery of cone photoresponses. The absence of GCAP2 does not seem to affect the restoration of the cone response recovery. The 2-times slower recovery in GCAP-/- mice compared with wild-type mice is much smaller than the delay seen in GRK1-/- (40 times slower) and RGS9-/- (60 times slower) mice (18, 29). These results suggest that the default pathway for cGMP synthesis in the absence of acceleration is faster than the default pathways for rhodopsin and G-protein inactivation.
Our observation that the cone-driven b-wave in G1T3+GCAP-/- mice recovers faster than wild-type suggests that greater than normal levels of GCAP1 may be capable of accelerating the recovery of the photoreceptor. However, we did not observe an increase in the recovery of the cone-driven a-wave, a more direct measurement of photoreceptor activity. One explanation for this discrepancy is that the small amplitude of the a-wave prevents discerning the subtle difference. Alternatively, GCAP1 may play a role in unidentified pathways at the cone synapse. In wild-type mice, both GCAP1 and GC1 are found in photoreceptor terminals and could modulate cGMP channels at the synapse (8, 26, 33, 34). G1T3+GCAP-/- mice exhibit high levels of GCAP1 in the cone somata and pedicles (Fig. 1), suggesting the possibility of an effect on synaptic transmission. Finally, the altered b-wave kinetics in G1T3+GCAP-/- mice may suggest a role for GCAP2 in transmission of the cone signal to second order neurons.
In recent years, there has been great interest in determining the relative speed of enzymes involved in recovery, particularly to determine the rate-limiting process governing recovery. In rods, it has been suggested that the rate-limiting process of photoreceptor response recovery is primarily determined by the inactivation of rhodopsin or transducin and not by the synthesis of cGMP (35, 36). Recent work showing that there is no change in the dominant time constant in rod recordings from GCAP-/- mice further supports the idea that GCAPs are not rate limiting in rods (37). We were not able to measure the dominant time constant of the cones, but the increase in cone sensitivity in GCAP-/- mice suggests that the GCAPs act relatively fast in cones and are not rate limiting. The changes in cone sensitivity and recovery kinetics measured by our experiments were similar to those observed in rods by using ERGs and single cell recording (11, 12).
Several mutations in the GCAP1 gene have been associated with autosomal dominant cone dystrophy in human patients (38, 39, 40). Two of these mutations (Y99C, E115G) affect Ca2+ binding and lead to a persistent stimulation of cone GC1 and elevated levels of cGMP in cones. Whereas these GCAP1 mutations incur gain of function events leading to disease (41), loss of GCAP1 alters the recovery kinetics in GCAP-/- mice but does not lead to a degenerative disorder. Although GCAP2 has been identified in human cone inner segments (26) and at low levels in inner retinal cells (15), no mutations in the GCAP2 gene have been associated with retina disease (42). The high levels of GCAP1 in cone outer segments (15, 43) and its ability to restore response recovery argue that GCAP1 is the predominant Ca2+-dependent GC activator in other mammalian cones as well.
Acknowledgments
We thank Ed Pugh, Jason Chen, and Ted Wensel for critical reading of the manuscript. We are indebted to Jill Church-Kopish for providing excellent technical assistance. We thank Jeanie Chen at the University of Southern California for providing the GCAP-/- mice. We thank Pal Röhlich for cone-specific antibodies. This work was supported by National Institutes of Health Grants EY04446 (to S.M.W.), EY08123 (to W.B.), NIH Vision Core EY02520, and R03EY014120 (to K.A.H.). Additional support came from the Retina Research Foundation of Houston, the International Retina Research Foundation, the Macular Vision Research Foundation, Research to Prevent Blindness, Inc., and a Center Grant from the Foundation Fighting Blindness to the University of Utah. M.E.P. was supported by Baylor Research Advocates for Student Scientists (BRASS) and Medical Scientist Training Program Training Grant GM07330. S.M.W. and W.B. are the recipients of a Senior Investigator Award from Research to Prevent Blindness and a Ralph and Mary Tuck endowment to the Department of Ophthalmology at the University of Utah.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GC, guanylate cyclase; ERG, electroretinogram; GCAP, GC-activating protein; ISI, interstimulus interval.
Footnotes
Naarendorp, F., Lyubarsky, A. L. & Pugh, E. N., Jr. (2000) Soc. Neurosci. Abstr. 26, 1192.
References
- 1.Lagnado, L. (2002) Curr. Biol. 12, R215-R217. [DOI] [PubMed] [Google Scholar]
- 2.Krizaj, D. & Copenhagen, D. R. (2002) Front. Biosci. 7, d2023-d2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okano, T., Fukada, Y., Shichida, Y. & Yoshizawa, T. (1992) Photochem. Photobiol. 56, 995-1001. [DOI] [PubMed] [Google Scholar]
- 4.Ebrey, T. & Koutalos, Y. (2001) Prog. Retin. Eye Res. 20, 49-94. [DOI] [PubMed] [Google Scholar]
- 5.Zhao, X., Huang, J., Khani, S. C. & Palczewski, K. (1998) J. Biol. Chem. 273, 5124-5131. [DOI] [PubMed] [Google Scholar]
- 6.McGinnis, J. F., Stepanik, P. L., Baehr, W., Subbaraya, I. & Lerious, V. (1992) FEBS Lett. 302, 172-176. [DOI] [PubMed] [Google Scholar]
- 7.Zhang, K., Howes, K. A., He, W., Bronson, J. D., Pettenati, M. J., Chen, C., Palczewski, K., Wensel, T. G. & Baehr, W. (1999) Gene 240, 23-34. [DOI] [PubMed] [Google Scholar]
- 8.Liu, X., Seno, K., Nishizawa, Y., Hayashi, F., Yamazaki, A., Matsumoto, H., Wakabayashi, T. & Usukura, J. (1994) Exp. Eye Res. 59, 761-768. [DOI] [PubMed] [Google Scholar]
- 9.Yang, R. B., Robinson, S. W., Xiong, W. H., Yau, K. W., Birch, D. G. & Garbers, D. L. (1999) J. Neurosci. 19, 5889-5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palczewski, K., Polans, A. S., Baehr, W. & Ames, J. B. (2000) Bioessays 22, 337-350. [DOI] [PubMed] [Google Scholar]
- 11.Mendez, A., Burns, M. E., Sokal, I., Dizhoor, A. M., Baehr, W., Palczewski, K., Baylor, D. A. & Chen, J. (2001) Proc. Natl. Acad. Sci. USA 98, 9948-9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howes, K. A., Pennesi, M. E., Sokal, I., Church-Kopish, J., Schmidt, B., Margolis, D., Frederick, J. M., Rieke, F., Palczewski, K., Wu, S. M., et al. (2002) EMBO J. 21, 1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeseleer, F., Sokal, I., Li, N., Pettenati, M., Rao, N., Bronson, D., Wechter, R., Baehr, W. & Palczewski, K. (1999) J. Biol. Chem. 274, 6526-6535. [DOI] [PubMed] [Google Scholar]
- 14.Imanishi, Y., Li, N., Sokal, I., Sowa, M. E., Lichtarge, O., Wensel, T. G., Saperstein, D. A., Baehr, W. & Palczewski, K. (2002) Eur. J. Neurosci. 15, 63-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuenca, N., Lopez, S., Howes, K. & Kolb, H. (1998) Invest. Ophthalmol. Vis. Sci. 39, 1243-1250. [PubMed] [Google Scholar]
- 16.Howes, K., Bronson, J. D., Dang, Y. L., Li, N., Zhang, K., Ruiz, C., Helekar, B., Lee, M., Subbaraya, I., Kolb, H., et al. (1998) Invest. Ophthalmol. Vis. Sci. 39, 867-875. [PubMed] [Google Scholar]
- 17.Szel, A. & Röhlich, P. (1992) Exp. Eye Res. 55, 47-52. [DOI] [PubMed] [Google Scholar]
- 18.Lyubarsky, A. L., Chen, C., Simon, M. I. & Pugh, E. N. (2000) J. Neurosci. 20, 2209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepperberg, D. R., Birch, D. G. & Hood, D. C. (1997) Vis. Neurosci. 14, 73-82. [DOI] [PubMed] [Google Scholar]
- 20.Lyubarsky, A. L. & Pugh, E. N. (1996) J. Neurosci. 16, 563-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyubarsky, A. L., Falsini, B., Pennesi, M. E., Valentini, P. & Pugh, E. N. (1999) J. Neurosci. 19, 442-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Applebury, M. L., Antoch, M. P., Baxter, L. C., Chun, L. L., Falk, J. D., Farhangfar, F., Kage, K., Krzystolik, M. G., Lyass, L. A. & Robbins, J. T. (2000) Neuron 27, 513-523. [DOI] [PubMed] [Google Scholar]
- 23.Birch, D. G. & Sandberg, M. A. (1996) Doc. Ophthalmol. 92, 269-280. [DOI] [PubMed] [Google Scholar]
- 24.Peachey, N. S., Goto, Y., al-Ubaidi, M. R. & Naash, M. I. (1993) Neurosci. Lett. 162, 9-11. [DOI] [PubMed] [Google Scholar]
- 25.Jeffrey, B. G., Mitchell, D. C., Gibson, R. A. & Neuringer, M. (2002) Invest. Ophthalmol. Vis. Sci. 43, 2806-2814. [PubMed] [Google Scholar]
- 26.Otto-Bruc, A., Fariss, R. N., Haeseleer, F., Huang, J., Buczylko, J., Surgucheva, I., Baehr, W., Milam, A. H. & Palczewski, K. (1997) Proc. Natl. Acad. Sci. USA 94, 4727-4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szel, A., Takacs, L., Monostori, E., Diamantstein, T., Vigh-Teichmann, I. & Röhlich, P. (1986) Exp. Eye Res. 43, 871-883. [DOI] [PubMed] [Google Scholar]
- 28.Birch, D. G., Hood, D. C., Nusinowitz, S. & Pepperberg, D. R. (1995) Invest. Ophthalmol. Vis. Sci. 36, 1603-1614. [PubMed] [Google Scholar]
- 29.Lyubarsky, A. L., Naarendorp, F., Zhang, X., Wensel, T., Simon, M. I. & Pugh, E. N., Jr. (2001) Mol. Vis. 7, 71-78. [PubMed] [Google Scholar]
- 30.Baylor, D. A. (1987) Invest. Ophthalmol. Vis. Sci. 28, 34-49. [PubMed] [Google Scholar]
- 31.Sieving, P. A., Murayama, K. & Naarendorp, F. (1994) Vis. Neurosci. 11, 519-532. [DOI] [PubMed] [Google Scholar]
- 32.Bush, R. A. & Sieving, P. A. (1996) J. Opt. Soc. Am. A 13, 557-565. [DOI] [PubMed] [Google Scholar]
- 33.Cooper, N., Liu, L., Yoshida, A., Pozdnyakov, N., Margulis, A. & Sitaramayya, A. (1995) J. Mol. Neurosci. 6, 211-222. [DOI] [PubMed] [Google Scholar]
- 34.Rieke, F. & Schwartz, E. A. (1994) Neuron 13, 863-873. [DOI] [PubMed] [Google Scholar]
- 35.Pepperberg, D. R., Jin, J. & Jones, G. J. (1994) Vis. Neurosci. 11, 53-62. [DOI] [PubMed] [Google Scholar]
- 36.Sagoo, M. S. & Lagnado, L. (1997) Nature 389, 392-395. [DOI] [PubMed] [Google Scholar]
- 37.Burns, M. E., Mendez, A., Chen, J. & Baylor, D. A. (2002) Neuron 36, 81-91. [DOI] [PubMed] [Google Scholar]
- 38.Payne, A. M., Downes, S. M., Bessant, D. A., Taylor, R., Holder, G. E., Warren, M. J., Bird, A. C. & Bhattacharya, S. S. (1998) Hum. Mol. Genet. 7, 273-277. [DOI] [PubMed] [Google Scholar]
- 39.Downes, S. M., Holder, G. E., Fitzke, F. W., Payne, A. M., Warren, M. J., Bhattacharya, S. S. & Bird, A. C. (2001) Arch. Ophthalmol. 119, 96-105. [PubMed] [Google Scholar]
- 40.Wilkie, S. E., Li, Y., Deery, E. C., Newbold, R. J., Garibaldi, D., Bateman, J. B., Zhang, H., Lin, W., Zack, D. J., Bhattacharya, S. S., et al. (2001) Am. J. Hum. Genet. 69, 471-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sokal, I., Li, N., Surgucheva, I., Warren, M. J., Payne, A. M., Bhattacharya, S. S., Baehr, W. & Palczewski, K. (1998) Mol. Cell 2, 129-133. [DOI] [PubMed] [Google Scholar]
- 42.Payne, A. M., Downes, S. M., Bessant, D. A., Plant, C., Moore, T., Bird, A. C. & Bhattacharya, S. S. (1999) J. Med. Genet. 36, 691-693. [PMC free article] [PubMed] [Google Scholar]
- 43.Kachi, S., Nishizawa, Y., Olshevskaya, E., Yamazaki, A., Miyake, Y., Wakabayashi, T., Dizhoor, A. & Usukura, J. (1999) Exp. Eye Res. 68, 465-473. [DOI] [PubMed] [Google Scholar]