Abstract
After pollen grains germinate on the stigma, pollen tubes traverse the extracellular matrix of the style on their way to the ovules. We previously characterized two pollen-specific, receptor-like kinases, LePRK1 and LePRK2, from tomato (Lycopersicon esculentum). Their structure and immunolocalization pattern and the specific dephosphorylation of LePRK2 suggested that these kinases might interact with signaling molecules in the style extracellular matrix. Here, we show that LePRK1 and LePRK2 can be coimmunoprecipitated from pollen or when expressed together in yeast. In yeast, their association requires LePRK2 kinase activity. In pollen, LePRK1 and LePRK2 are found in an ≈400-kDa protein complex that persists on pollen germination, but this complex is disrupted when pollen is germinated in vitro in the presence of style extract. In yeast, the addition of style extract also disrupts the interaction between LePRK1 and LePRK2. Fractionation of the style extract reveals that the disruption activity is enriched in the 3- to 10-kDa fraction. A component(s) in this fraction also is responsible for the specific dephosphorylation of LePRK2. The style component(s) that dephosphorylates LePRK2 is likely to be a heat-stable peptide that is present in exudate from the style. The generally accepted model of receptor kinase signaling involves binding of a ligand to extracellular domains of receptor kinases and subsequent activation of the signaling pathway by receptor autophosphorylation. In contrast to this typical scenario, we propose that a putative style ligand transduces the signal in pollen tubes by triggering the specific dephosphorylation of LePRK2, followed by dissociation of the LePRK complex.
There are >600 receptor kinases in Arabidopsis (1) with diverse types of extracellular domains. The largest group of plant receptor kinases have extracellular domains composed of variable numbers of leucine-rich repeats (LRRs). LRR kinases mediate diverse pathways, including meristem maintenance (2), abscission (3), male gametogenesis and seed development (4, 5), and somatic embryogenesis (6). Other LRR kinases mediate perception of steroid hormones (7), phytosulfokine (8), or bacteria (9). Relatively little is known about how these receptors transduce their exogenous signals, and only a few protein complexes have been characterized both mutationally and biochemically. For example, the CLAVATA complex, which is involved in maintaining meristem size, is composed of a LRR-receptor kinase, CLV1 (2), a probable coreceptor, CLV2 (10), and the ligand, CLV3 (11). The CLAVATA complex also contains a small GTPase, ROP, and a protein phosphatase, KAPP, that is a negative regulator of CLV1 signaling (12). Expression of CLV1 and CLV2 in yeast showed that a functional kinase domain of CLV1 is required for CLV3 binding (13). Similarly, both the extracellular domain and kinase activity are required for ligand binding and signaling through FLS2, the LRR-receptor kinase that is involved in detecting signals from bacteria (9). Perception of brassinosteroids is mediated through the LRR-receptor kinases BRI1 and BAK1 (14, 15). BRI1 and BAK1 interact in vitro and when expressed in yeast cells and can phosphorylate each other in vitro. WAK1, a member of a different group of receptor kinases that are cell wall-associated, is found in a protein complex of ≈500 kDa, comprising KAPP, the putative ligand AtGRP3, and other unknown proteins (16). SRK, a receptor kinase expressed in stigma cells, and SCR, its ligand from pollen, mediate self-incompatibility in Brassica (17–19). Both chemical cross-linking analysis and sucrose gradient separations showed that SRK forms protein complexes in the absence of ligand (20).
Pollen–pistil interactions offer an excellent model for studying cell signaling (21). As pollen tubes grow through the style, guidance cues from the extracellular matrix of the female tissue presumably are perceived by receptors in the pollen tube to facilitate cytoskeletal changes (22) and other cytoplasmic events (23) required for tip growth. To begin dissecting the signaling pathways that mediate pollen tube growth, we characterized three pollen-specific LRR-receptor kinases from tomato: LePRK1 and LePRK2 (24) and LePRK3 (25). These LePRKs localize at the plasma membrane/cell wall of pollen tubes in partially overlapping patterns (25). LePRK2, but not LePRK1, was shown to be phosphorylated in membrane preparations and to be dephosphorylated specifically on the addition of tomato style extract (24). Yeast two-hybrid screens were used to identify candidate ligands for the LePRKs (26). One of these, LAT52, is a small, cysteine-rich extracellular protein from pollen that interacts with the extracellular domain of LePRK2 before, but not after, pollen germination (26). This suggests that binding partners for the extracellular domains of the LePRKs might be different before and after pollen germination, which is a reasonable expectation, considering pollen tube guidance.
Here, we used coimmunoprecipitation to show that LePRK1 and LePRK2 interact with each other in pollen and when expressed in yeast. We also demonstrate that, in yeast, this interaction is impaired when LePRK2 is mutated at an amino acid residue required for kinase activity. In both mature pollen and pollen germinated in vitro, LePRK1 and LePRK2 are found in a protein complex of ≈400 kDa, together with other, still unknown proteins. However, the multimeric LePRK complex dissociates into LePRK1 and LePRK2 monomers if pollen is germinated in vitro for 4 h in the presence of style extract. Furthermore, style extract also can disrupt the LePRK1–LePRK2 interaction in yeast. For both dephosphorylation of LePRK2 in pollen and for disruption of the LePRK1–LePRK2 interaction in yeast, the activity is enriched in the 3- to 10-kDa fraction of the style extract. For LePRK2 dephosphorylation, the active component is likely to be a heat-stable protein that is present in the style exudate.
Materials and Methods
Plant Material. Lycopersicon esculentum cv. VF36 and Nicotiana tabacum cv. Xanthi D8 plants were grown under standard greenhouse conditions. Tomato pollen was obtained as described (24). Tomato or tobacco pistils were harvested from mature flowers, dissected into component parts (stigma-style, ovary), and stored at -80°C.
Pollen Protein Extraction. Microsomal membranes from both mature and germinated pollen were prepared as described (24), with the following change in the buffer composition. Tissue (100 mg) was disrupted by using 1 ml of extraction buffer [50 mM Tris·HCl, pH 7.4/1 mM EDTA/50 mM NaCl/1× protease inhibitor mixture (Complete; Roche Molecular Biochemicals)]. For immunoprecipitation experiments, microsomal membranes (P100) were resuspended thoroughly in nondenaturing immunoprecipitation (ND-IP) buffer (50 mM Tris·HCl, pH 8.0/150 mM NaCl/0.5% Nonidet P-40) by stirring on a magnetic stirrer at 4°C for 1 h and adjusted to a final protein concentration of 15 μg/μl.
To obtain both cytoplasmic and membrane proteins for gel filtration (FPLC extract), the tissue was disrupted by using 1 ml of extraction buffer containing detergent (0.5% Triton X-100). The resulting homogenate was stirred on a magnetic stirrer at 4°C for 1 h and centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant (S10) was fractionated by centrifugation at 100,000 × g for 3h at 4°C. The S100 fraction then was loaded onto the gel-filtration FPLC Superdex 200 HR column (Amersham Biosciences).
Germination of Pollen. Freshly collected pollen was dispersed (100 mg of pollen per 10 ml) onto germination medium (24) and incubated for 3–4 h at 28°C on a rotating shaker. Pollen tube integrity was monitored periodically. Pollen germination efficiency in different experiments varied from 60% to 90%. Pollen tubes were filtered under vacuum by using filter paper, and microsomal membranes or FPLC extract were obtained as outlined above.
For some experiments, frozen stigma-style tissue from 20–30 VF36 flowers was homogenized in 1 ml of germination medium. The homogenate was centrifuged at 10,000 × g for 10 min at 4°C, and the S10 fraction, termed style extract, was added to the germination medium together with the pollen.
Gel Filtration. Extracts from mature pollen or germinated pollen were loaded onto a FPLC Superdex 200 HR column equilibrated in a column buffer (50 mM Tris·HCl, pH 7.4/1 mM EDTA/100 mM NaCl/0.1% Triton X-100). Fractionation was performed at a 0.3-ml/min flow rate, and 60 fractions (0.5 ml) were collected, concentrated three times by Microcon YM-10 filters (Amicon, Millipore), and stored at -20°C. The Superdex 200 HR column was calibrated with ferritin (440 kDa), yeast alcohol dehydrogenase (150 kDa), and BSA (66 kDa).
Expression of Recombinant LePRK1 and LePRK2 in Yeast. BJ2168 yeast (208277 from American Type Culture Collection) was transformed separately (or in pairs if noted in figure legend) with LePRK1 (cloned in YCpIF3), LePRK2 (cloned in YCpIF6), LePRK1-(K396R) (cloned in YCpIF12), or LePRK2-(K372R) (cloned in YCpIF12) (27). Each cloned gene was under the control of the GAL1 promoter and, therefore, was activated in cells grown in the presence of galactose. Yeast transformation was performed as described (28). Cultures were grown at 30°C in a minimal glucose medium to a cellular density of 108 cells per ml (i.e., for 30–40 h), and pelleted cells were resuspended in minimal medium with galactose and grown for an additional 24 h. Cells were pelleted and then resuspended in 2 vol of yeast buffer (20 mM Tris·HCl, pH 7.9/10 mM MgCl2/1 mM EDTA/5% glycerol/1 mM DTT/300 mM (NH4)2SO4/1× protease inhibitor mixture). After adding 4 vol of chilled 0.5-mm glass beads (Sigma), cells were broken by agitating each tube on a Vortex mixer five times for 1 min at 4°C. The supernatant was decanted. The glass beads were washed with 1 vol of the buffer, and both supernatants were pooled. The supernatants were centrifuged at 10,000 × g for 10 min at 4°C, and the S10 fractions were fractionated by centrifugation at 100,000 × g for 3 h at 4°C. For immunoprecipitation experiments, yeast microsomal membranes (P100) were resuspended in ND-IP buffer and adjusted to a final protein concentration of 15 μg/μl.
Immunoprecipitations. The resuspended pollen (950 μg of protein) or yeast (750 μg of protein) membranes were incubated with 2 μl of the corresponding antibody for 3 h at 4°C. The mixture was centrifuged at 10,000 × g for 10 min at 4°C. Then, 100 μl of 10% protein A-Sepharose (Sigma; preequilibrated in ND-IP buffer) was added to the supernatant and incubated for 1 h at 4°C. The beads were pelleted, washed twice with ND-IP buffer, resuspended in 60 μl of 1× Laemmli SDS/PAGE buffer, and boiled for 3 min. After pelleting the beads, the proteins were separated by SDS/PAGE, blotted to nitrocellulose, and immunoblotted.
SDS/PAGE and Immunoblotting. Protein (≈15 μg) from each Superdex 200 HR fraction and 60 μg of the microsomal fractions were separated by SDS/PAGE and blotted to nitrocellulose. The membranes were blocked first with 6% gelatin in 1× Tris-buffered saline (TBS) for 30 min and then in 1× TBS with 0.2% Triton X-100/6% nonfat dry milk/4% glycine for another 30 min. The blocked membranes were incubated with antibodies diluted to 1:1,000 in 1× TBS with 0.2% Triton X-100, 0.3% nonfat dry milk, and 0.3% glycine for 1 h with shaking at room temperature. After six washes of 10 min each with 1× TBS with 0.2% Triton X-100, the membranes were incubated with sheep anti-mouse polyclonal secondary antibodies conjugated with horseradish peroxidase (Amersham Biosciences), washed, and developed by using the enhanced chemiluminescence kit (Amersham Biosciences).
LePRK2 Dephosphorylation Assay. The dephosphorylation assay was as described (24), except that the labeling reaction was stopped with a 20-μl mixture of 100 mM EDTA/10 mM ATP/5× Laemmli SDS/PAGE sample buffer. The entire reaction was loaded on a SDS/PAGE gel, blotted to nitrocellulose, and subjected to autoradiography or exposed with a Storm 820 PhosphorImager (Molecular Dynamics).
Style Extract and Exudate Preparation and Size Fractionation. Frozen tobacco styles (three styles) or tomato styles (30 styles) were homogenized in 250 μl of 50 mM Tris·HCl (pH 7.4). The extract was centrifuged at 10,000 × g for 10 min at 4°C, and the supernatant, S10 (style extract), was used (8 μg/μl). To prepare style exudate, two tobacco styles were cut transversely into 5-mm pieces and stirred for 2 h at 4°C with 250 μl of 50 mM Tris·HCl, pH 7.4/100 mM NaCl. The crude exudate was centrifuged at 10,000 × g for 10 min at 4°C, dialyzed for 24 h against water by using dialysis membrane with a cutoff of <6 kDa, and lyophilized and resuspended to a final concentration of 2 μg/μl (final volume, 20 μl).
Complex Dissociation in Yeast. Yeast cells expressing both LePRK1 and LePRK2 were incubated at 30°C for 10 min with tomato style extract (480 μg of protein) and then processed for immunoprecipitation as described above. Alternatively, yeast microsomal membranes (P100, 450 μg of protein) were resuspended in ND-IP buffer to a concentration of 10 μg/μl and then were incubated with style extract (480 μg of protein) at 4°C for 30 min. After incubation, yeast membranes were collected by centrifugation, washed with ND-IP buffer, and immunoprecipitated as described.
Results and Discussion
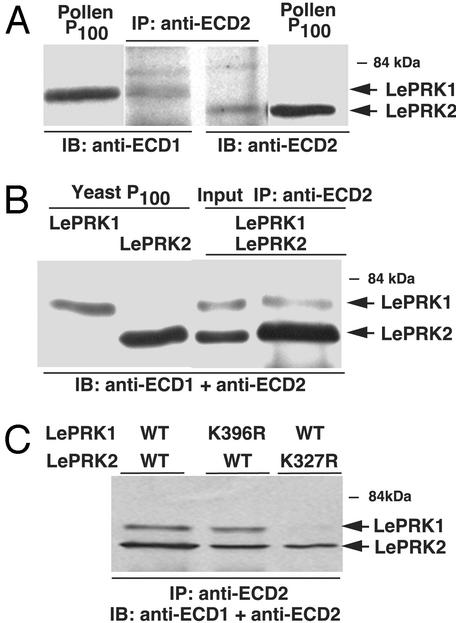
LePRK1 and LePRK2 Associate in Pollen and When Expressed Together in Yeast. We used coimmunoprecipitation to determine whether LePRK1 and LePRK2 associate in pollen. Membrane proteins from pollen were immunoprecipitated with anti-extracellular domain of LePRK2 (ECD2; Fig. 1A, second and third lanes), and immunoblots were developed with anti-extracellular domain of LePRK1 (ECD1; Fig. 1 A, first and second lanes) or anti-ECD2 (third and fourth lanes). Membrane proteins (Fig. 1 A, first and fourth lanes), loaded for reference, showed that each antibody is specific and did not recognize the other LePRK. Because a band corresponding to LePRK1 was detected after immunoprecipitation with anti-ECD2 (Fig. 1 A, second lane), we conclude that LePRK1 and LePRK2 associate in pollen.
Fig. 1.
LePRK1 and LePRK2 associate with each other in pollen and in yeast membranes. (A) Membrane proteins from pollen (first and fourth lanes) were immunoprecipitated (second and third lanes) by using anti-ECD2 antibody. The proteins were separated by SDS/PAGE, and immunoblots were developed with anti-ECD1 antibody (first and second lanes) and anti-ECD2 antibody (third and fourth lanes). (B) Membrane proteins from yeast expressing LePRK1 and LePRK2 were immunoprecipitated by using anti-ECD2 antibody. The precipitated proteins were subjected to SDS/PAGE, and the immunoblot was developed with both anti-ECD1 and anti-ECD2 antibodies (fourth lane). First lane, yeast membrane preparations (P100) expressing LePRK1; second lane, yeast membrane preparations expressing LePRK2; third lane, yeast membrane preparations expressing LePRK1 and LePRK2. (C) LePRK2 kinase activity is required for complex formation. Membrane proteins from yeast expressing LePRK1 and LePRK2 (first lane), LePRK1-(K396R) and LePRK2 (second lane), and LePRK1 and LePRK2-(K372R) (third lane) were immunoprecipitated by using anti-ECD2 antibody. The precipitated proteins were subjected to SDS/PAGE, transferred to membranes, and immunoblotted with both anti-ECD1 and anti-ECD2 antibodies.
To test whether the interaction of LePRK1 and LePRK2 requires other pollen proteins, we expressed LePRK1 and LePRK2 in yeast, separately or together, by using yeast cloning vectors (27). LePRK1 and LePRK2 were present in the membrane fraction of yeast (Fig. 1B, first, second, and third lanes), but not in the soluble fraction (data not shown). Fig. 1B (fourth lane) shows that LePRK1 was immunoprecipitated, when using anti-ECD2, from yeast expressing both LePRK1 and LePRK2. This result indicates that there are no other pollen-specific proteins required for the interaction of LePRK1 and LePRK2 but does not exclude the possibility that other yeast proteins might be required. In this respect, the LePRK1–LePRK2 complex therefore appears to be similar to SRK (20) but different from at least two other characterized receptor kinase complexes (16, 13), where the corresponding ligands (CLV3 and AtGRP3) are required for the formation and maintenance of the active protein complex.
We tested whether kinase activity was required for complex formation of LePRK1 and LePRK2 (Fig. 1C). Trotochaud et al. (13) showed that CLV1 kinase activity was required for CLV3 binding, but there are conflicting reports (14, 15) as to whether kinase activity is required for the association of BRI1 and BAK1. Mutated LePRK1-(K396R) and LePRK2-(K372R) were shown to be inactive kinases (24). Membrane proteins from yeast cells expressing both LePRK1 and LePRK2 (Fig. 1C, first lane), both mutated LePRK1 and LePRK2 (second lane), or both LePRK1 and mutated LePRK2 (third lane) were immunoprecipitated by using anti-ECD2 antibody and immunoblotted with both anti-ECD1 and anti-ECD2. Because LePRK2 was in the immunopellet when LePRK1 was mutated, we conclude that LePRK1 kinase activity is not required for the interaction. Because LePRK1 was not in the immunopellet when the mutated form of LePRK2 was present, we conclude that LePRK2 kinase activity is required for LePRK1 binding. This experiment (Fig. 1C, third lane) further confirms that the coimmunoprecipitation of LePRK1 (see Fig. 1A, second lane; Fig. 1B, fourth lane; and Fig. 1C, first and second lanes) was specific.
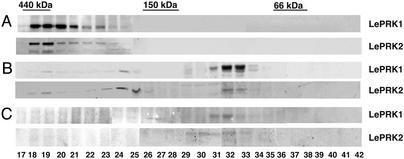
LePRK1 and LePRK2 Are Present in an ≈400-kDa Protein Complex in Pollen Membranes. To determine the apparent size of the LePRK complex in pollen, proteins from mature pollen were solubilized in the presence of 0.5% Triton X-100 (FPLC extract), fractionated by Superdex 200 HR gel-filtration chromatography, and immunoblotted separately with anti-ECD1 and anti-ECD2. Fig. 2A shows that both LePRK1 and LePRK2 eluted in the ≈400-kDa region. Neither kinase was observed in the 70-kDa region, where monomers of LePRK1 and LePRK2 would elute. Considering that ROP and KAPP were associated with the CLAVATA complex (12), and because KAPP was also associated with the Wak1 protein complex (16), we tested whether ROP and KAPP were present in the ≈400-kDa LePRK complex. ROP eluted in the same fractions as LePRK1 and LePRK2 but was not detected in fractions corresponding to the monomeric size of ROP (data not shown). KAPP was found in the same fractions as LePRK1 and LePRK2 (data not shown) as well as in other lower molecular mass fractions, suggesting that KAPP might be present in various protein complexes in pollen membranes. As an aside, LAT52 did not elute in the same fractions as the LePRK complex because the extraction buffer contained EDTA, which abolishes the LAT52–LePRK2 interaction (26).
Fig. 2.
LePRK1 and LePRK2 exist in pollen extract as oligomeric complexes that are dissociated by tomato style extract. Gel-filtration fractions of the Superdex 200 HR from mature pollen (A) and pollen germinated in the presence of style extract (B and C) were collected and separated by SDS/PAGE. The presence of LePRK1 and LePRK2 was determined by immunoblot analysis. The positions of the molecular mass standards are indicated. Numbers indicate the fraction number as eluted sequentially.
The LePRK Complex Dissociates When Pollen Is Germinated in the Presence of Style Extract. We previously proposed a model (24) whereby ligand(s) from the style would interact with the extracellular domains of the LePRKs and consequently transduce the signal into pollen tubes. Recently, we have shown that LAT52 interacts with LePRK2 before, but not after, pollen germination (26). We reasoned that we might detect a difference in the composition of the LePRK complex after style components interact with germinating pollen. To test whether pollen germination itself induces a change in the composition of the LePRK complex, we germinated pollen in vitro for 4 h, prepared an FPLC extract, and fractionated the proteins. Both receptor kinases still were detected only in the ≈400-kDa region (data not shown). However, if pollen was germinated in the presence of extract prepared from tomato styles, the LePRK complex was partially or completely dissociated, as shown in Fig. 2 B and C. Although a minor amount of LePRK1 and LePRK2 was detected in the ≈400-kDa region, the majority was in the ≈70-kDa region, as indicated by the intensity of the signal in Fig. 2B (compare fractions 32 and 33 with fractions 18–20). In two other experiments (Fig. 2C and data not shown), only the monomeric forms were detected. Altogether, these results suggest that some component of the style extract caused the dissociation of the LePRK complex. More specifically, these results suggest that LePRK complex dissociation is due to some component of the style and not due to pollen germination per se.
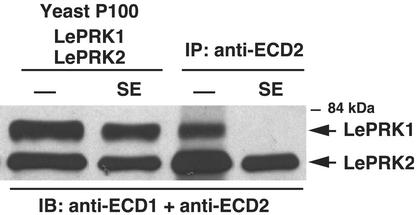
Style Extract Disrupts the LePRK1–LePRK2 Association in Yeast Membranes. To test whether the style extract was specifically disrupting the LePRK1–LePRK2 interaction, we used yeast. It is known that receptors in the yeast plasma membrane are accessible to peptide-mating factors after diffusion through the yeast cell wall (29). By analogy, we thought that small molecules in the style extract would be accessible to the extracellular domains of LePRK1 and LePRK2 expressed in yeast. Yeast cells expressing both LePRK1 and LePRK2 were incubated for 10 min with tomato style extract, and the P100 fraction was immunoprecipitated with anti-ECD2. As shown in Fig. 3 (fourth lane), LePRK1 was not coimmunoprecipitated when using anti-ECD2 if yeast cells first were incubated in the presence of tomato style extract, but could be coimmunoprecipitated from yeast cells that were not incubated with style extract (Fig. 3, third lane). This result shows that component(s) of the tomato style extract are also effective in disrupting the LePRK1–LePRK2 interaction in yeast. The first and second lanes of Fig. 3 correspond to membrane preparations (P100) from yeast expressing LePRK1 and LePRK2, incubated in the absence (-) or presence (SE) of tomato style extract, showing that the dissociation of the complex is not due to degradation.
Fig. 3.
Tomato style extract disrupts the association of LePRK1 and LePRK2 in yeast. The first and second lanes correspond to membrane proteins (P100) from yeast expressing LePRK1 and LePRK2, which had been incubated in the absence (first lane; -) or presence (second lane; SE) of tomato style extract. Duplicate samples (third and fourth lanes) were immunoprecipitated with anti-ECD2 antibody. The proteins were subjected to SDS/PAGE, transferred to membranes, and immunoblotted with both anti-ECD1 and anti-ECD2 antibodies.
Immunofluorescence studies (data not shown) indicated that LePRK1 and LePRK2 localize at the margins of the yeast cells. We hypothesized that the dissociation occurred via interaction of the style component(s) with the extracellular domains of LePRK1 and/or LePRK2. The LePRK1–LePRK2 interaction also was disrupted when membranes from yeast expressing both LePRK1 and LePRK2 were incubated directly with style extract (not shown). For convenience, the following experiments were done with yeast membranes instead of with intact yeast cells.
The Style Component(s) Is Likely to Be a Heat-Stable Peptide That Is Present in the Style Exudate. Tomato and tobacco are closely related. Tobacco has three pollen receptor kinases that are closely related to the LePRKs; indeed, the amino acid sequence of LePRK2 and its tobacco homolog are nearly identical (25). Tobacco styles are considerably larger than tomato styles; one tobacco style yields as much protein as 10 tomato styles. We determined that tobacco style extract had a similar effect on LePRK1–LePRK2 dissociation in yeast (data not shown); so, for convenience, tobacco style extract was used for some experiments.
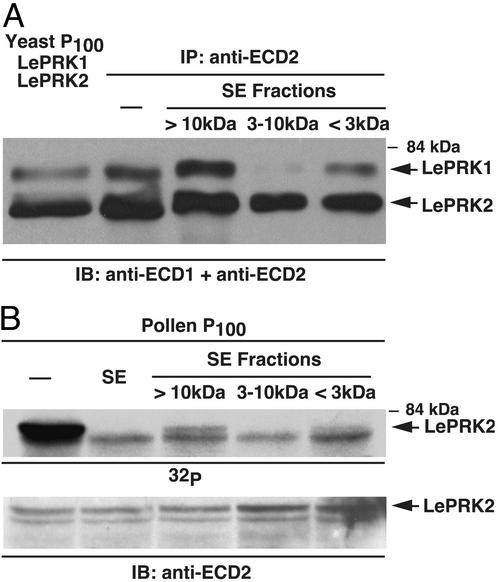
To characterize the style component responsible for the disruption of the LePRK1–LePRK2 interaction, we used Microcon filters to crudely size-fractionate tobacco style extract. Fig. 4A shows that the 3- to 10-kDa size fraction was the most effective in disrupting the LePRK1–LePRK2 interaction in yeast.
Fig. 4.
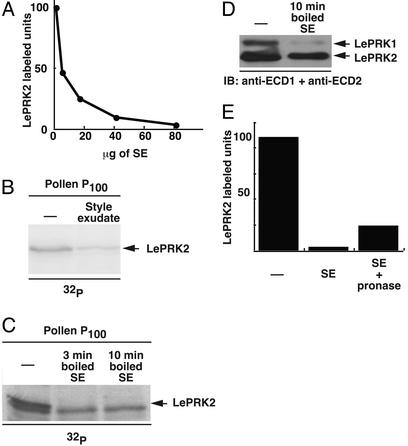
The effective style extract component contains 3- to 10-kDa molecules. Style extract was loaded on YM10 filters (cutoff, 10 kDa), and the eluate (<10 kDa) was loaded on YM3 filters (cutoff, 3 kDa). After centrifugation, the retentate of YM10 (>10 kDa), the retentate of YM3 (3–10 kDa), and the eluate of YM3 (<3 kDa) were assayed for the ability to disrupt the association of LePRK1 and LePRK2 in yeast (A) and for the ability to dephosphorylate LePRK2 in pollen (B). The position of LePRK2 is indicated by arrows. In B, the LePRK2 on the IB precisely aligned with the upper band on the radiography film, and the lower bands did not align.
We showed that tomato style extract specifically dephosphorylated LePRK2 in pollen membranes (24). To see whether the style extract component responsible for complex dissociation was also responsible for LePRK2 dephosphorylation, we tested the different Microcon size fractions of tomato style extract in a phosphorylation assay. Fig. 4B shows that tomato style extract specifically dephosphorylated LePRK2 (the top band in the doublet) and that the 3- to 10-kDa fraction was most effective. Note that the pollen membrane control (Fig. 4B, first lane) shows a higher degree of phosphorylation than the third and fifth lanes, where, supposedly, the effective style component should be absent. This could be explained by incomplete separation of components with molecular masses close to the cutoffs of Microcon filters. According to the manufacturer, 20% of the 3- to 10-kDa component might be present in the >10-kDa fraction and 10% in the <3-kDa fraction. It is also unlikely that the dephosphorylation of LePRK2 and disruption of the LePRK1–LePRK2 interaction were due to a nonspecific effect, such as a change in pH or in ion composition, because not all size fractions were equally effective. Furthermore, because the <3-kDa fraction was not effective, electrolytes probably are not involved. Additional support for this idea is shown by dilution of the style extract (Fig. 5A). Dephosphorylation of LePRK2 was reduced dramatically when the style extract protein content was decreased, although the pH and ion composition remain identical.
Fig. 5.
Style factor is likely to be a heat-stable peptide that is present in the style exudate. (A) Different amounts of total style extract were used to dephosphorylate LePRK2. (B) Pollen membranes (P100) were incubated in phosphorylation buffer with [γ-32]ATP in the absence (first lane; -) or presence (second lane; style exudate) of tobacco style exudate (20 μg of protein). Total proteins were separated by SDS/PAGE, blotted to nitrocellulose, and then subjected to autoradiography. The position of LePRK2 is indicated by an arrow. (C) Total tobacco style extract first was subjected to 95°C for 3 min (second lane) and 10 min (third lane) and then assayed for dephosphorylation of LePRK2. The position of LePRK2 is indicated by an arrow. (D) Total tobacco style extract (100 μg of protein) first was subjected to 95°C for 10 min (second lane) and then assayed for the ability to disrupt the association of LePRK1 and LePRK2 in yeast. (E) Total style extract (100 μg of protein) first was incubated or not with pronase (50 μg; 2.5 μg/μl)for 3h at 37°C, subjected to 95°C for 10 min, and then assayed for dephosphorylation of LePRK2. For A and E, the relative amounts of labeled LePRK2 in each treatment were estimated by scanning the gel with a Storm 820 PhosphorImager (Molecular Dynamics), and the values obtained were compared with signal of the treatment without style extract (-) as reference.
Pollen tubes grow through the extracellular matrix of the style, but the style extract contains both extracellular and cytoplasmic proteins, as well as metabolites. If the style component indeed interacts with the extracellular domains of the LePRKs, it should be present in style exudate. Fig. 5B shows that tobacco style exudate also induced LePRK2 dephosphorylation, suggesting that the component in the style extract is the same as that in the style exudate.
Boiling of the tobacco style extract does not affect its ability to dephosphorylate LePRK2 (Fig. 5C) or its ability to disrupt the LePRK1–LePRK2 interaction in yeast (Fig. 5D), suggesting that if the active component is a protein, it is heat-stable. It is noteworthy in this context that the candidate ligand LAT52 remains soluble after boiling (30), but, after boiling, LAT52 is unable to interact with LePRK2 (26). To test whether the active component in the style extract was proteinaceous, we used protease treatment. Preincubating tobacco style extract with pronase reduced, but did not eliminate, the dephosphorylation of LePRK2 (Fig. 5E). This result suggests that a protein is involved in LePRK2 dephosphorylation but does not exclude the possibility that some other molecule also might be involved. It is possible that pronase cannot fully prevent style-specific LePRK2 dephosphorylation because pronase cannot digest all proteins completely, because partial digestion products of the putative peptide still may be able to induce dephosphorylation of LePRK2, or because the active component(s) could be composed of a peptide as well as some other nonproteinaceous factors. This idea has precedence: In lily styles, both a small (9-kDa) peptide and a large, pectic polysaccharide are required for pollen tube adhesion (31, 32).
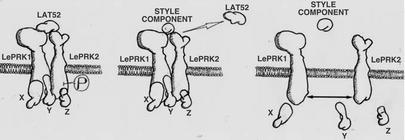
LePRK Signaling in Pollen. The model shown in Fig. 6 attempts to explain how the LePRKs signal in pollen. In mature, pregerminated pollen, the LePRKs are present in a high-molecular-mass complex. Tang et al. (26) showed that LAT52 interacts with LePRK2 in mature pollen, so we propose that in pregerminated pollen, LAT52 is part of the LePRK complex, playing a role before or during pollen hydration. The coimmunoprecipitation results (Fig. 1B) indicate that LAT52 is not needed for the complex to form in yeast. After pollen hydration and germination, LAT52 no longer interacts with LePRK2 (26). We suggest that in germinated pollen the displacement of LAT52 by still unknown pistil ligand(s) would induce the dephosphorylation of LePRK2 and LePRK complex dissociation. We cannot speculate about which form is the active state for the LePRKs; we can only suggest that LePRK1 and LePRK2 would transduce the style signal through the specific dephosphorylation of LePRK2 and LePRK complex dissociation. In another signaling scenario, the LePRK complex would act only in pregerminated pollen, monitoring pollen status before germination. However, this model does not explain why LePRK2 significantly increases after germination (24), suggesting that it has a continued role. It is not yet clear how all these events occur in germinating pollen, which other players are involved, or in what chronology they occur. It is unresolved as to whether only LePRK2 dephosphorylation is responsible for LePRK complex dissociation or whether LePRK1 also is involved in binding to the style component and in LePRK complex dissociation.
Fig. 6.
Model for LePRK1–LePRK2 signaling. LePRK1 and LePRK2 associate in mature pollen membranes (pregermination) as part of a multimeric protein complex (Left). In this complex, LAT52 interacts as an extracellular partner and hypothetical pollen proteins (X, Y, and Z) interact with the cytoplasmic domains of LePRK1 and of phosphorylated LePRK2. Upon germination on the stigma, the still unknown pistil ligand displaces LAT52 and, on binding, induces the dephosphorylation of LePRK2 (Center). The LePRK complex then dissociates, releasing the cytoplasmic partners (X, Y, and Z) and transducing the signal to the pollen tube (Right).
What is noteworthy in this LePRK-signaling system is the pattern of signal and response. For other plant-receptor kinases (33), and also in many signaling pathways in animals, binding of the ligand to the extracellular domain triggers receptor autophosphorylation and recruitment of cytoplasmic factors, leading to the formation of an active protein complex. For example, BRI1 is phosphorylated only when its ligand is present (34) and SRK phosphorylation is induced on its interaction with the ligand SCR (35, 19). Conversely, we show here that in pollen, the LePRKs seem to work in a different fashion, because the pistil ligand would transduce the signal through LePRK2 dephosphorylation.
In plants, the ligands AtGRP3 (16) and CLV3 (12) are necessary for the assembly and maintenance of the high-molecular-mass receptor kinase complexes. In animal cells, oligomerization of receptor kinases is one of the regulatory steps required for signal activation. For example, Fas (CD95) is a cell-surface receptor that, when engaged by its ligand (FasL, CD95L), causes death of the cell that bears it. Preassembly of Fas receptors may be required for the binding of FasL (36, 37). The two IFN-γ receptors (IFN-γR1 and IFN-γR2) are preassembled on cell membranes before the ligand IFN binds and activates the receptor complex (38). Here, we showed that the putative style ligand dissociates the LePRK complex into LePRK1 and LePRK2 monomers. Although atypical, a few examples of this kind of regulation have been described. In Drosophila, the EGF receptor (DER) interacts with two ligands: one activating, Spitz, and one inhibitory, Argos. Argos inhibits the binding of Spitz to DER, thereby inhibiting DER dimerization and the subsequent phosphorylation of DER induced by Spitz. Argos, therefore, is a negative autocrine ligand that acts sequentially to limit the duration of DER signaling (39). In Arabidopsis, ethylene receptors repress downstream signaling responses in the absence of the hormone, but when ethylene binds to the receptors, this repression is released, with the consequent activation of the ethylene-response pathway (40).
Here, we demonstrate that LePRK1 and LePRK2 seem to transduce the style signal through the specific dephosphorylation of LePRK2 and LePRK complex dissociation. The nature of the style component and the role of the LePRK complex will need to be defined to determine precisely how LePRKs signal during pollen tube growth.
Acknowledgments
We thank Zhenbiao Yang and Steve Clark for providing the ROP and KAPP antisera; Marta Bravo and Guillermo Alonso for their help in FPLC Superdex fractionation; Ignacio Nojek and Norberto Malarini for drawing the model shown in Fig. 6; and Dior Kelley and Michele Engel for critical reading of the manuscript. This work was supported in part by International Foundation for Science Grant C2865-1, Fundación Antorchas, Universidad de Buenos Aires-Ciencia y Técnica, Proyectos de Investigación Científica y Tecnológica 1998/Fondo para la Investigación Científica y Tecnológica/Agencia Naciónal de Promoción Científica y Tecnológica Grant 01-04142, and by U.S. Department of Agriculture-Current Research Information Service Grant 5335-21000-011-00D.
Abbreviations: LRR, leucine-rich repeat; ND-IP, nondenaturing immunoprecipitation; ECD2, extracellular domain of LePRK2; ECD1, extracellular domain of LePRK1.
References
- 1.Shiu, S. H. & Bleecker, A. B. (2001) Science STKE 2001, RE22. [DOI] [PubMed] [Google Scholar]
- 2.Clark, S. E., Williams, R. W. & Meyerowitz, E. M. (1997) Cell 89, 575-585. [DOI] [PubMed] [Google Scholar]
- 3.Jinn, T. L., Stone, J. M. & Walker, J. C. (2000) Genes Dev. 14, 108-117. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao, D. Z., Wang, G. F., Speal, B. & Ma, H. (2002) Genes Dev. 16, 2021-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canales, C., Bhatt, A. M., Scott, R. & Dickinson, H. (2002) Curr. Biol. 12, 1718-1727. [DOI] [PubMed] [Google Scholar]
- 6.Shah, K., Gadella, T. W., van Erp, H., Hecht, V. & de Vries, S. C. (2001) J. Mol. Biol. 309, 641-655. [DOI] [PubMed] [Google Scholar]
- 7.Li, J. & Chory, J. (1997) Cell 90, 929-938. [DOI] [PubMed] [Google Scholar]
- 8.Matsubayashi, Y., Ogawa, M., Morita, A. & Sakagami, Y. (2002) Science 296, 1470-1472. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Gomez, L., Bauer, Z. & Boller, T. (2001) Plant Cell 13, 1155-1163. [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong, S., Trotochaud, A. E. & Clark, S. E. (1999) Plant Cell 11, 1925-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher, J. C., Brand, U., Running, M. P., Simon, R. & Meyerowitz, E. M. (1999) Science 283, 1911-1914. [DOI] [PubMed] [Google Scholar]
- 12.Trotochaud, A. E., Hao, T., Wu, G., Yang, Z. & Clark, S. E. (1999) Plant Cell 11, 393-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotochaud, A. E., Jeong, S. & Clark, S. E. (2000) Science 289, 613-617. [DOI] [PubMed] [Google Scholar]
- 14.Li, J., Wen, J., Lease, K. A., Doke, J. T., Tax, F. E. & Walker, J. C. (2002) Cell 110, 213-222. [DOI] [PubMed] [Google Scholar]
- 15.Nam, K. H. & Li, J. (2002) Cell 110, 203-212. [DOI] [PubMed] [Google Scholar]
- 16.Park, A. R., Cho, S. K., Yun, U. J., Jin, M. Y., Lee, S. H., Sachetto Martins, G. & Park, O. K. (2001) J. Biol. Chem. 276, 26688-26693. [DOI] [PubMed] [Google Scholar]
- 17.Kachroo, A., Schopfer, C. R., Nasrallah, M. E. & Nasrallah, J. B. (2001) Science 293, 1824-1826. [DOI] [PubMed] [Google Scholar]
- 18.Schopfer, C. R., Nasrallah, M. E. & Nasrallah, J. B. (1999) Science 286, 1697-1700. [DOI] [PubMed] [Google Scholar]
- 19.Takayama, S., Shimosato, H., Shiba, H., Funato, M., Che, F. S., Watanabe, M., Iwano, M. & Isogai, A. (2001) Nature 413, 534-548. [DOI] [PubMed] [Google Scholar]
- 20.Giranton, J. L., Dumas, C., Cock, J. M. & Gaude, T. (2000) Proc. Natl. Acad. Sci. USA 97, 3759-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wheeler, M. J., Franklin Tong, V. E. & Franklin, F. C. H. (2001) New Phytol. 151, 565-584. [DOI] [PubMed] [Google Scholar]
- 22.Franklin Tong, V. E. (1999) Curr. Opin. Plant Biol. 2, 490-495. [DOI] [PubMed] [Google Scholar]
- 23.Feijo, J. A., Sainhas, J., Holdaway Clarke, T., Cordeiro, M. S., Kunkel, J. G. & Hepler, P. K. (2001) BioEssays 23, 86-94. [DOI] [PubMed] [Google Scholar]
- 24.Muschietti, J., Eyal, Y. & McCormick, S. (1998) Plant Cell 10, 319-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim, H. U., Cotter, R., Johnson, S., Senda, M., Dodds, P., Kulikauskas, R., Tang, W., Ezcurra, I., Herzmark, P. & McCormick, S. (2002) Plant Mol. Biol. 50, 1-16. [DOI] [PubMed] [Google Scholar]
- 26.Tang, W., Ezcurra, I., Muschietti, J. & McCormick, S. (2002) Plant Cell 14, 2277-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foreman, P. K. & Davis, R. W. (1994) Gene 144, 63-68. [DOI] [PubMed] [Google Scholar]
- 28.Gietz, R. D. & Woods, R. A. (2002) Methods Enzymol. 350, 87-96. [DOI] [PubMed] [Google Scholar]
- 29.White, J. M. & Rose, M. D. (2001) Curr. Biol. 11, R16-R20. [DOI] [PubMed] [Google Scholar]
- 30.Muschietti, J., Dircks, L., Vancanneyt, G. & McCormick, S. (1994) Plant J. 6, 321-338. [DOI] [PubMed] [Google Scholar]
- 31.Mollet, J. C., Park, S. Y., Nothnagel, E. A. & Lord, E. M. (2000) Plant Cell 12, 1737-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park, S. Y., Jauh, G. Y., Mollet, J. C., Eckard, K. J., Nothnagel, E. A., Walling, L. L. & Lord, E. M. (2000) Plant Cell 12, 151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsubayashi, Y., Yang, H. & Sakagami, Y. (2001) Trends Plant Sci. 6, 573-577. [DOI] [PubMed] [Google Scholar]
- 34.Wang, Z. Y., Seto, H., Fujioka, S., Yoshida, S. & Chory, J. (2001) Nature 410, 380-383. [DOI] [PubMed] [Google Scholar]
- 35.Cabrillac, D., Cock, J. M., Dumas, C. & Gaude, T. (2001) Nature 410, 220-223. [DOI] [PubMed] [Google Scholar]
- 36.Siegel, R. M., Frederiksen, J. K., Zacharias, D. A., Chan, F. K., Johnson, M., Lynch, D., Tsien, R. Y. & Lenardo, M. J. (2000) Science 288, 2354-2357. [DOI] [PubMed] [Google Scholar]
- 37.Papoff, G., Hausler, P., Eramo, A., Pagano, M. G., Di Leve, G., Signore, A. & Ruberti, G. (1999) J. Biol. Chem. 274, 38241-38250. [DOI] [PubMed] [Google Scholar]
- 38.Krause, C. D., Mei, E., Xie, J., Jia, Y., Bopp, M. A., Hochstrasser, R. M. & Pestka, S. (2002) Mol. Cell Protein 1, 805-815. [DOI] [PubMed] [Google Scholar]
- 39.Jin, M. H., Sawamoto, K., Ito, M. & Okano, H. (2000) Mol. Cell. Biol. 20, 2098-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang, C. & Stadler, R. (2001) BioEssays 23, 619-627. [DOI] [PubMed] [Google Scholar]