Abstract
Unlike a Tokyo isolate of hepatitis B virus variants, we found a Shanghai isolate that secretes few virions with an immature genome despite its core I97L mutation. Core mutations P5T and I97L were found to be mutually compensatory in offsetting their respective distinct effects on virion secretion.
Hepatitis B virus (HBV) replicates by using reverse transcriptase (11, 24). Due to the low fidelity of the polymerase, error-prone replication and natural selection in vivo lead to the accumulation of multiple mutations in HBV variants predominant in chronic carriers (20, 22). The most frequent natural mutation in the HBV core protein occurs at amino acid 97 (5, 6, 9, 10, 13). It remains a challenge to elucidate the functional significance of these prevalent and predominant mutations, since there is no a priori knowledge about what kind of assays should be used. Recently, a so-called “immature secretion” phenotype was demonstrated to be a global phenotype in tissue culture by introducing a 97L mutation into the core gene of a wild-type HBV genetic background of subtype adr and ayw origins (31, 33). This phenotype of HBV variant 97L is interesting, because it represents an exception to the dogma of preferential export of virions containing mature genomes (higher-molecular-weight viral DNA in relaxed circle form) in wild-type hepadnaviruses (24). Unlike the wild-type HBV, the 97L mutant secretes similar amounts of mature and immature genomes (lower-molecular-weight viral DNA in single-strand form). This phenotype is not caused by any deficiency in reverse transcription (33) or any instability of core proteins and particles (31, 33; M. Newman, F. M. Suk, and C. Shih, unpublished results).
Although an immature secretion-like phenotype has also been found to occur in vivo in woodchuck and snow goose hepadnaviruses (4, 26), it is puzzling that it has not been found so far in natural infection in humans (F. M. Suk, M. H. Lin, and C. Shih, unpublished results). Furthermore, it remains unclear whether an immature secretion phenotype can still be observed in the genetic context of naturally occurring variants, which often contain multiple mutations throughout the genome. To address these issues, we took a reciprocal approach by reverting the natural mutation at amino acid 97 in a naturally occurring HBV variant from leucine back to isoleucine (L97I) and asked whether the predicted immature secretion phenotype can be abolished by eliminating the leucine residue at position 97 of the HBV core antigen (HBcAg).
This parental HBV variant clone of adr subtype origin was isolated from a hepatocellular carcinoma patient from Shanghai, China (clone 14 in references 18 and 19). In addition to the hot spot mutation I97L, it contains multiple frequent mutations, including core mutations P5T and S87G, enhancer II mutations at nucleotides 1762 and 1764, and mutations truncating the X protein and abrogating the production of the pre-S2-containing M envelope protein (18). The 3.2-kb monomeric HBV genome in clone 14 was released from the pUC18 vector by SapI digestion, religated, and linearized by BamHI before being cloned into plasmid pBluescript. This HBV variant plasmid in pBluescript underwent tandem dimerization and is referred to as “Shanghai adr,” while a wild-type adr HBV plasmid, obtained from K. Koike (30), was recloned and dimerized in tandem in pBluescript and is referred to here as “Tokyo adr.”
HepG2 and Huh7 cells were transfected with 10 μg of plasmid DNA by the calcium phosphate method as previously described (31). Transfected cells were harvested on day 7 posttransfection and analyzed for HBV replication by Southern blotting. Medium was collected on days 5 and 7 posttransfection, and virus particles were purified through a 20% sucrose cushion followed by CsCl gradient ultracentrifugation. Fractions containing virion particles were collected, and HBV DNA was extracted and subjected to Southern blot analysis.
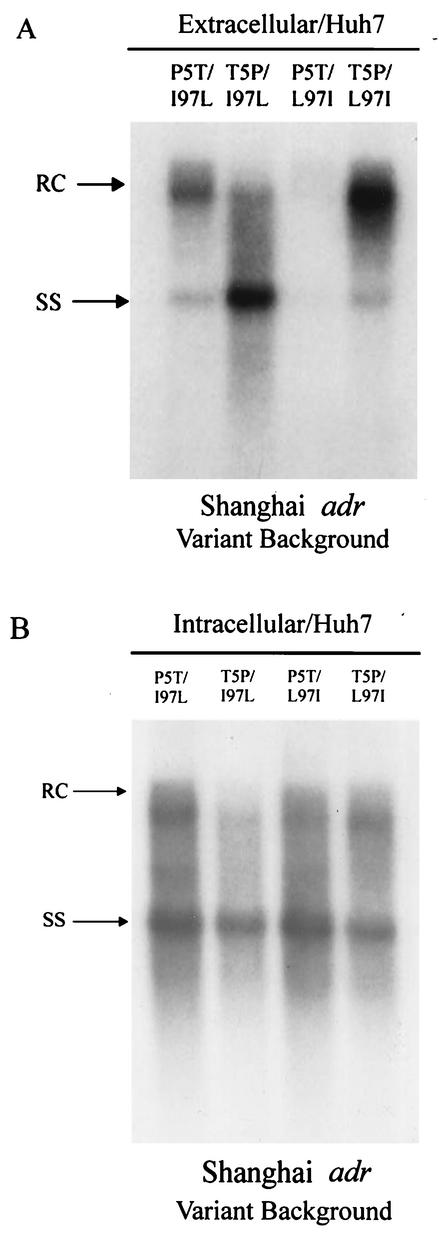
To our surprise, as shown in Fig. 1, the parental Shanghai adr isolate, abbreviated “P5T/I97L” here, does not exhibit any immature virion secretion, despite the presence of a leucine residue at HBcAg position 97 (31, 33). One interpretation of this intriguing result is that a leucine residue at amino acid 97 is not always dominant for the immature secretion phenotype. Indeed, we observed a suppression effect on immature secretion by a core natural mutation, P130T (32), or a pre-S1 artificial mutation, A119F (16). However, when the published DNA sequences of this Shanghai adr variant were examined, there were no P130T or A119F mutations (18) (GenBank accession no. AF411408). It is therefore tempting to speculate that there is another unknown compensatory mutation present somewhere in this Shanghai adr variant.
FIG. 1.
A frequent HBV core mutation, P5T, is responsible for the absence of the immature virion secretion phenotype of a naturally occurring variant containing another frequent core mutation, I97L, in the variant genetic background of a Shanghai adr strain. Culture media from Huh7-transfected cells were analyzed for secreted virion particles (A), while HBV core-associated DNAs from transfected cell lysate were subjected to Southern blot analysis (B). RC, relaxed circular; SS, single-stranded HBV DNA replicative intermediates.
As mentioned earlier, this Shanghai adr variant contains a number of mutations, including core mutations P5T, S87G, and I97L. This P5T mutation, changing a highly conserved proline to threonine at codon 5, was initially discovered during our sequencing studies of the e antigen mutation (13). In the genetic context of wild-type HBV, the naturally occurring core mutation P5T resulted in a low level of virion secretion (17). A similar phenotype of reduced level of virion secretion was observed when certain artificial mutations were introduced into HBcAg (e.g., P79A) (14, 21).
To test if the core mutation P5T could be compensatory for 97L-induced immature secretion, we introduced mutations into the Shanghai adr variant at core amino acids 5 and 97 by using the QuikChange XL site-directed mutagenesis kit (Strata gene, La Jolla, Calif.). The oligonucleotides used for mutagenesis are shown in Table 1. The HBV monomers were then dimerized in tandem to mimic the circular configuration of an HBV genome as previously described (31). All mutations were confirmed by sequencing.
TABLE 1.
Oligonucleotides used for mutagenesis
| Oligo-nucleotidea | Sequenceb |
|---|---|
| T5P (S) | 5′-ATGGACATTGACCCCTATAAAGAATTTGGA-3′ |
| T5P (AS) | 5′-TCCAAATTCTTTATAGGGGTCAATGTCCAT-3′ |
| L97I (S) | 5′-ATGGGCCTAAAAATCAGACAACTACTGTGG-3′ |
| L97I (AS) | 5′-CCACAGTAGTTGTCTGATTTTTAGGCCCAT-3′ |
| P5T (S) | 5′-ATGGACATTGACACGTATAAAGAATTTGGA-3′ |
| P5T (AS) | 5′-TCCAAATTCTTTATACGTGTCAATGTCCAT-3′ |
S, sense polarity; AS, antisense polarity.
Mutated codons are underlined, mutation sites are in boldface.
Interestingly, when the mutation T5P was introduced into the Shanghai adr gene, immature secretion was revealed in mutant T5P/I97L-transfected Huh7 cells (Fig. 1). In contrast, mutant P5T/L97I exhibited a “low-secretion” phenotype with almost no detectable virion secretion, a result consistent with our previous report of mutant P5T in the wild-type Tokyo adr background (17). Finally, when both positions 5 and 97 of HBcAg in Shanghai adr were replaced by wild-type amino acids, mutant T5P/L97I exhibited normal mature secretion at increased intensity (Fig. 1A). It should be noted here that there is no apparent correlation between the diverse virion secretion profiles of various genotypes in Fig. 1A and their respective intracellular activities of HBV replication in Fig. 1B. Such a lack of correlation strongly suggests that these extracellular phenotypes of virion secretion are not the result of any minor difference, if any, in their intracellular viral replication or capsid stability. Taken together, while the mutation P5T can rescue the immature secretion induced by mutation I97L, the low-secretion phenotype induced by the P5T mutation can be rescued by the I97L mutation. In other words, the P5T and I97L mutations appear to be mutually compensatory in the genetic background of the Shanghai adr variant.
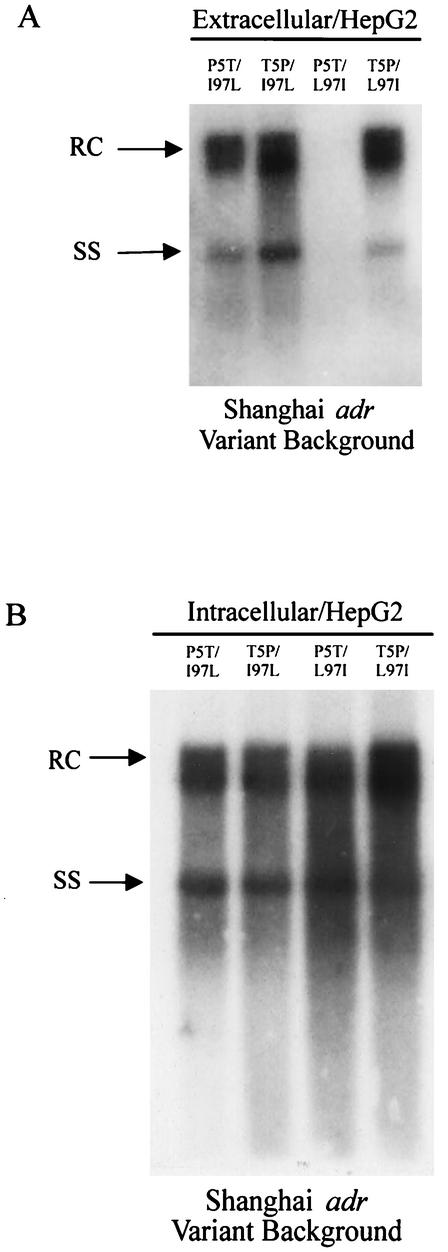
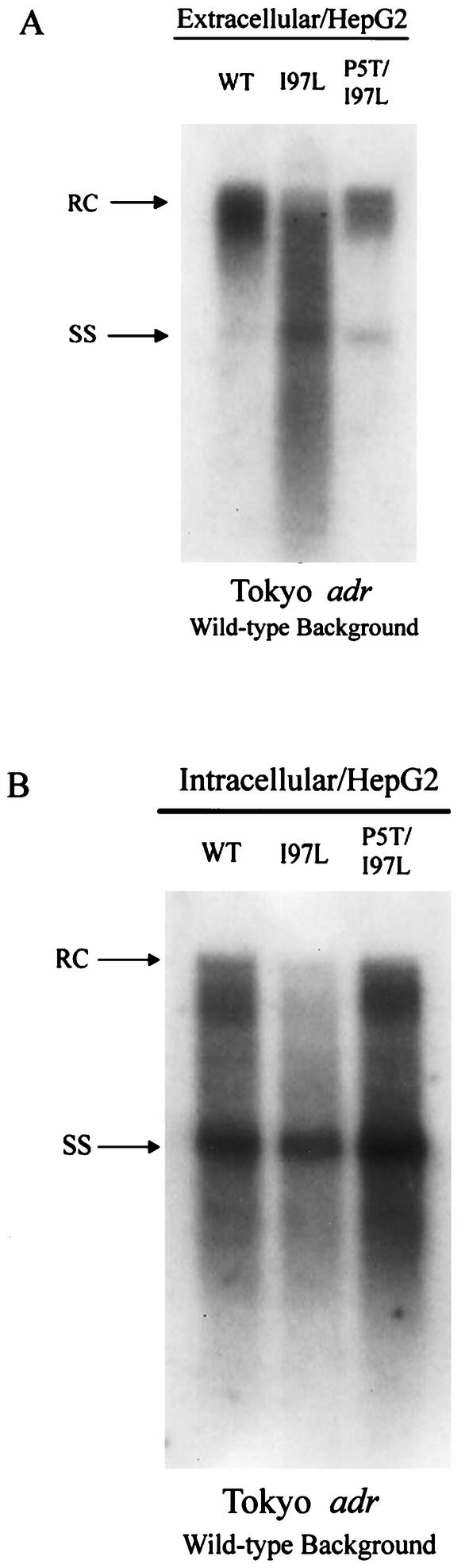
For reasons unclear at present, the immature secretion phenotype was much less pronounced in HepG2 cells (Fig. 2). The diminished phenotype of immature secretion of mutant T5P/I97L in the Shanghai adr background in HepG2 cells could be related to both host factors (HepG2) and viral factors (e.g., X and M protein deficiency) (18). Previously, we noted that both the extracellular immature secretion phenotype and the intracellular replication advantage phenotype tend to be more pronounced in Huh7 cells than in HepG2 cells (23, 33). However, host factors alone cannot entirely explain this phenomenon, since mutant I97L in the Tokyo adr genetic background can still exhibit immature virion secretion in HepG2 cells (Fig. 3A). On the other hand, viral factors alone cannot entirely explain this phenomenon either, since mutant T5P/I97L in the Shanghai adr genetic context can exhibit immature virion secretion in Huh7 cells (Fig. 1A).
FIG. 2.
The immature virion secretion phenotype of core mutation I97L in the genetic background of a Shanghai adr strain is not pronounced in HepG2 cells. Culture media from HepG2-transfected cells were analyzed for secreted virion particles (A), while HBV core-associated DNAs from transfected cell lysate were subjected to Southern blot analysis (B). RC, relaxed circular; SS, single-stranded HBV DNA replicative intermediates.
FIG. 3.
A frequent core mutation, P5T, is compensatory for the immature virion secretion caused by another frequent core mutation, I97L, in the wild-type (WT) HBV genetic background of a Tokyo adr strain. Southern blot analysis for secreted virion particles from culture media of transfected HepG2 cells (A) and assay of intracellular viral replication of transfected human HepG2 cells (B) were performed as described in the text. RC, relaxed circular; SS, single-stranded HBV DNA replicative intermediates.
The compensatory effect of mutation P5T on immature virion secretion was confirmed in the genetic background of the wild-type Tokyo adr HBV in HepG2 (Fig. 3) and Huh7 cells (data not shown). Instead of using the genetic context of the wild-type HBV, to our knowledge, this was the first study of the functional significance of HBV core mutations using the genetic context of a naturally occurring variant. Our study demonstrates that not only viral replication, but also the immature secretion phenotype, can occur in Huh7 cells independent from the expression of an M envelope and full-length wild-type X proteins (18).
In the literature, out of 65 sequences containing 97L mutations, 10 (15.4%) have concurrent P5T mutations (1, 2, 5, 9, 12, 13, 15, 25, 27, 28). Previously, we reported a compensatory core mutation, P130T, which also can rescue the immature secretion phenotype induced by the I97L mutation (32). In the sequences mentioned above, a total of 43 out of 65 (66.2%) sequences were found to contain both 97L and P130T. Of these, only 3 out of 65 (4.6%) sequences contain all three mutations 97L, P5T, and P130T. Taken together, approximately 76% of HBV 97L variants isolated from chronic carriers are found to contain compensatory mutations at codon 5 or 130. It is theoretically possible that some of the naturally compensatory mutations for 97L immature secretion may eventually be found in the envelope genes (16).
At present, the mechanisms of the compensatory effect on immature secretion by either core mutation P5T or P130T remain unclear. Based on the known three-dimensional structure of HBV capsid particles (3, 7, 8, 29), both amino acids 5 and 130 appear to be distant from amino acid 97 in the monomeric structure. Further examination of the core-envelope interaction (16), as well as the spatial and dynamic relationships between core amino acids 5, 97, and 130 in the structural context of dimer, 180-mer, 240-mer, or replicating icosahedral particles, is warranted.
Acknowledgments
This work was funded by NIH grants R01 CA 70336 and CA 84217 to C.S.
We thank colleagues in C. Shih's laboratory for careful reading of the manuscript.
REFERENCES
- 1.Akarca, U. S., and A. S. Lok. 1995. Naturally occurring hepatitis B virus core gene mutations. Hepatology 22:50-60. [PubMed] [Google Scholar]
- 2.Asahina, Y., N. Enomoto, Y. Ogura, M. Kurosaki, I. Sakuma, N. Izumi, F. Marumo, and C. Sato. 1996. Sequential changes in full-length genomes of hepatitis B virus accompanying acute exacerbation of chronic hepatitis B. J. Hepatol. 25:787-794. [DOI] [PubMed] [Google Scholar]
- 3.Bottcher, B., S. A. Wynne, and R. A. Crowther. 1997. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature 386:88-91. [DOI] [PubMed] [Google Scholar]
- 4.Chang, S. F., H. J. Netter, M. Bruns, R. Schneider, K. Frolich, and H. Will. 1999. A new avian hepadnavirus infecting snow geese (Anser caerulescens) produces a significant fraction of virions containing single-stranded DNA. Virology 262:39-54. [DOI] [PubMed] [Google Scholar]
- 5.Chuang, W. L., M. Omata, T. Ehata, O. Yokosuka, Y. Ito, F. Imazeki, S. N. Lu, W. Y. Chang, and M. Ohto. 1993. Precore mutations and core clustering mutations in chronic hepatitis B virus infection. Gastroenterology 104:263-271. [DOI] [PubMed] [Google Scholar]
- 6.Chuang, W. L., M. Omata, T. Ehata, O. Yokosuka, and M. Ohto. 1993. Concentrating missense mutations in core gene of hepatitis B virus. Evidence for adaptive mutation in chronic hepatitis B virus infection. Dig. Dis. Sci. 38:594-600. [DOI] [PubMed] [Google Scholar]
- 7.Conway, J. F., N. Cheng, A. Zlotnick, P. T. Wingfield, S. J. Stahl, and A. C. Steven. 1997. Visualization of a 4-helix bundle in the hepatitis B virus capsid by cryo-electron microscopy. Nature 386:91-94. [DOI] [PubMed] [Google Scholar]
- 8.Crowther, R. A., N. A. Kiselev, B. Bottcher, J. A. Berriman, G. P. Borisova, V. Ose, and P. Pumpens. 1994. Three-dimensional structure of hepatitis B virus core particles determined by electron cryomicroscopy. Cell 77:943-950. [DOI] [PubMed] [Google Scholar]
- 9.Ehata, T., M. Omata, W. L. Chuang, O. Yokosuka, Y. Ito, K. Hosoda, and M. Ohto. 1993. Mutations in core nucleotide sequence of hepatitis B virus correlate with fulminant and severe hepatitis. J. Clin. Investig. 91:1206-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehata, T., M. Omata, O. Yokosuka, K. Hosoda, and M. Ohto. 1992. Variations in codons 84-101 in the core nucleotide sequence correlate with hepatocellular injury in chronic hepatitis B virus infection. J. Clin. Investig. 89:332-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganem, D., and R. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2970. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 12.Gunther, S., S. Baginski, H. Kissel, P. Reinke, D. H. Kruger, H. Will, and H. Meisel. 1996. Accumulation and persistence of hepatitis B virus core gene deletion mutants in renal transplant patients are associated with end-stage liver disease. Hepatology 24:751-758. [DOI] [PubMed] [Google Scholar]
- 13.Hosono, S., P. C. Tai, W. Wang, M. Ambrose, D. G. Hwang, T. T. Yuan, B. H. Peng, C. S. Yang, C. S. Lee, and C. Shih. 1995. Core antigen mutations of human hepatitis B virus in hepatomas accumulate in MHC class II-restricted T cell epitopes. Virology 212:151-162. [DOI] [PubMed] [Google Scholar]
- 14.Koschel, M., D. Oed, T. Gerelsaikhan, R. Thomssen, and V. Bruss. 2000. Hepatitis B virus core gene mutations which block nucleocapsid envelopment. J. Virol. 74:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, Y. I., G. M. Hur, D. J. Suh, and S. H. Kim. 1996. Novel pre-C/C gene mutants of hepatitis B virus in chronic active hepatitis: naturally occurring escape mutants. J. Gen. Virol. 77:1129-1138. [DOI] [PubMed] [Google Scholar]
- 16.Le Pogam, S., and C. Shih. 2002. Influence of a putative intermolecular interaction between core and the pre-S1 domain of the large envelope protein on hepatitis B virus secretion. J. Virol. 76:6510-6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Pogam, S., T. T.-T. Yuan, G. K. Sahu, S. Chatterjee, and C. Shih. 2000. Low-level secretion of human hepatitis B virus virions caused by two independent, naturally occurring mutations (P5T and L60V) in the capsid protein. J. Virol. 74:9099-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin, X., Z. M. Ma, X. Yao, Y. P. Zhang, and Y. M. Wen. 2002. Replication efficiency and sequence analysis of full-length hepatitis B virus isolates from hepatocellular carcinoma tissues. Int. J. Cancer 102:487-491. [DOI] [PubMed] [Google Scholar]
- 19.Lin, X., Z.-H. Yuan, L. Wu, J.-P. Ding, and Y.-M. Wen. 2001. A single amino acid in the reverse transcriptase domain of hepatitis B virus affects virus replication efficiency. J. Virol. 75:11827-11833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou, J. H. 1997. Molecular biology of hepatitis B virus e antigen. J. Gastroenterol. Hepatol. 12:S178-S187. [DOI] [PubMed] [Google Scholar]
- 21.Ponsel, D., and V. Bruss. 2003. Mapping of amino acid side chains on the surface of hepatitis B virus capsids required for envelopment and virion formation. J. Virol. 77:416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shih, C. 2003.. Functional significance of naturally occurring hepatitis B virus variants, p. 23-41. In S. Locarnini and C. L. Lai (ed.), Human virus guides—human hepatitis B viruses. International Medical Press, London, United Kingdom.
- 23.Suk, F.-M., M.-H. Lin, M. Newman, S. Pan, S.-H. Chen, J.-D. Liu, and C. Shih. 2002. Replication advantage and host factor-independent phenotypes attributable to a common naturally occurring capsid mutation (I97L) in human hepatitis B virus. J. Virol. 76:12069-12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summers, J., and W. S. Mason. 1982. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell 29:403-415. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi, K., Y. Akahane, K. Hino, Y. Ohta, and S. Mishiro. 1998. Hepatitis B virus genomic sequence in the circulation of hepatocellular carcinoma patients: comparative analysis of 40 full-length isolates. Arch. Virol. 143:2313-2326. [DOI] [PubMed] [Google Scholar]
- 26.Tencza, M. G., and J. E. Newbold. 1997. Heterogeneous response for a mammalian hepadnavirus infection to acyclovir: drug-arrested intermediates of minus-strand viral DNA synthesis are enveloped and secreted from infected cells as virion-like particles. J. Med. Virol. 51:6-16. [PubMed] [Google Scholar]
- 27.Uchida, T., T. T. Aye, S. O. Becker, M. Hirashima, T. Shikata, F. Komine, M. Moriyama, Y. Arakawa, S. Takase, and S. Mima. 1993. Detection of precore/core-mutant hepatitis B virus genome in patients with acute or fulminant hepatitis without serological markers for recent HBV infection. J. Hepatol. 18:369-372. [DOI] [PubMed] [Google Scholar]
- 28.Uchida, T., T. T. Aye, T. Shikata, M. Yano, H. Yatsuhashi, M. Koga, and S. Mima. 1994. Evolution of the hepatitis B virus gene during chronic infection in seven patients. J. Med. Virol. 43:148-154. [DOI] [PubMed] [Google Scholar]
- 29.Wynne, S. A., R. A. Crowther, and A. G. Leslie. 1999. The crystal structure of the human hepatitis B virus capsid. Mol. Cell 3:771-780. [DOI] [PubMed] [Google Scholar]
- 30.Yaginuma, K., Y. Shirakata, M. Kobayashi, and K. Koike. 1987. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc. Natl. Acad. Sci. USA 84:2678-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan, T. T.-T., G. K. Sahu, W. E. Whitehead, R. Greenberg, and C. Shih. 1999. The mechanism of an immature secretion phenotype of a highly frequent naturally occurring missense mutation at codon 97 of human hepatitis B virus core antigen. J. Virol. 73:5731-5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan, T. T.-T., and C. Shih. 2000. A frequent, naturally occurring mutation (P130T) of human hepatitis B virus core antigen is compensatory for immature secretion phenotype of another frequent variant (I97L). J. Virol. 74:4929-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan, T. T.-T., P.-C. Tai, and C. Shih. 1999. Subtype-independent immature secretion and subtype-dependent replication deficiency of a highly frequent, naturally occurring mutation of human hepatitis B virus core antigen. J. Virol. 73:10122-10128. [DOI] [PMC free article] [PubMed] [Google Scholar]