Abstract
Circadian rhythms are widespread in nature and reflect the activity of an endogenous biological clock. In metazoans, the circadian system includes a central circadian clock in the brain as well as distinct clocks in peripheral tissues such as the retina or liver. Similarly, plants have distinct clocks in different cell layers and tissues. Here, we show that two different circadian clocks, distinguishable by their sensitivity to environmental temperature signals, regulate the transcription of genes that are expressed in the Arabidopsis thaliana cotyledon. One oscillator, which regulates CAB2 expression, responds preferentially to light–dark versus temperature cycles and fails to respond to the temperature step associated with release from stratification. The second oscillator, which regulates CAT3 expression, responds preferentially to temperature versus light–dark cycles and entrains to the release from stratification. Finally, the phase response curves of these two oscillators to cold pulses are distinct. The phase response curve of the oscillator component TOC1 to cold pulses is similar to that of CAB2, indicating that CAB2 is regulated by a TOC1-containing clock. The existence of two clocks, distinguishable on the basis of their sensitivity to temperature, provides an additional means by which plants may integrate both photoperiodic and temperature signals to respond to the changing seasons.
The circadian clock is an endogenous oscillator with an approximate period of 24 hr that can be synchronized, or entrained, to the exact period of daily oscillations in light and temperature (1). This enables an organism to phase its biological activities to the correct time of day. The sleep–wake cycle in humans, activity and eclosion rhythms in flies, and conidiation in Neurospora are rhythmic processes that display distinct phase angles with the environment (2). In the flowering plant Arabidopsis thaliana, the circadian clock phases the peak mRNA abundance of many genes to distinct times of day (3). Furthermore, the phase angle of specific circadian rhythms with the environmental light–dark (LD) cycle controls seasonal behavior such as flowering (4–6).
The clock is temperature-compensated, meaning that the pace of the clock is more or less constant across a range of temperatures and fails to exhibit the acceleration with temperature that characterizes typical enzymatic reactions. Nonetheless, temperature serves as an important environmental time cue and entrainment to temperature cycles has been demonstrated in a number of systems (7, 8). It is commonly assumed that light is the dominant environmental time cue for circadian clocks, although few studies have systematically compared light and temperature (9–12). In fact, temperature cycles applied antiphase to LD cycles set the phase angle of multiple rhythms, including CO2 assimilation in Kalanchoë (13), ethylene production in sorghum (14), and conidiation in Neurospora (15).
We compared the relative strengths of temperature cycles and LD cycles as determinants of the phase of rhythmic transcription of CATALASE 3 (CAT3), which peaks in the evening (16), and of CHLOROPHYLL A/B BINDING PROTEIN 2 (CAB2), which peaks in midmorning (17). We found that temperature and light differentially regulate the phase of these two rhythms: the CAT3 rhythm is more sensitive to temperature than is the CAB2 rhythm. The phase response of CAB2 to cold pulses was similar to that of TOC1, which encodes a central clock component, and was dissimilar to that of CAT3. The spatial expression patterns of these three genes in the Arabidopsis cotyledon overlap, which suggests that single tissues contain at least two circadian oscillator mechanisms, distinguishable on the basis of relative sensitivity to temperature versus light signals.
Methods
Plant Material and Growth Conditions. Arabidopsis seed stocks were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). All experiments were performed in temperature-controlled (±0.5°C) chambers. Temperature steps required ≈15 min to reach target temperature. To ensure that plants received the identical temperature cycles, they were grown simultaneously in the same chamber and LD cycles were administered manually with light-tight boxes.
For the temperature phase response curve (tPRC), plants were grown for 7 days in 12 hr light (white fluorescent, 50 μmol·m-2·s-1) and 12 hr dark, transferred to black 96-well microtiter dishes containing Murashige and Skoog (18) agar (0.8% wt/vol) medium [amended with 2% (wt/vol) sucrose] and 35 μl of 0.5 mM luciferin (Biosynth, Basel) per well, further entrained for 2 days in 12 hr light (white fluorescent, 15–30 μmol·m-2·s-1) and 12 hr dark on a Packard TopCount luminometer (Perkin–Elmer Life Sciences), and then released into continuous light (LL) (white fluorescent, 15–30 μmol·m-2·s-1) for 1 day. On the third day, starting at subjective dawn [circadian time (CT) 0], seedlings received a temperature pulse by removing individual microtiter plates every 4 hr and placing them at 12°C under the same light conditions (white fluorescent, 15–30 μmol·m-2·s-1) for 4 hr. As controls to determine the phase of the oscillator in the absence of a temperature pulse, one plate was moved to an identical growth chamber at 22°C (white fluorescent, 15–30 μmol·m-2·s-1) and one plate stayed on the TopCount during the entire run.
Luciferase (LUC) promoter fusions and methods were as described (16). The [-221/-103]2 CAT3 promoter fragment was cloned upstream of a promoterless uidA gene, encoding β-glucuronidase (GUS), in vector pCAMBIA-1381Z, which also carries the hygromycin phosphotransferase gene for plant selection. Resistant seedlings were selected on 1% (wt/vol) agar half-strength Murashige and Skoog plates with 1% sucrose, 25 μg/ml hygromycin, and 150 μg/ml carbenicillin (Sigma). Seedlings were stained for GUS activity (19), then dehydrated through a graded ethanol series.
Data Analysis. LUC activity was analyzed as described (16). At least 3 days of data were collected and rhythms were detected by fast Fourier transform nonlinear least squares (FFT-NLLS) (20). FFT-NLLS fits sine waves to the data and estimates the period, phase, and amplitude with associated confidence intervals. Additionally, for each trace it gives the relative amplitude error, which is a measure of rhythmic robustness with a value of 0 being a perfect sine wave and a value of 1 being arrhythmic. Analysis was restricted to traces exhibiting periods of 20–30 hr. Phase is expressed in circadian time (CT phase = sidereal phase/ period × 24 hr), which normalizes the periods to 24 hr so that the phases may be directly compared between traces in which the periods differ.
Results
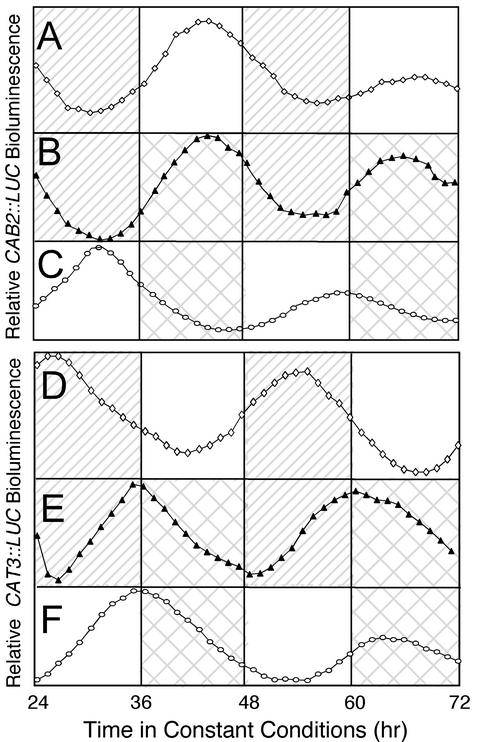
The Phases of CAT3::LUC and CAB2::LUC Respond Differently to Temperature Cycles. Both CAB2::LUC and CAT3::LUC are entrained to LD and temperature cycles (7, 16). However, transcription of these two transgenes is phased differently; CAB2::LUC peak activity occurs during the middle of the subjective light or hot period (CT 6; Fig. 1 A and C), and CAT3::LUC peak activity occurs at the beginning of the subjective dark or cold period (CT 12; Fig. 1 D and F). Thus, CAB2::LUC and CAT3::LUC exhibit a stable phase difference, or phase angle, after entrainment to LD and temperature cycles. This is consistent with other systems in which the response to high temperatures mimics that to light (hot days) and the response to low temperatures mimics that to dark (cold nights) (10, 11).
Fig. 1.
Temperature cycles set the phase of CAT3 but not of CAB2 over light cycles. Plants were grown for 14 days in 12 hr dark/12 hr light at 22°C (A and D; ⋄), 12 hr light/12 hr dark antiphase to 12 hr 22°C/12 hr 12°C (B and E; ▴), or 12 hr at 22°C/12 hr 12°C in LL (C and F; ○). Plants were then monitored for LUC activity on a Packard TopCount luminometer in LL at a constant temperature (22°C). Striped boxes represent dark and hatched boxes represent cold (equivalent to night); white boxes represent either light or hot. Representative CAB2::LUC traces in A–C and CAT3::LUC traces in D–F were taken from two independent experiments that yielded consistent results.
Several observations suggested that different oscillators might regulate CAB2 and CAT3. First, in LL CAB2::LUC and CAT3::LUC display the same period after entrainment to temperature cycles; yet after entrainment to LD cycles, they display different periods (Table 1; see Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org). The longer period of CAB2::LUC after LD entrainment is also observed when temperature cycles are applied concurrently, either in phase or antiphase, with the LD cycle (Table 1, Fig. 6). This difference in period suggests that the two rhythms respond to separate oscillators (21–23). Second, Millar and Kay (24) established that the phase of CAB2 is modulated after entrainment to various photoperiods such that the peak in mRNA abundance and transcription occurs in the middle of the subjective light period (Fig. 2A, Table 2). In contrast, CAT3 phase is fixed relative to subjective dawn in either long or short photoperiods (Fig. 2B, Table 2). This differential response of CAB2 and CAT3 phase angles to photoperiod is consistent with their regulation by distinct circadian oscillators.
Table 1. Period and phase differences between CAB2::LUC and CAT3::LUC in continuous conditions after entrainment to different LD and temperature cycles.
| Condition | Reporter gene | Period ± SEM, hr | P* |
|---|---|---|---|
| LL hot/cold | CAT3::LUC | 25.15 ± 0.18 | 0.8240 |
| CAB2::LUC | 25.21 ± 0.20 | ||
| LD continuous hot | CAT3::LUC | 24.56 ± 0.28 | <0.0001 |
| CAB2::LUC | 26.29 ± 0.31 | ||
| LD hot/cold | CAT3::LUC | 25.19 ± 0.18 | <0.0001 |
| CAB2::LUC | 26.41 ± 0.20 | ||
| LD cold/hot | CAT3::LUC | 24.18 ± 0.21 | <0.0001 |
| CAB2::LUC | 26.65 ± 0.27 |
As determined by Student's t test.
Fig. 2.
The phase of CAB2 is sensitive but that of CAT3 is insensitive to photoperiod. CAB2::LUC (A) and CAT3::LUC (B) plants were grown at constant temperature (22°C) in long (16 hr light/8 hr dark; ○) or short (8 hr light/16 hr dark; ▪) days for 7 days, then transferred to LL. Data presented represent averages from two independent experiments. Hatched boxes represent subjective night; white boxes represent subjective day.
Table 2. Period and phase differences between CAB2::LUC and CAT3::LUC in continuous conditions after entrainment to LD cycles with different photoperiods.
| Photoperiod | Reporter gene | Period ± SEM, hr | Phase ± SEM, hr |
|---|---|---|---|
| 16 hr light/8 hr dark | CAT3::LUC | 26.55 ± 0.30 | 11.54 ± 0.52 |
| CAB2::LUC | 28.14 ± 0.45 | 10.34 ± 0.80 | |
| 8 hr light/16 hr dark | CAT3::LUC | 26.57 ± 0.38 | 11.59 ± 0.79 |
| CAB2::LUC | 28.86 ± 0.45 | 3.69 ± 1.04 |
To test the hypothesis that two distinct oscillators regulate CAB2 and CAT3 transcription, we analyzed how light and temperature interact to set the phase of CAB2::LUC and CAT3::LUC. We first performed a competition experiment in which plants were entrained to light and temperature provided in antiphase (cold days/hot nights): 12 hr light at 18°C and 12 hr dark at 22°C [4°C difference in temperature (DIF)] or 12 hr light at 12°C and 12 hr dark at 22°C (10°C DIF). When the plants were entrained with a 4°C DIF antiphase to light, the entraining light cue predominated. CAB2::LUC expression peaked in the middle of the light/cold period and CAT3::LUC expression peaked at the beginning of the dark/hot period, indicating that the phase of expression of both genes was set by LD cycle (data not shown). Similarly, at 10°C DIF CAB2::LUC was phased to the middle of the light/cold period (Fig. 1B), indicating that the phase of CAB2::LUC was still set by light. These results are consistent with observations in a number of systems. For example, the circadian phases of the nitrogenase and protein synthesis rhythms of Synechococcus RF-1 are set by light cycles in preference to temperature cycles (25). However, at 10°C DIF CAT3::LUC was phased to the beginning of the light/cold period (Fig. 1E), indicating that CAT3::LUC was responding to the temperature cycle in preference to the LD cycle. These experiments establish that the phase of CAT3 expression is more sensitive to temperature cycles than that of CAB2: 10°C DIF temperature cycles set the phase preferentially to antiphase LD cycles. In contrast, the phase of CAB2 expression, which can be set by temperature cycles, responds preferentially to LD cycles when presented in antiphase to temperature cycles. Thus, temperature can act over light to entrain some circadian rhythms in Arabidopsis.
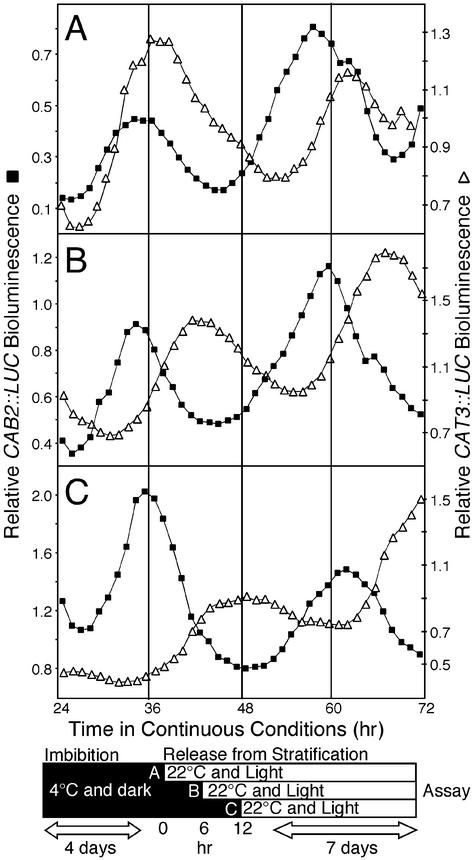
In addition to responding to temperature cycles, the phase of the circadian clock can also be reset by temperature steps (9, 11). In our experiments, we imbibe (hydrate) the seeds and then stratify at 4°C in the dark to synchronize germination before release into light at 22°C. We have previously shown that the phase of morning-specific CAT2 transcript is set by imbibition and fails to respond to the subsequent temperature step and/or light onset associated with release from stratification (26). Therefore, we tested whether CAB2::LUC and CAT3::LUC are equally responsive to imbibition and unresponsive to release from stratification and/or light onset. Three sets of seedlings were imbibed and plated [Zeitgeber time (ZT) = 0], stratified at 4°C for ≈4 days, and released into LL at 22°C at 6-hr intervals of ZT 6, 12, and 18 (Fig. 3 A, B, and C, respectively). In each case, CAB2::LUC expression occurred at a similar phase relative to imbibition and failed to respond to the signals associated with release from stratification, as seen with CAT2 (26). Transcription from the CAB2 promoter was shown to be insensitive to light onset for the first 60 hr after germination (27, 28). Consistent with these results, we find that CAB2::LUC is insensitive to both the temperature step and light onset associated with release from stratification. In striking contrast, the phases of CAT3::LUC expression maintained a constant relationship (phase angle) to the time of release from stratification, indicating that the phase was reset by the release from stratification. At this time, we cannot distinguish between the effects of the temperature step (4°C to 22°C) or light onset in establishing the phase of CAT3::LUC expression. However, we have changed the phase angle between CAB2 and CAT3, which suggests that they are regulated by distinct oscillators.
Fig. 3.
The phase of CAT3 but not of CAB2 is set by release from stratification. Plants were plated at 8 a.m., stratified at 4°C in the dark for 4 days, and released into 22°C and LL at 6-hr intervals at 2 p.m. (A), 8 p.m. (B), and 2 a.m. (C). Plants were grown in LL at 22°C for 7 days, and LUC activity was measured on a Packard TopCount luminometer in LL at a constant temperature (22°C). Representative CAT3::LUC (▵) and CAB2::LUC (▪) traces are displayed. Two independent experiments yielded similar results.
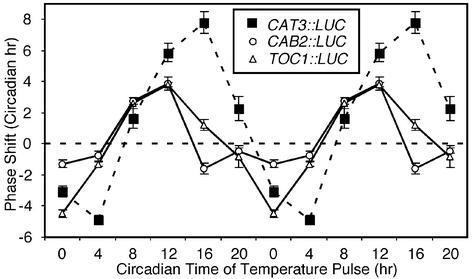
tPRC: State of the Circadian Oscillator(s). Our results suggested that CAB2 and CAT3 respond to two distinct circadian clocks that can be distinguished on the basis of differential sensitivity to temperature. The phase response curve is a powerful tool with which to probe the circadian clock and reflects the general state of the circadian oscillator rather than input or output parameters of the oscillator (29). To address the possibility that different clocks regulate CAB2 and CAT3, we generated tPRCs in which we monitored CAB2::LUC and CAT3::LUC phases in seedlings that were entrained to LD cycles and released into LL at 22°C. After 1 day in continuous conditions, the seedlings were subjected to a series of cold pulses (12°C for 4 hr) presented at 4-hr intervals spanning a complete 24-hr cycle. We also tested a LUC fusion to the promoter of TIMING OF CAB EXPRESSION 1 (TOC1::LUC) (16) to directly monitor a central oscillator component. An Arabidopsis central oscillator has been shown to be a negative feedback loop comprised of the positive element TOC1 and the negative elements CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) (30–33).
The tPRCs measured with TOC1::LUC and CAB2::LUC are similar (Fig. 4), suggesting that they respond to the same oscillator. This is consistent with the identification of toc1-1 in a screen using CAB2::LUC (17). However, the tPRC measured with CAT3::LUC shows stronger advances in response to cold pulses administered in the subjective night. In particular, the phase of CAT3::LUC is advanced ≈8 hr at CT 16, when CAB2::LUC exhibits a modest (≈2 hr) delay and TOC1::LUC exhibits only a weak (≈1 hr) advance. Similarly, CAT3::LUC is advanced at CT 20, whereas both CAB2::LUC and TOC1::LUC are only weakly affected. These results suggest that CAT3 responds to an oscillator that can be distinguished on the basis of temperature sensitivity from the TOC1 oscillator that regulates its own transcription, as well as that of CAB2.
Fig. 4.
tPRCs reveal that the response of CAT3 to a temperature pulse is distinct from the responses of CAB2 and TOC1. Plants were grown in LD cycles for 7 days and placed on a Packard TopCount luminometer for 1 day of LD. A series of 4-hr 12°C temperature pulses was applied at 4-hr intervals over a 24-hr day after the first full day in LL. Period and phase were determined by fast Fourier transform nonlinear least squares (16), and phase advances and delays were determined compared with a mock-treated control. Data for CAT3::LUC (▪), CAB2::LUC (○), and TOC1::LUC (▵) represent mean ± SEM for three independent experiments and are double plotted for visualization purposes.
CAT3 and TOC1 Exhibit Overlapping Spatial Expression Patterns. The existence of multiple oscillators in multicellular plants is well established. Multiple rhythms have been shown to run with different periods (internal desynchronization) in several species (21–23, 34–36). However, these data cannot easily distinguish between the presence of two distinct molecular oscillators within a single cell or a single oscillator that exhibits organ- or cell type-specific differences in period. Although CHALCONE SYNTHASE (CHS), PHYTOCHROME B (PHYB), and CAB transcription rhythms show distinct periods, the period of each rhythm is shortened by toc1 and de-etiolated 1 mutations, suggesting that they are regulated by a similar, if not identical, clock (21, 23). However, careful analysis of the expression patterns of these genes showed that CAB::LUC is expressed in the photosynthetic leaf mesophyll, whereas CHS::LUC and PHYB::LUC expression is primarily derived from the leaf epidermis and roots, suggesting that they are responding to tissue-specific clocks. That toc1 mutation affects the periods of both CAB and CHS rhythms indicates that TOC1 must be expressed in both mesophyll and epidermal tissues, or that TOC1 expression in one tissue affects clock function in the other tissue (21, 23).
Because our results indicate that CAT3 is under the control of an oscillator distinct from that regulating CAB2, we sought to characterize the expression pattern of the CAT3 promoter fragment used in this study. To this end, we used the same CAT3 promoter fragment to drive the uidA reporter gene, encoding GUS (19). Individual seedlings were stained for GUS activity, which is strong in the cotyledons and the vasculature of cotyledons and leaves, and is detected at a lower level in the hypocotyl (Fig. 5A). Both mesophyll and epidermal cells of the cotyledons exhibit GUS activity (Fig. 5 B and C; the pink color is characteristic of GUS staining under dark field). Under DIC microscopy, GUS activity is evident in all cells of the cotyledons of transformed, but not of untransformed, seedlings. Therefore, the CAT3 promoter used in this study is expressed in both mesophyll and epidermal cells of cotyledons, overlapping with TOC1 in both tissues and with CAB2 in the mesophyll. It is important to note that, although the circadian expression of the CAT3::LUC reporter is consistent with other longer promoter fragments and with endogenous mRNA levels (16), the behavior of this reporter has not been shown to completely recapitulate the behavior of the endogenous CAT3 gene. Nonetheless, this does not affect our conclusion that the oscillator regulating expression of this small CAT3 promoter fragment differs from the oscillator regulating the CAB2 promoter fragment -321/+1 (17, 24).
Fig. 5.
CAT3 is expressed in both mesophyll and epidermis of aerial tissues. Hygromycin-resistant seedlings (ecotype Columbia) transformed with a CAT3::uidA reporter were stained for GUS activity (19). After chlorophyll removal, intact seedlings were imaged. (A) From left to right, three individual hygromycin-resistant and one untransformed seedling, which shows no GUS signal. Cot indicates the cotyledons. (B) Cotyledon, leaf, and hypocotyl images at higher magnification. Note how the blue precipitate is found in round mesophyll cells and in the hypocotyl. (C) Ten-micrometer sections of paraffin-embedded seedlings viewed by dark-field microscopy (two images on left), under which a GUS-positive cotyledon shows the characteristic pink color of the GUS product that is absent from a GUS-negative cotyledon. On the right, the blue precipitate is visible in all cells in the GUS-positive cotyledon under differential interference contrast (DIC) microscopy.
Discussion
Circadian clocks, without exception, respond to light (37), which is the most potent and best characterized entraining stimulus in plants (38, 39). However, temperature is also an important environmental timing cue that can act independently of light to entrain the Arabidopsis circadian clock (7, 16). In this study we show that temperature cues can be more important than light cues in determining the phase of the oscillator that regulates CAT3 transcription. We further demonstrate that two molecular oscillators can be distinguished based on temperature sensitivity: a TOC1 oscillator that drives CAB2::LUC as well as its own transcription, and a second oscillator that drives CAT3::LUC.
Spatial expression patterns for these three genes overlap in the mesophyll and epidermis of the cotyledons, indicating that these two oscillators are expressed in the same tissues. This raises the question of whether these two distinct circadian oscillators function within a single cell. The presence of two circadian oscillators within a single cell has been established in the unicellular dinoflagellate Lingulodinium polyedrum (formerly Gonyaulax polyedra; refs. 40 and 41), but nowhere else. However, we feel that our data are insufficient to demonstrate the presence of two clocks within a single cell. First, the three LUC constructs driven by the CAB2, CAT3, and TOC1 promoters were all examined in different transgenic lines and we are not yet able to simultaneously monitor and distinguish expression of two LUC transgenes within a single seedling. Second, although all three genes are expressed in the mesophyll and CAT3 and TOC1 are expressed in the epidermis, we have not established that the LUC signals we have measured originate in a common tissue. For example, it is possible that our CAT3::LUC signal is predominantly derived from the epidermis and our TOC1::LUC signal is predominantly derived from the mesophyll. This ambiguity allows for the possibility that CAT3 expression in the mesophyll exhibits the same relative temperature and light sensitivity as does CAB2 expression, but that the strong CAT3 signal originating from the epidermis obscures the pattern of mesophyll expression of CAT3. This same logic means that a mesophyll-derived TOC1::LUC signal might prevent our detection of a weaker epidermal signal that exhibits the same light and temperature sensitivity as CAT3. This ambiguity also exists at the cellular level; there may be cellular heterogeneity in the expression of these genes within single tissues. Detection of two oscillators within a single cell may require single-cell monitoring of two transgenes, perhaps distinguished on the basis of wavelength of light emission.
This logic further indicates that the CAT3 oscillator need not be independent of TOC1. Expression of CAB2, TOC1, and CAT3 is rendered arrhythmic by overexpression of CCA1 (30, 42). CCA1 and LHY are thought to be partially redundant, because loss of function of either alone shortens the period but does not eliminate rhythmicity. This is consistent with a model in which multiple oscillators differentially and only partially rely on CCA1 or LHY. This multiplicity of oscillators may also underlie the continued cycling of ELF3 transcript in LHY-overexpressing lhy mutants (43).
Multiple coupled circadian oscillators may provide a mechanism by which a plant can integrate environmental light and temperature cycles. This is likely to contribute to dynamic seasonal acuity. In nature, daily temperature cycles have a distinct phase angle with daily LD cycles, and this relationship is modulated in a seasonal fashion (44). Photoperiod and ambient temperature are integrated through the autonomous pathway to regulate flowering time in Arabidopsis (45). Recent work has strongly supported the external coincidence model of flowering (46), in which an endogenous oscillation in CONSTANS expression must coincide with the external LD cycle to induce flowering (4, 6). However, the integration of temperature signals into the floral transition may also entail the internal coincidence of multiple oscillators that exhibit different sensitivities to light and temperature. Integration of temperature and photoperiodic signals is likely to enhance seasonal sensitivity and thus contribute to the enhancement of fitness by the circadian system that has been demonstrated in Arabidopsis (47), as well as in cyanobacteria (48), Drosophila (49), and mammals (50).
Supplementary Material
Acknowledgments
We thank S. E. Bickel and R. D. Sloboda for help with imaging, K. L. Cottingham for statistical advice, and M. L. Guerinot for helpful discussions. This work was supported by National Science Foundation Grants MCB-9723482 and MCB-0091008.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CT, circadian time; DIF, difference between day and night temperature; GUS, β-glucuronidase; LD, light–dark; LL, continuous light; LUC, luciferase; tPRC, temperature phase response curve.
References
- 1.Dunlap, J. C. (1999) Cell 96, 271-290. [DOI] [PubMed] [Google Scholar]
- 2.Young, M. W. & Kay, S. A. (2001) Nat. Rev. Genet. 2, 702-715. [DOI] [PubMed] [Google Scholar]
- 3.Harmer, S. L., Hogenesch, J. B., Straume, M., Chang, H.-S., Han, B., Zhu, T., Wang, X., Kreps, J. A. & Kay, S. A. (2000) Science 290, 2110-2113. [DOI] [PubMed] [Google Scholar]
- 4.Suárez-López, P., Wheatley, K., Robson, F., Onouchi, H., Valverde, F. & Coupland, G. (2001) Nature 410, 1116-1120. [DOI] [PubMed] [Google Scholar]
- 5.Roden, L. C., Song, H.-R., Jackson, S., Morris, K. & Carré, I. A. (2002) Proc. Natl. Acad. Sci. USA 99, 13313-13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanovsky, M. J. & Kay, S. A. (2002) Nature 419, 308-312. [DOI] [PubMed] [Google Scholar]
- 7.Somers, D. E., Webb, A. A. R., Pearson, M. & Kay, S. A. (1998) Development (Cambridge, U.K.) 125, 485-494. [DOI] [PubMed] [Google Scholar]
- 8.Lüttge, U. (2000) Planta 211, 761-769. [DOI] [PubMed] [Google Scholar]
- 9.Pittendrigh, C. S. (1960) Cold Spring Harbor Symp. Quant. Biol. 25, 159-184. [DOI] [PubMed] [Google Scholar]
- 10.Bruce, V. G. (1960) Cold Spring Harbor Symp. Quant. Biol. 25, 29-48. [Google Scholar]
- 11.Sweeney, B. & Hastings, J. W. (1960) Cold Spring Harbor Symp. Quant. Biol. 25, 87-104. [DOI] [PubMed] [Google Scholar]
- 12.Underwood, H. & Calaban, M. (1987) J. Biol. Rhythms 2, 179-193. [DOI] [PubMed] [Google Scholar]
- 13.Oltmanns, O. (1960) Planta 54, 233-264. [Google Scholar]
- 14.Finlayson, S. A., Lee, I.-J. & Morgan, P. W. (1998) Plant Physiol. 116, 17-25. [PMC free article] [Google Scholar]
- 15.Liu, Y., Merrow, M., Loros, J. J. & Dunlap, J. C. (1998) Science 281, 825-829. [DOI] [PubMed] [Google Scholar]
- 16.Michael, T. P. & McClung, C. R. (2002) Plant Physiol. 130, 627-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millar, A. J., Carré, I. A., Strayer, C. A., Chua, N.-H. & Kay, S. A. (1995) Science 267, 1161-1163. [DOI] [PubMed] [Google Scholar]
- 18.Murashige, T. R. & Skoog, F. (1962) Physiol. Plant. 15, 473-497. [Google Scholar]
- 19.Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. (1987) EMBO J. 6, 3901-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plautz, J. D., Straume, M., Stanewsky, R., Jamison, C. F., Brandes, C., Dowse, H. B., Hall, J. C. & Kay, S. A. (1997) J. Biol. Rhythms 12, 204-217. [DOI] [PubMed] [Google Scholar]
- 21.Hall, A., Kozma-Bognar, L., Bastow, R. M., Nagy, F. & Millar, A. J. (2002) Plant J. 32, 529-537. [DOI] [PubMed] [Google Scholar]
- 22.Hennessey, T. L. & Field, C. B. (1992) J. Biol. Rhythms 7, 105-113. [DOI] [PubMed] [Google Scholar]
- 23.Thain, S. C., Murtas, G., Lynn, J. R., McGrath, R. B. & Millar, A. J. (2002) Plant Physiol. 130, 102-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millar, A. J. & Kay, S. A. (1996) Proc. Natl. Acad. Sci. USA 93, 15491-15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin, R.-F., Chou, H.-M. & Huang, T.-C. (1999) Planta 209, 202-206. [DOI] [PubMed] [Google Scholar]
- 26.Zhong, H. H., Painter, J. E., Salomé, P. A., Straume, M. & McClung, C. R. (1998) Plant Cell 10, 2005-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolar, C., Ádám, É., Schäfer, E. & Nagy, F. (1995) Proc. Natl. Acad. Sci. USA 92, 2174-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolar, C., Fejes, E., Ádám, É., Schäfer, E., Kay, S. & Nagy, F. (1998) Plant J. 13, 563-569. [DOI] [PubMed] [Google Scholar]
- 29.Johnson, C. H. (1999) Chronobiol. Intl. 16, 711-743. [DOI] [PubMed] [Google Scholar]
- 30.Alabadí, D., Oyama, T., Yanovsky, M. J., Harmon, F. G., Má, P. & Kay, S. A. (2001) Science 293, 880-883. [DOI] [PubMed] [Google Scholar]
- 31.Alabadí, D., Yanovsky, M. J., Más, P., Harmer, S. L. & Kay, S. A. (2002) Curr. Biol. 12, 757-761. [DOI] [PubMed] [Google Scholar]
- 32.Mizoguchi, T., Wheatley, K., Hanzawa, Y., Wright, L., Mizoguchi, M., Song, H.-R., Carré, I. A. & Coupland, G. (2002) Dev. Cell 2, 629-641. [DOI] [PubMed] [Google Scholar]
- 33.Makino, S., Matsushika, A., Kojima, M., Yamashino, T. & Mizuno, T. (2002) Plant Cell Physiol. 43, 58-69. [DOI] [PubMed] [Google Scholar]
- 34.Sai, J. & Johnson, C. H. (1999) Proc. Natl. Acad. Sci. USA 96, 11659-11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer, W. & Sadleder, D. (1972) Planta 108, 173-178. [DOI] [PubMed] [Google Scholar]
- 36.King, R. W. (1975) Can. J. Bot. 53, 2631-2638. [Google Scholar]
- 37.Roenneberg, T. & Foster, R. G. (1997) Photochem. Photobiol. 66, 549-561. [DOI] [PubMed] [Google Scholar]
- 38.Devlin, P. F. & Kay, S. A. (2001) Annu. Rev. Physiol. 63, 677-694. [DOI] [PubMed] [Google Scholar]
- 39.Fankhauser, C. & Staiger, D. (2002) Planta 216, 1-16. [DOI] [PubMed] [Google Scholar]
- 40.Roenneberg, T. & Morse, D. (1993) Nature 362, 362-364. [DOI] [PubMed] [Google Scholar]
- 41.Morse, D., Hastings, J. W. & Roenneberg, T. (1994) J. Biol. Rhythms 9, 263-274. [DOI] [PubMed] [Google Scholar]
- 42.Wang, Z.-Y. & Tobin, E. M. (1998) Cell 93, 1207-1217. [DOI] [PubMed] [Google Scholar]
- 43.Hicks, K. A., Albertson, T. M. & Wagner, D. R. (2001) Plant Cell 13, 1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittendrigh, C. S. & Bruce, V. G. (1959) in Photoperiodism and Related Phenomena in Plants and Animals, ed. Withrow, R. B. (Am. Assoc. Advance. Sci., Washington, DC), pp. 475-505.
- 45.Blázquez, M. A., Ahn, J. H. & Weigel, D. (2003) Nat. Genet. 33, 168-171. [DOI] [PubMed] [Google Scholar]
- 46.Bünning, E. (1936) Ber. Dtsch. Bot. Ges. 54, 590-607. [Google Scholar]
- 47.Green, R. M., Tingay, S., Wang, Z.-Y. & Tobin, E. M. (2002) Plant Physiol. 129, 576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ouyang, Y., Andersson, C. R., Kondo, T., Golden, S. S. & Johnson, C. H. (1998) Proc. Natl. Acad. Sci. USA 95, 8660-8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaver, L. M., Gvakharia, B. O., Vollintine, T. S., Hege, D. M., Stanewsky, R. & Giebultowicz, J. M. (2002) Proc. Natl. Acad. Sci. USA 99, 2134-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeCoursey, P. J., Walker, J. K. & Smith, S. A. (2000) J. Comp. Physiol. A 186, 169-180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.