Abstract
Genes for trehalose metabolism are widespread in higher plants. Insight into the physiological role of the trehalose pathway outside of resurrection plant species is lacking. To address this lack of insight, we express Escherichia coli genes for trehalose metabolism in Arabidopsis thaliana, which manipulates trehalose 6-phosphate (T6P) contents in the transgenic plants. Plants expressing otsA [encoding trehalose phosphate synthase (TPS)] accumulate T6P whereas those expressing either otsB [encoding trehalose phosphate phosphatase (TPP)] or treC [encoding trehalose phosphate hydrolase (TPH)] contain low levels of T6P. Expression of treF (encoding trehalase) yields plants with unaltered T6P content and a phenotype not distinguishable from wild type when grown on soil. The marked phenotype obtained of plants accumulating T6P is opposite to that of plants with low T6P levels obtained by expressing either TPP or TPH and consistent with a critical role for T6P in growth and development. Supplied sugar strongly inhibits growth of plants with reduced T6P content and leads to accumulation of respiratory intermediates. Remarkably, sugar improves growth of TPS expressors over wild type, a feat not previously accomplished by manipulation of metabolism. The data indicate that the T6P intermediate of the trehalose pathway controls carbohydrate utilization and thence growth via control of glycolysis in a manner analogous to that in yeast. Furthermore, embryolethal A. thaliana tps1 mutants are rescued by expression of E. coli TPS, but not by supply of trehalose, suggesting that T6P control over primary metabolism is indispensable for development.
Trehalose is an ancient sugar consisting of two glucose molecules alpha-1,1-linked and thus with no reducing ends. It is one of only two nonreducing sugars found widely in nature, the other being sucrose. In bacteria, fungi, and insects, trehalose functions as a storage carbohydrate and protects against a variety of stresses (1). In plants, this role has been largely replaced by sucrose, although trehalose does protect against desiccation in certain specialized resurrection plants (2, 3). Absence or trace amounts of trehalose in most plants precludes a role as a reserve or stress protectant.
Since 1998, when genes encoding trehalose pathway constituents were first reported in Arabidopsis thaliana (4, 5), it has become clear that trehalose metabolism is ubiquitous in plants. There are as many as 11 trehalose phosphate synthase (TPS, EC 2.4.1.15) homologues in A. thaliana (6). Expression of TPS and trehalose phosphate phosphatase (TPP, EC 3.1.3.12) genes has been detected in all organs tested (4, 5, 7, 8), and the expression of some displays circadian control (9). TPS genes in particular are expressed at very low levels [Arabidopsis Functional Genomics Consortium (AFGC) and Genomic Arabidopsis Resource Network (GARNet) Affymetrix databases], including AtTPS1, which is expressed in all tissues (ref. 4; A.v.D., H.S., and S.S., unpublished results). Low ubiquitous expression is typical of genes of regulatory metabolisms; the adenylate cyclase gene for cAMP metabolism is an example in yeast (10). Further, AtTPS1 is essential during embryogenesis (7), suggesting an important role for trehalose metabolism in plants. However, it is unclear whether this essential role is played by the intermediate in the pathway T6P, trehalose, or some other regulatory property of TPS1. A wider role of trehalose metabolism in plants remains largely obscure, although the work of Eastmond et al. (7) did suggest an interaction with sucrose metabolism.
In yeast species including Saccharomyces cerevisiae, T6P plays a critical role in the regulation of carbon flux into glycolysis. Yeast fdp1, cif1, and byp1 mutants, where the primary lesion is in the TPS1 gene (11–13), cannot grow on glucose due to unregulated glycolysis, which results in Pi sequestration in accumulated phosphorylated intermediates and consequential inhibition of ATP synthesis (14–16). T6P inhibits yeast hexokinase II activity in vitro at physiological concentrations (17), and the tps1 mutant phenotype can be rescued by reducing hexokinase activity by deletion of the ScHXKII gene (18). In yeast, therefore, T6P is an important component of glycolytic regulation. T6P does not, however, seem to inhibit plant hexokinases in vitro (7, 19), and the role of this metabolite in plant metabolism remains in question.
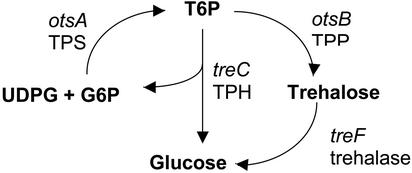
We combined biochemical and genetic approaches to address the function of the trehalose pathway in plants. We engineered A. thaliana for expression of genes encoding enzymes of the Escherichia coli trehalose pathway (Fig. 1), otsA, otsB, treC, and treF. OtsA encodes a TPS, otsB a TPP (20, 21), treC a trehalose-6-phosphate (T6P) phospho hydrolase (TPH; EC 3.2.1.93) (22). TreF is the cytoplasmic trehalase (EC 3.2.1.28; ref. 23). Expression of E. coli genes in plants has the advantage that enzymes encoded by these genes are unlikely to be subject to endogenous plant regulatory mechanisms. OTSA and OTSB have previously been shown to be active in tobacco plants (24).
Fig. 1.
Reactions catalyzed by the E. coli enzymes used for this study. otsA encodes a trehalose phosphate synthase (TPS), otsB a trehalose phosphate phosphatase (TPP), treC a trehalose phosphate hydrolase (TPH), and treF a trehalase.
Expression of TPS, TPP, or TPH affected T6P content and phenotype in parallel; expression of active trehalase did neither affect T6P content nor alter development of the plants. Sugar-feeding experiments suggest that changes in phenotype could be ascribed to T6P regulating carbohydrate utilization for growth, a feat not accomplished before by metabolic engineering. Complementation of the tps1 mutant by E. coli TPS expression but not by exogenous trehalose supply further confirms that T6P is essential in A. thaliana as in yeast.
Methods
Plasmids. CaMV35S::otsA and otsB constructions were pMog 799 and 1010 described in patents WO95/01446 and WO97/42326. CaMV35S::treC and treF were constructed by insertion of PCR-amplified treC or treF into NcoI blunt BstEII pCambia 1304 (Cambia, Australia). TreC and treF fragments were also cloned behind the ethanol-inducible switch (25) into pZM1423 (Syngenta, Bracknell, U.K.). AtTPS1 cDNA was PCR amplified from cDNA, then used to screen a LambdaGEM-11 phage (Promega) library of genomic Arabidopsis DNA. One phage isolated contained 3-kb AtTPS1 promoter, which was cloned as an EcoR1-blunt BstEII fragment to drive otsA expression.
Plant Material. A. thaliana plants were Col.0. tps1-2 is described in ref. 7. Seeds were sown on soil, stratified at 4°C for 3 days, then transferred to 22°C and plants grown in 16 h/day at 200 μmol·m-2·s-1 irradiance. For growth on plates, seeds were sterilized (26), then sown in plates onto 0.8% wt/vol agar solidified medium containing half-strength Murashige and Skoog (MS; ref. 27). Media containing sugars or antibiotic were prepared by mixing filter-sterilized stock solutions with pre-cooled agar/MS. Transformations were as in ref. 26 by using Agrobacterium tumefaciens strains GVG 2260 and EHA105. Selection of the first generation transgenic (T1) was on agar solidified 1/2 MS with either 20 mg/liter hygromycin or 25 mg/liter kanamycin. Lines with a transgenic generation 2 (T2) segregation consistent with single locus integration were chosen to produce T3 seed; only homozygous seed from T3 or generations beyond T3 was used for experimentation. T1 plants containing CaMV35S::otsA were mostly infertile; 11 of 31 plants set seed. Line A19.3 had the strongest phenotype among lines with sufficient seed and two copies of the transgene in a single locus and had high and stable otsA expression over five generations. Line B12.1 has at least three copies of CaMV35S::otsB in a single locus with high and stable otsB expression over five generations. Lines C10.10 and F11.2, expressing treC and treF behind the ethanol switch, also express the transgenes without ethanol induction.
Plant DNA Analysis. Seedling or leaf material was frozen in liquid N2, pulverized with glass beads for 2 min at 3,000 rpm in a Dismembranator (Braun, Melsungen, Germany), then DNA extracted by using Pure gene DNA isolation kit (Gentra Systems). PCR detection of wild-type (wt) TPS1 and tps1-2 mutation was with primers RB (GGCTTGTGTGGAACTTACTATG) located in the transposon Right Border, tps4d (TGTGAGCGTATGCCTGGAAATAA), and tps47u (AGCCCATTGTATCCATCTG). Primers tps4d and 47u amplify a 570-bp fragment of wt AtTPS1 that is disrupted by transposon insertion in tps1-2, whereas primers tps47u and RB amplify a 1,056-bp fragment present only in tps1-2.
Metabolite Analysis. Seedlings were grown on plates for 7 days, transferred to the dark for 2 h, then collected, weighed, snap frozen, and ground in liquid N2. Powder (50–100 mg of tissue) was extracted in 5 vol 5% perchloric acid on ice for 30 min, then neutralized with 5 M KOH in 1 M triethanolamine and metabolites measured by using enzyme-linked assays as in ref. 28. Brief ly, glucose-6-phosphate (G6P), fructose-6-phosphate (F6P), glucose-1-phosphate (G1P), and uridine-diphosphate glucose (UDPG) were assayed together in 50 mM Hepes (pH 7) with 5 mM MgCl2, 0.25 mM NADP, and 0.12 mM sodium pyrophosphate. Metabolites were measured by sequential addition of 0.1 units each of G6P dehydrogenase, phosphoglucose isomerase, phopshoglucomutase, and UDPG pyrophosphorylase. ATP was measured in 50 mM Hepes (pH 7), 5 mM MgCl2, 0.25 mM NADP, 0.8 mM glucose, 0.1 units of G6P dehydrogenase, and 0.1 units of phosphoglucose isomerase. Reaction was started with 0.1 units of hexokinase. Citrate was measured in Tris·HCl (pH 7.6), 0.15 mM NADH, 0.04 mM ZnCl2, and 0.1 units of malic dehydrogenase, and started with 0.1 units of citrate lyase. All measurements were performed on a dual-wavelength spectrophotometer 340 nm/410 nm, on 50- to 100-μl tissue extract in 1 ml of the above reagents. T6P was extracted from frozen pulverized leaf tissue (1 g fresh weight). Powder was extracted 20 min with 5 ml of 80% boiling ethanol and centrifuged 10 min at 10,000 × g, and the supernatant obtained was vacuum desiccated. The dry sample was extracted with 1.2 ml of boiling 0.1 M NaOH for 1 h and centrifuged 10 min at 13,000 rpm, and the supernatant obtained was neutralized with 12 ml of 200 mM triethanolamine (pH 7.6), then split into two equal portions. One unit of alkaline phosphatase (type IV-S from bovine intestinal mucosa, Sigma), 10 mM MgCl2, and 1 mM ZnCl2 were added to one portion to efficiently remove T6P (zero control) at 25°C for 20 min, followed by 90°C for 45 min and centrifugation to remove the phosphatase activity. T6P was assayed based on inhibition of Yarrowia lipolytica hexokinase (29); assays were in quadruplicate on microtiter plates and included an internal standard curve of 0–800 pmol T6P for each sample. S. cerevisiae expressing this hexokinase, but lacking its own, was used (30). Yeast cake was extracted in 20 mM imidazole buffer by vortexing with glass beads and centrifuged 10 min at 700 × g, and the supernatant was used for hexokinase inhibition assay with 0.2 units of phosphoglucose isomerase, 0.2 units of glucose-6-phosphate dehydrogenase, 1 mM ATP, 0.5 mM NADP, and 0.1 mM fructose final concentration. Assays were linear for 30 min and could resolve T6P levels of less than 50 pmol T6P. Recoveries of T6P were in excess of 80%. Amounts of T6P quantified this way were confirmed by HPLC by using a method modified from ref. 31. Trehalose was determined as in ref. 24.
Results
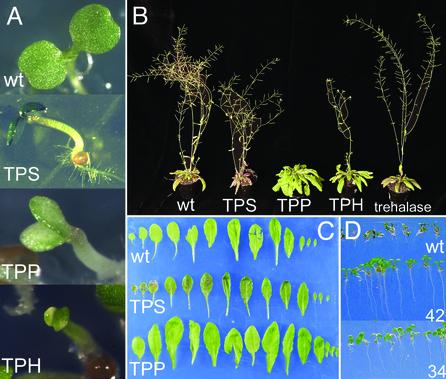
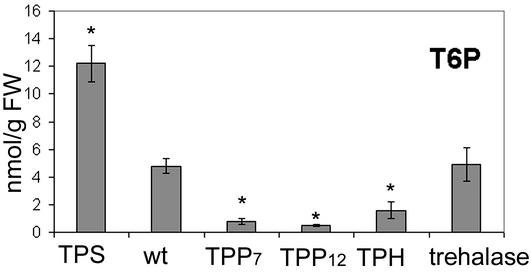
Contrasting Phenotypes Parallel T6P Content. Seedlings expressing TPS with the CaMV35S promoter are dark green with anthocyanin accumulation along the rim of the cotyledons (Fig. 2A). Rosette leaves of the developing plantlets are smaller and dark green compared with wt (Fig. 2 B and C). Mature plants are bushy, and seed set in early flowers is poor except under continuous light. Seedlings expressing TPP or TPH with the CaMV35S promoter have cotyledons that expand and green 1–3 days later than wt and display characteristic bleached areas (Fig. 2 A). Leaves of the mature plants are lighter green and larger than wt (Fig. 2 B and C). The mature plants bolt up to 3 wk later than wt and have a pronounced apical dominance (Fig. 2B). Seed set is plentiful. Seedlings expressing trehalase with the CaMV35S promoter develop extensive roots and primary leaves when grown on 100 mM trehalose compared with wt seedlings, which do not grow on trehalose and accumulate anthocyanin (Fig. 2D). Seedlings and mature plants expressing trehalase and grown in soil are indistinguishable from wt (Fig. 2B). The phenotypes of the transgenics therefore center on the metabolism of T6P rather than trehalose, and we proceeded to measure T6P and trehalose. Leaves from wt typically contain 5 nmol T6P per g fresh weight (FW; Fig. 3), 20 times less than amounts of G6P (Fig. 5). TPS expression results in increased T6P content in leaves, typically 2 to 3 times the amount in wt plants with high otsA transcription (line A19.3, Fig. 3). Plants expressing TPP and TPH have low T6P levels, typically more than two-fold lower than wt (Fig. 3). T6P content of trehalase expressors was the same as wt. Trehalose in all plants was less than T6P and below a detection limit of 2 nmol·g-1 FW.
Fig. 2.
Typical seedling and mature plant phenotypes of Arabidopsis Col.0 expressing E. coli TPS, TPP, THP, and trehalase using the CaMV35S promoter. (A) Seedlings grown for 5 days on 1/2 MS medium. (B) Plants grown for 6 wk in long day conditions. (C) Leaves from rosettes 1 wk after bolting, wt, TPS line A19, and TPP line B12. (D) Seedlings grown for 7 days on 1/2 MS with 100 mM trehalose. wt, trehalase expressor lines 42 and 34.
Fig. 3.
T6P content in lines expressing TPS (line A19), TPP (lines 7 and 12), TPH, and trehalase. The mean content of three independent lines is shown in the case of TPH and trehalase expressors. Plants were grown on soil for 4 wk, and leaf material was harvested as described in Methods. A minimum of three independent determinations were carried out for each line. *, Significantly different from wt (P < 0.05).
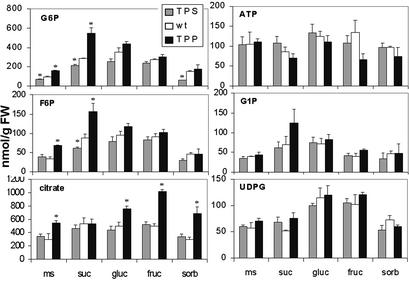
Fig. 5.
Profiles of metabolites in seedlings without or with sugars in the medium at 7 days. Seedlings were grown on 1/2 MS without or with 100 mM glucose (gluc), fructose (fruc), sucrose (suc), or sorbitol (sorb). Data shown are typical for a series of experiments. TPS, and TPP were as in Fig. 4. *, Significantly different from wt under the same growth conditions (P < 0.05).
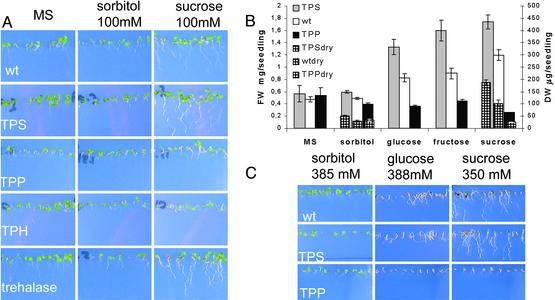
T6P Levels Determine the Capacity to Use Sugar Supplied. To test the hypothesis that T6P regulates carbohydrate utilization in plants as in yeast (15), we examined the response of seedlings to sugar feeding. First, seedlings were grown for 7 days on agar solidified 1/2 MS medium with or without addition of 100 mM sorbitol, glucose, fructose, or sucrose (Fig. 4A). Seedlings of all lines are not affected by 100 mM sorbitol. Seedlings expressing TPP and TPH develop much slower than wt on 100 mM glucose, fructose, or sucrose, with reduced cotyledon expansion and greening, reflected in the lower fresh and dry weight after 7 days (Fig. 4B). Seedlings expressing TPS grow faster than wt, displaying optimum growth on sucrose (Fig. 4B). Second, seedlings were grown on agar-solidified 1/2 MS medium with 7% wt/vol sorbitol (384 mM) or glucose (388 mM) or 12% wt/vol sucrose (351 mM; Fig. 4C). wt and transgenic seedlings all develop a little slower on 7% wt/vol sorbitol than on medium lacking sugar. On 7% wt/vol glucose and 12% wt/vol sucrose, cotyledons of wt seedlings do not expand or green even though root growth is prolific. TPS expressors are similarly sensitive to the high sugar concentration, with no greening or expansion of the cotyledons after 14 days. However, growth of TPP expressors is completely arrested by high sugar (Fig. 4C), with no hypocotyl expansion or root growth even after several months; this result also holds for TPH expressors (data not shown). Growth inhibition of seedlings expressing TPP or TPH gradually increases with rising sugar concentration (data not shown).
Fig. 4.
Growth of seedlings expressing TPS (line A19), TPP (line B12), TPH (line C10.10), and trehalase (line F12.1) on media with or without sugars. (A) Seedling responses to 100 mM sorbitol and sucrose. Seedlings were grown for 7 days in plates as described in Methods.(B) Fresh weight (FW) and dry weight (DW) of 7-day seedlings on 1/2 MS (MS) without or with 100 mM sorbitol, glucose, fructose, and sucrose. TPSdry, wtdry, and TPPdry correspond to DW determinations. Differences between TPS, wt, and TPP are significant for all FW determinations, except those from seedlings grown in 1/2 MS, and for DW determinations on sucrose (P < 0.005; n = 6). (C) Seedling responses at 14 days to high concentrations of sorbitol and metabolizable sugars. TPH seedlings are not shown because they resemble TPP seedlings.
T6P Levels Determine the Accumulation of Respiratory Intermediates in Seedlings. In yeast tps1 mutants, inability to grow on sugar is associated with a large build up of phosphorylated intermediates. We therefore measured dark levels of respiratory metabolites in response to feeding 100 mM sorbitol, sucrose, fructose, or glucose. Without added sugar, expressing TPS leads to a generally lower content of G6P (ms, Fig. 5), compared with wt. Conversely, expressing TPP leads to increased levels of G6P, F6P, and citrate (Fig. 5). G1P, UDPG, and ATP levels are similar in all lines. Overall, differences in G6P are greatest and inversely proportional to levels of T6P in the lines. Feeding 100 mM sorbitol does not affect the levels of sugar phosphates in all lines when compared with growth on 1/2 MS (Fig. 5). Feeding sucrose, fructose, and glucose to wt seedlings leads to an increase in G6P and F6P. Feeding sucrose leads to hexose phosphate contents that are opposite to those of T6P: accumulation in plants with low T6P and depletion in plants with high T6P content. There are also trends in ATP content that is lowest in plants expressing TPP. Feeding fructose does not result in the significant hexose phosphate changes observed for sucrose, although fructose is as effective as sucrose for promoting growth of TPS expressors. Feeding fructose to TPP expressors leads to a large accumulation of citrate, but there is no depletion of citrate in TPS expressors on fructose, and this effect may therefore not be directly linked to T6P levels. Feeding glucose results in metabolite profiles that are “in between” sucrose and fructose feeding. Sugar feeding does not lead to clear differences in UDPG or G1P content between the lines. Taken together, manipulation of T6P levels affects pools of respiratory intermediates, with the effect most evident at the level of G6P and F6P analogous to the effect described in bakers yeast (15). Feeding fructose, however, circumvents the hexokinase step and uncovers an effect further downstream in respiration. This downstream effect needs yet to be directly related to T6P. But fructose feeding leads to increased growth of TPS expressors over wt and decreased growth of TPP and TPH expressors over wt, and this effect downstream in respiration might hence also be due to changes in T6P content.
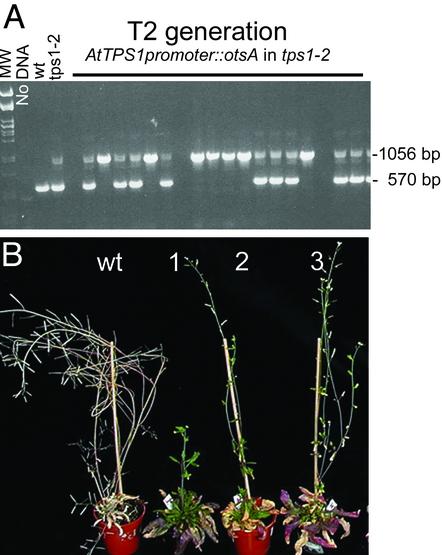
T6P Is Indispensable. Embryos homozygous for the tps1 mutation develop slower than wt and are arrested at torpedo stage, the stage at which developing seeds accumulate vast amounts of sucrose for synthesis of storage lipid and protein (7). In light of the above results suggesting a role for T6P in carbohydrate utilization, we wondered whether T6P was critically lacking in tps1 embryos even though another 10 TPS homologues are present in the Arabidopsis genome. To test this, we introduced E. coli TPS behind a 3-kb AtTPS1 promoter previously validated in complementation experiments (data not shown) and transformed heterozygous tps1-2. The first generation transgenic (T1) was selected on hygromycin, then grown on soil, and siliques of 40 T1 plants were analyzed for presence of arrested embryos. Ten T1 had no arrested embryos consistent with a TPS1/TPS1 genotype. Four T1 had an arrested to wt embryo ratio of 1/3 consistent with a heterozygous tps1–2/TPS1 genotype. Sixteen T1 had an arrested to wt embryo ratio of much less than 1/3. T2 plants were selected on phosphinotricin for presence of the tps1-2 transposon insertion and simultaneously on hygromycin for presence of the E. coli TPS construct. PCR analysis of DNA from this T2 generation reveals that 24 of 75 T2 plants tested are homozygous for the tps1-2 mutation (Fig. 6A). E. coli TPS expression hence rescues tps1-2 embryos, and viable plants are obtained at the expected frequency. E. coli TPS, consisting of a single catalytic domain, is unlikely to engage in cellular signaling processes; the result therefore points to T6P as the indispensable component in arrested tps1 embryos. Plants rescued by E. coli TPS expression and homozygous for the tps1-2 mutation appear like wt in 10 of 18 plants analyzed; the remaining plants are late-flowering to varying extent, with formation of aerial rosettes and no seed set in three plants (Fig. 6B). Expression behind the AtTPS1 promoter is extremely low (data not shown), and the phenotypes observed are consistent with the delayed flowering observed in low T6P plants generated by expressing TPP and TPH.
Fig. 6.
Complementation of tps1-2 with AtTPS1promoter::otsA. (A) Segregation of TPS1 and tps1-2 in the T2 generation containing tps1-2 and AtTPS1promoter::otsA. Heterozygous tps1-2 plants were transformed with the AtTPS1promoter::otsA construct described in Methods on a T-DNA with a hygromycin selectable marker. The first transgenic generation, T1, was selected hygromycin, and 42 T1 were chosen randomly for analysis of their offspring, the T2 generation. T2 plants resistant to hygromycin and phosphinotricin [the bar resistance gene is linked to the transposon insertion in tps1-2 (7)] were then grown from each of the T1 and DNA was extracted from the individual T2 plants and analyzed by PCR for presence of tps1-2 and TPS1 genes as described in Methods. A 570-bp fragment is amplified from AtTPS1 but not from the gene disrupted by transposon insertion in tps1-2 whereas a 1056-bp fragment is amplified from the transposon border present in tps1-2. MW is pst1-digested lambda phage; NoDNA is PCR without template DNA; then PCR with DNA from wt Col.0 (wt), tps1-2 (tps1-2), and from six offspring of the T1 plant 201-1, from eight offspring of the T1 plant 201-8, and from three offspring of the T1 plant 201-19 (T2 generation). (B) Examples of late-flowering T2 plants obtained from tps1-2 complementation with AtTPS1promoter::otsA. T2 offspring were grown until flowering, which, for 8 of 18 otsA complemented homozygous tps1-2, was late. Three examples of these late-flowering plants (1, 2, and 3) are compared with a wt plant.
Discussion
T6P Has a Critical Role in Plant Growth and Development. Our data provide several lines of evidence to support a regulatory role for T6P in plant growth and development. First, expression of TPP and TPH produces the same visual phenotype. TPP and TPH enzymes cleave T6P to different end products: trehalose in the case of TPP and glucose and G6P in the case of TPH. This finding suggests that cleavage of T6P rather than synthesis of the end-products is responsible for the large pale leaf phenotype of the adult plants. Also, this finding rules out a nonspecific phosphatase effect in the TPP transgenics because the phenotype obtained by the hydrolytic cleavage of T6P is the same. Second, expression of E. coli TPS, which catalyzes synthesis of T6P, produces a phenotype distinct from and opposite that of the plants expressing TPP and TPH. Plants have smaller darker green leaves in contrast to the large pale leaves of the TPP and TPH expressors. Third, expression of E. coli cytoplasmic trehalase, which results in enhanced metabolism of trehalose demonstrated through growth on 100 mM trehalose, produces a phenotype identical to wt when grown on soil. This finding further suggests that T6P, rather than trehalose, causes the opposed phenotypes of TPS and TPP or TPH expressors. The conclusion arising from the above genetic experiments was confirmed by determination of T6P and trehalose contents of leaves. TPS expressors had elevated T6P whereas TPP and TPH expressors had reduced T6P levels compared with wt. Expression of trehalase did not affect T6P content. At the same time, the trehalose content of leaves was below the detection limit and below that of T6P. This finding confirms the work of Goddijn et al. (24), who were unable to detect trehalose in vegetative tissue of tobacco expressing otsA and otsB except when plants were fed validamycin A to inhibit endogenous extracellular trehalase. Similar observations were made in wt Arabidopsis (32). The correlation of T6P content with phenotype in transgenics produced from three different transgenes provides strong evidence that T6P is responsible for the alterations in growth and development observed. Consistent with a regulatory role of T6P is the low micromolar range concentration measured.
An absolute requirement for AtTPS1 during embryogenesis and hence for the trehalose pathway in plants has been observed (7). However, it was unclear whether T6P, trehalose, or some other regulatory feature of TPS1, or a combination of these, were necessary. Trehalose supplied to tps1 embryos in culture failed to rescue the mutation (7), and the problem unlikely lies with trehalose import into the cells as we demonstrate that trehalose is taken up by plant cells: intracellular trehalase expression protects seedlings from the toxic effect of high trehalose in the medium (Fig. 2D). Moreover, alpha-glycoside transporters are likely expressed during embryo development (33), and those that have been characterized best in yeast are notoriously promiscuous (34, 35). The tps1 mutant phenotype can be fully rescued with E. coli TPS (Fig. 6), however, suggesting that T6P is the critical requirement. Additional regulatory functions of the yeast TPS1 complex have been suggested (36, 37), and the large multiple domain AtTPS1 protein may well have several functions. Yet, these AtTPS1 functions seem to be dispensable for rescue of the tps1 phenotype by the single domain E. coli TPS. That T6P is indispensable for the breakdown of sucrose supplied to the embryo (7) is compatible with our observation that T6P content controls carbohydrate utilization.
T6P Levels Regulate Carbohydrate Utilization for Growth. Quite remarkably, growth of TPS expressors on sugars was significantly improved over wt whereas it was strongly inhibited in TPP and TPH expressors (Fig. 4 A, B, and C). We are unaware of any other manipulation in metabolism that has resulted in such spectacular control of carbon resource utilization. These results may provide novel opportunities for improving crop yield via modification of metabolism.
To understand the basis of this response in plants, we tested the only known biological function of T6P except as an intermediate in trehalose biosynthesis, that as a regulator of the flux through glycolysis in yeast. Yeast mutants with lesions in TPS1 accumulate respiratory intermediates in response to glucose feeding; the resulting Pi sequestration inhibits ATP synthesis and hence growth (14, 15, 16). Arabidopsis expressing TPP might be expected to display a similar phenotype if T6P were acting in an analogous fashion. These lines already showed signs of perturbed respiratory metabolism in the absence of sugar feeding as levels of G6P, F6P, and citrate were clearly higher than in wt (Fig. 5, ms). After sucrose feeding, contents in G6P and F6P were inversely proportional to T6P. Differences in G1P and UDPG were small. This finding indicates an effect on glycolytic flux rather than on reactions upstream of hexokinase. Fructose feeding did not lead to changes in G6P and F6P, thus confirming that hexokinase could be responsible for accumulation of these compounds. Furthermore, sugar feeding produced trends in ATP content in parallel with effects on growth. Results therefore point to glycolysis as a site of T6P regulation in Arabidopsis. This finding would be analogous to more comprehensive studies carried out in yeast (36, 38). In contrast to mammalian hexokinases, both yeast and plant hexokinases are not subject to physiologically relevant feedback regulation by G6P (39, 40). In yeast, regulation by T6P may have evolved specifically to cope with sudden and large variations in carbohydrate availability, increasing the dynamic range of regulation under highly variable environmental conditions, in contrast to mammals where glucose levels are relatively constant (40). The same could apply to plants. However, despite similarities with the yeast model, there is no evidence that T6P inhibits plant hexokinases or fructokinases assayed in vitro so far (refs. 7 and 19; unpublished results).
The Arabidopsis genome contains at least six hexokinase homologues, and EST databases show that five of these are expressed. Biochemical assays of Arabidopsis extracts do not distinguish different isoforms. We can therefore not rule out a direct control by T6P on hexokinase activity in Arabidopsis. Indirect control of hexokinase activity through regulatory systems acting at multiple sites is also a possibility. Feeding fructose led to essentially the same growth reactions as sucrose yet to no changes in G6P and F6P contents. Fructose feeding circumvents hexokinase and uncovers another site of respiratory regulation downstream of glycolysis. In yeast, the possibility of another T6P/Tps1 regulated step beyond glycolysis has also been proposed (37). Although our study does not allow assigning this effect to T6P yet, the possibility that T6P controls a step beyond citrate is attractive.
Respiration is required to allow efficient redox exchange between cell compartments to avoid overreduction of the photosynthetic system (41). T6P control over respiration might therefore begin to explain the altered photosynthetic capacity measured in the plants presented here (M.P., unpublished results) and in tobacco plants expressing the same genes (42). We examined the levels of expression of genes known to be affected by hexokinase-mediated sugar repression (43). Expression of neither the Rubisco small subunit nor the chlorophyll a/b binding proteins in 5-day-old seedlings was affected in the transgenics (data not shown). T6P may therefore not control this particular function of hexokinase. This result is consistent with the hypothesized link between T6P-mediated control of respiration and photosynthesis. In yeast, the exact nature of the interaction of T6P and TPS with carbohydrate utilization is only partially understood (6, 44), and clearly much remains to be discovered in plants, too. Interestingly, a cauliflower TPS interacts with 14-3-3 proteins (45), now known as a class II TPS (J. Harthill and C. Mackintosh, personal communication). 14-3-3 binding depends on the nutritional status of cells and determines the stability of the target protein in response to carbon supply (46). Furthermore, Arabidopsis TPSs possess putative SnRK1 phosphorylation sites, and SnRK1 is thought to be subject to regulation by G6P (47). Such interactions provide the basis for sophisticated regulation of T6P synthesis in response to signals in the cell. If the role of T6P is conserved, the links between environmental cues such as stress and trehalose metabolism may be as well. The requirement for carefully regulated T6P synthesis to regulate carbohydrate utilization in different tissues under different conditions and at different developmental stages may explain the plethora of TPS genes found in the Arabidopsis genome.
Our results in Arabidopsis show that the T6P component of the trehalose pathway is active and indispensable in plants by regulating carbohydrate utilization and growth. This central role of T6P may be more widely conserved than previously imagined. Beyond yeasts and plants, it could well be conserved in insects: Drosophila mutated in the TPS has recently been shown to be embryo lethal (48). There exists a particular pressing need to find the site and details of the T6P interaction in all these organisms.
Acknowledgments
We thank Carlos Cancedo for providing us with Yarrowia lipolytica hexokinase. We acknowledge with gratitude discussions with Klaus Peter Krause, Oscar Goddijn, Anne Ponstein, and Deborah Keith from Syngenta. A.v.D. and H.S. acknowledge financial support from the Dutch Science Foundation (Chemical Science/Foundation for Applied Science Project 349-4657). Rothamsted Research receives grant-aided support from the Biotechnological and Biological Sciences Research Council of the United Kingdom. T.P. acknowledges a Biotechnology and Biological Sciences Research Council case studentship awarded to Rothamsted and Syngenta Mogen.
Abbreviations: T6P, trehalose-6-phosphate; TPS, trehalose-6-phosphate synthase; TPP, trehalose-6-phosphate phosphatase; TPH, trehalose-6-phosphate hydrolase; MS, Murashige and Skoog; G6P, glucose-6-phosphate; F6P, fructose-6-phosphate; G1P, glucose-1-phosphate; UDGP, uridine-diphosphate glucose; wt, wild type.
References
- 1.Goddijn, O. J. & van Dun, K. (1999) Trends Plant Sci. 4, 315-319. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi, G., Gamba, A., Limiroli, R., Pozzi, N., Elster, R., Salamini, F. & Bartels, D. (1993) Physiol. Plant 87, 223-226. [Google Scholar]
- 3.Drennan, P. M., Smith, M. T., Goldsworthy, D. & van Staden, J. (1993) J. Plant Physiol. 142, 493-496. [Google Scholar]
- 4.Blazquez, M., Santos, E., Flores, C., Martinez-Zapater, J., Salinas, J. & Gancedo, C. (1998) Plant J. 13, 685-689. [DOI] [PubMed] [Google Scholar]
- 5.Vogel, G., Aeschbacher, R. A., Muller, J., Boller, T. & Wiemken, A. (1998) Plant J. 13, 673-683. [DOI] [PubMed] [Google Scholar]
- 6.Leyman, B., Van Dijck, P. & Thevelein, J. M. (2001) Trends Plant Sci. 6, 510-513. [DOI] [PubMed] [Google Scholar]
- 7.Eastmond, P. J., van Dijken, A. J., Spielman, M., Kerr, A., Tissier, A. F., Dickinson, H. G., Jones, J. D., Smeekens, S. C. & Graham, I. A. (2002) Plant J. 29, 225-235. [DOI] [PubMed] [Google Scholar]
- 8.Vogel, G., Fiehn, O., Jean-Richard-dit-Bressel, L., Boller, T., Wiemken, A., Aeschbacher, R. A. & Wingler, A. (2001) J. Exp. Bot. 52, 1817-1826. [DOI] [PubMed] [Google Scholar]
- 9.Harmer, S. L., Hogenesch, J. B., Straume, M., Chang, H. S., Han, B., Zhu, T., Wang, X., Kreps, J. A. & Kay, S. A. (2000) Science 290, 2110-2113. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka, T., Broek, D. & Wigler, M. (1985) Cell 43, 493-505. [DOI] [PubMed] [Google Scholar]
- 11.Bell, W., Klaassen, P., Ohnacker, M., Boller, T., Herweijer, M., Schoppink, P., Van der Zee, P. & Wiemken, A. (1992) Eur. J. Biochem. 209, 951-959. [DOI] [PubMed] [Google Scholar]
- 12.Thevelein, J. M. & Hohmann, S. (1995) Trends Biochem. Sci. 20, 3-10. [DOI] [PubMed] [Google Scholar]
- 13.Vuorio, O. E., Kalkkinen, N. & Londesborough, J. (1993) Eur. J. Biochem. 216, 849-861. [DOI] [PubMed] [Google Scholar]
- 14.Hohmann, S., Bell, W., Neves, M. J., Valckx, D. & Thevelein, J. M. (1996) Mol. Microbiol. 20, 981-991. [DOI] [PubMed] [Google Scholar]
- 15.Van Aelst, L., Hohmann, S., Bulaya, B., de Koning, W., Sierkstra, L., Neves, M. J., Luyten, K., Alijo, R., Ramos, J. & Coccetti, P. (1993) Mol. Microbiol. 8, 927-943. [DOI] [PubMed] [Google Scholar]
- 16.Van de Poll, K. & Schambart, D. (1974) Mol. Gen. Genet. 154, 61-66. [DOI] [PubMed] [Google Scholar]
- 17.Blazquez, M., Lagunas, R., Gancedo, C. & Gancedo, J. (1993) FEBS Lett. 329, 51-54. [DOI] [PubMed] [Google Scholar]
- 18.Hohmann, S., Neves, M. J., de Koning, W., Alijo, R., Ramos, J. & Thevelein, J. M. (1993) Curr. Genet. 23, 281-289. [DOI] [PubMed] [Google Scholar]
- 19.Wiese, A., Groner, F., Sonnewald, U., Deppner, H., Lerchl, J., Hebbeker, U., Flugge, U. & Weber, A. (1999) FEBS Lett. 461, 13-18. [DOI] [PubMed] [Google Scholar]
- 20.Gibson, R. P., Lloyd, R. M., Charnock, S. J. & Davies, G. J. (2002) Acta Crystallogr. D Biol. Crystallogr. 58, 349-351. [DOI] [PubMed] [Google Scholar]
- 21.Kaasen, I., Falkenberg, P., Styrvold, O. B. & Strom, A. R. (1992) J. Bacteriol. 174, 889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rimmele, M. & Boos, W. (1994) J. Bacteriol. 176, 5654-5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horlacher, R., Uhland, K., Klein, W., Ehrmann, M. & Boos, W. (1996) J. Bacteriol. 178, 6250-6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goddijn, O. J., Verwoerd, T. C., Voogd, E., Krutwagen, R. W., de Graaf, P. T., van Dun, K., Poels, J., Ponstein, A. S., Damm, B. & Pen, J. (1997) Plant Physiol. 113, 181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caddick, M. X., Greenland, A. J., Jepson, I., Krause, K., Qu, N., Riddell, K., Salter, M., Schuch, W., Sonnewald, U. & Tomsett, A. (1998) Nat. Biotechnol. 16, 177-180. [DOI] [PubMed] [Google Scholar]
- 26.Clough, S. J. & Bent, A. F. (1998) Plant J. 16, 735-743. [DOI] [PubMed] [Google Scholar]
- 27.Murashige, T. & Skoog, F. (1962) Physiol. Plant 15, 473-497. [Google Scholar]
- 28.Stitt, M., Lilley, R. M., Gerhardt, R. & Heldt, H. W. (1989) Methods Enzymol. 174, 518-552. [Google Scholar]
- 29.Blazquez, M. A., Gancedo, J. M. & Gancedo, C. (1994) FEMS Microbiol. Lett. 121, 223-227. [DOI] [PubMed] [Google Scholar]
- 30.Petit, T. & Gancedo, C. (1999) Yeast 15, 1573-1584. [DOI] [PubMed] [Google Scholar]
- 31.Paul, M., Driscoll, S., Andralojc, P., Knight, J., Gray, J. & Lawlor, D. (2000) Planta 2000 211, 112-119. [DOI] [PubMed] [Google Scholar]
- 32.Muller, J., Aeschbacher, R. A., Wingler, A., Boller, T. & Wiemken, A. (2001) Plant Physiol. 125, 1086-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruuska, S. A., Girke, T., Benning, C. & Ohlrogge, J. B. (2002) Plant Cell 14, 1191-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han, E. K., Cotty, F., Sottas, C., Jiang, H. & Michels, C. A. (1995) Mol. Microbiol. 17, 1093-1107. [DOI] [PubMed] [Google Scholar]
- 35.Wieczorke, R., Krampe, S., Weierstall, T., Freidel, K., Hollenberg, C. P. & Boles, E. (1999) FEBS Lett. 464, 123-128. [DOI] [PubMed] [Google Scholar]
- 36.Bonini, B. M., Van Vaeck, C., Larsson, C., Gustafsson, L., Ma, P., Winderickx, J., Van Dijck, P. & Thevelein, J. M. (2000) Biochem J. 350, 261-268. [PMC free article] [PubMed] [Google Scholar]
- 37.Noubhani, A., Bunoust, O., Rigoulet, M. & Thevelein, J. M. (2000) Eur. J. Biochem. 267, 4566-4576. [DOI] [PubMed] [Google Scholar]
- 38.Neves, M. J., Hohmann, S., Bell, W., Dumortier, F., Luyten, K., Ramos, J., Cobbaert, P., de Koning, W., Kaneva, Z. & Thevelein, J. M. (1995) Curr. Genet. 27, 110-122. [DOI] [PubMed] [Google Scholar]
- 39.Renz, A. & Stitt, M. (1993) Planta 190, 166-175. [Google Scholar]
- 40.Teusink, B., Walsh, M. C., van Dam, K. & Westerhoff, H. V. (1998) Trends Biochem. Sci. 23, 162-169. [DOI] [PubMed] [Google Scholar]
- 41.Dutilleul, C., Driscoll, S., Cornic, G., De Paepe, R., Foyer, C. H. & Noctor, G. (2003) Plant Physiol. 131, 264-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul, M., Pellny, T. & Goddijn, O. (2001) Trends Plant Sci. 6, 197-200. [DOI] [PubMed] [Google Scholar]
- 43.Martin, T., Oswald, O. & Graham, I. A. (2002) Plant Physiol. 128, 472-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silva-Udawatta, M. N. & Cannon, J. F. (2001) Mol. Microbiol. 40, 1345-1356. [DOI] [PubMed] [Google Scholar]
- 45.Moorhead, G., Douglas, P., Cotelle, V., Harthill, J., Morrice, N., Meek, S., Deiting, U., Stitt, M., Scarabel, M., Aitken, A., et al. (1999) Plant J. 18, 1-12. [DOI] [PubMed] [Google Scholar]
- 46.Cotelle, V., Meek, S. E., Provan, F., Milne, F. C., Morrice, N. & MacKintosh, C. (2000) EMBO J. 19, 2869-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toroser, D., Plaut, Z. & Huber, S. C. (2000) Plant Physiol. 123, 403-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen, Q., Ma, E., Behar, K. L., Xu, T. & Haddad, G. G. (2002) J. Biol. Chem. 277, 3274-3279. [DOI] [PubMed] [Google Scholar]