Abstract
Gammaherpesviruses such as Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus are important human pathogens that establish long-term latent infections. Understanding of the initiation and maintenance of latent infections has important implications for the prevention and treatment of gammaherpesvirus-related diseases. Although much is known about gammaherpesvirus pathogenesis, it is unclear how the infectious dose of a virus influences its ability to establish latent infection. To examine the relationship between the infectious dose and gammaherpesvirus latency, we inoculated wild-type mice with 0.01 to 106 PFU of murine gammaherpesvirus 68 (γHV68) and quantitatively measured latency and acute-phase replication. Surprisingly, during latency, the frequencies of ex vivo reactivation were similar over a 107-fold range of doses for i.p. infection and over a 104-fold range of doses for intranasal infection. Further, the frequencies of cells harboring viral genome during latency did not differ substantially over similar dose ranges. Although the kinetics of acute-phase replication were delayed at small doses of virus, the peak titer did not differ significantly between mice infected with a large dose of virus and those infected with a small dose of virus. The results presented here indicate that any initiation of infection leads to substantial acute-phase replication and subsequent establishment of a maximal level of latency. Thus, infections with doses as small as 0.1 PFU of γHV68 result in stable levels of acute-phase replication and latent infection. These results demonstrate that the equilibrium level of establishment of gammaherpesvirus latency is independent of the infectious dose and route of infection.
Gammaherpesviruses such as Epstein-Barr virus (EBV) are ubiquitous and efficiently establish long-term latent infections. Although the frequency of cells positive for EBV genome during latency differs significantly among individuals (11), it is stable over time. It is unknown whether this reflects differences in infecting doses, variations between viruses, or variations between hosts regardless of the dose. In addition, it is unclear how acute-phase replication of gammaherpesviruses relates to the level of latency. For example, data demonstrating that vaccinations reduce acute-phase replication but do not alter long-term latency (13, 19) suggest that efficient acute infection is not a mandatory step for establishment of latency by a gammaherpesvirus. For alphaherpesviruses such as herpes simplex virus type 1 (HSV-1), establishment of latency correlates with the ability to initiate acute-phase replication (9). A detailed analysis demonstrated that the virus efficiently establishes a stable level of latency once acute-phase replication occurs above a threshold level (9, 17, 18). In contrast, for the betaherpesvirus cytomegalovirus (CMV), the extent of acute-phase replication determines the load of latent viral genome (14, 15). To determine how the infectious dose of a gammaherpesvirus relates to the efficiency of establishment of latency, we quantitatively examined both latency and acute-phase replication following infection of mice with murine gammaherpesvirus 68 (γHV68).
Latent infection.
According to previous reports, acute γHV68 infection in wild-type mice is cleared within 9 to 15 days (4, 22, 28). Thus, early latency is typically monitored after 15 days of infection while late latency is measured after 42 days. To assess both the establishment and the maintenance of latent infection, we assayed latency at 16 and 42 days postinfection. As standard measures of latency, we determined (i) the frequency of cells that reactivate virus from latency ex vivo (frequency of reactivation) and (ii) the frequency of cells containing viral genome. Latency was assessed in peritoneal cells and splenocytes, two important reservoirs of γHV68 latency (23, 29, 30).
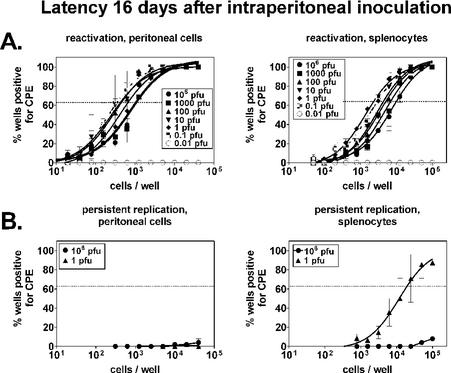
The frequency of cells reactivating virus from latency ex vivo was quantified by limiting-dilution reactivation analysis as previously described (6, 25, 27). Following intraperitoneal (i.p.) infection with 106 PFU of γHV68, the frequencies of reactivation were 1 in 1,000 for peritoneal cells and 1 in 9,700 for splenocytes at 16 days postinfection (Fig. 1A). Notably, these frequencies did not differ more than fourfold over infecting doses ranging from 0.1 to 106 PFU and were similar to data previously published for 106 PFU (25). That infection was detectable with doses as small as 0.1 PFU (as determined by plaque assay on 3T12 cells) likely reflects the fact that mice are a more sensitive indicator of infection with γHV68 than are cultured 3T12 fibroblasts. This is also seen when viral stocks are serially diluted on mouse embryonic fibroblasts, an assay that detects 0.2 PFU of γHV68 (28, 29). In contrast to the 0.1-to-106-PFU doses, little or no reactivation could be detected after infection with 0.01 PFU. Thus, establishment of latency was similar over a 107-fold range of infectious doses.
FIG. 1.
Establishment of latency (16 days) following i.p. infection is independent of the infectious dose. B6 mice were infected i.p. with the indicated doses of γHV68, and 16 days later, peritoneal cells and splenocytes were evaluated. Cells from five mice were pooled and analyzed for each experiment (n = 2). (A) Ex vivo reactivation from latency. A limiting-dilution ex vivo reactivation assay was used to determine the frequencies of peritoneal cells and splenocytes reactivating from latency. Data points indicate the percentage of wells at a given cell dilution that scored positive for a cytopathic effect (CPE) on a mouse embryo fibroblast monolayer (24 wells per dilution). The horizontal line indicates the 63.2% Poisson distribution line at which the frequency of reactivation was calculated (the point at which one reactivation event is likely to occur per well). (B) Preformed infectious virus. Samples parallel to those in the reactivation assay were mechanically disrupted to test for the presence of preformed virus. Disruption destroys cells but does not appreciably alter the viral titer (26). Disrupted samples were plated in a limiting dilution of fibroblast monolayers and scored for cytopathic effect. On the basis of the ability of the assay to detect 0.2 PFU of γHV68 (26) and a very conservative estimate of 1 PFU per productively infected cell, the presence of preformed virus in splenocytes from the 1-PFU sample group corresponded to productive infection of, at most, 1 in 99,500 cells (0.2 PFU at 19,900 cells = 1 PFU in 99,500 cells).
To determine whether preformed infectious virus was present in peritoneal cells or splenocytes at small or large doses of γHV68, we mechanically disrupted duplicate cell samples from the 106- and 1-PFU groups and analyzed them in parallel. Preformed virus was not detectable in peritoneal cells or splenocytes from mice infected with 106 PFU of γHV68, as we have previously reported (25). No preformed virus was detectable in peritoneal cells from mice infected with 1 PFU of γHV68. However, splenocytes contained small amounts of preformed virus (corresponding to ≤1 in 99,500 cells productively infected; Fig. 1B, legend). This was not at a high enough level to constitute a significant contribution to the frequency of reactivation (1 in 2,300). Although it is unknown why preformed virus is detectable in mice inoculated with 1 PFU of γHV68 but not in mice inoculated with 106 PFU of γHV68, it is conceivable that the immune system is not as rapidly induced in the context of a small dose of virus and, consequently, that acute infection is not as rapidly cleared (see below).
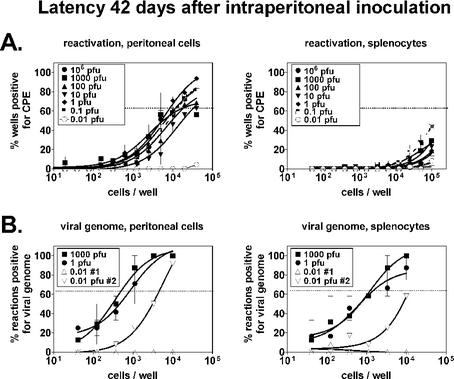
At a later stage of latency (42 days postinfection), results from ex vivo reactivation assays were similar to the day 16 data, with doses over the 0.1-to-106-PFU range resulting in similar levels of latency (Fig. 2A). The frequency of reactivation for peritoneal cells was 1 in 11,900 following infection with 106 PFU, and this did not differ more than threefold over doses ranging from 0.1 to 106 PFU. Results were similar for splenocytes, although the frequencies of reactivation could not be calculated because the curves fell below the 63.2% Poisson distribution line (the point at which one reactivation event is likely to occur per well). We have not detected preformed virus in wild-type mice at any dose 42 days postinfection (data not shown). On the basis of measurement of ex vivo reactivation, the establishment (16 days) and maintenance (42 days) of γHV68 latency did not differ over a 107-fold range of doses.
FIG. 2.
Maintenance of latency (42 days) following i.p. infection is independent of the infectious dose. B6 mice were infected i.p. for 42 days and harvested as described in the legend to Fig. 1. (A) Ex vivo reactivation from latency. A limiting-dilution ex vivo reactivation assay was used to determine the frequencies of peritoneal cells and splenocytes reactivating from latency, as described in the legend to Fig. 1A. (B) Presence of viral genome in latently infected cells. A limiting-dilution nested PCR assay specific for γHV68 gene 72 was used to determine the frequencies of peritoneal cells and splenocytes containing viral genome. Data points indicate the percentage of reaction mixtures at a given cell dilution that scored positive for the presence of viral genome (12 wells per dilution). The horizontal line indicates the 63.2% Poisson distribution line at which the frequency of cells bearing viral genome was calculated. CPE, cytopathic effect.
To further examine maintenance of latent infection, we analyzed cell samples from the 1,000-, 1-, and 0.01-PFU groups for the presence of γHV68 genome (Fig. 2B). The frequency of cells bearing viral genome was determined as previously described (6, 25, 27, 29), by using a limiting-dilution nested PCR assay specific for γHV68 gene 72. The frequencies were similar in the 1,000- and 1-PFU samples for both peritoneal cells (1 in 490 at 1,000 PFU, 1 in 660 at 1 PFU) and splenocytes (1 in 930 at 1,000 PFU, 1 in 1,200 at 1 PFU), similar to results previously published for 106 PFU (25, 29). In contrast, the 0.01-PFU samples contained significantly less viral genome. Data obtained at this dose were inconsistent, with detectable genome in one sample set (0.01-PFU sample set 2) but not in another (0.01-PFU sample set 1), suggesting that 0.01 PFU does not infect 100% of the mice. The low level of detectable viral genome in sample set 2 may reflect infection of some, but not all, of the mice in that group or perhaps a lower level of latency established in all of the mice. Regardless, the presence of latent genome and the ability to reactivate from latency were comparable over a wide range of doses greater than 0.01 PFU, demonstrating that latency is independent of the infectious dose when virus is administered systemically.
Latency following i.n. inoculation.
Since viral genes such as M2 play a role specifically after intranasal (i.n.) inoculation (10), we considered it possible that the relationship between the challenge dose and latency may vary depending on the route of infection. It has also been suggested that latency established after a small-dose i.n. challenge may be more representative of normal gammaherpesvirus physiology than latency established after a large-dose systemic challenge. As it is unclear what the natural route of infection is for γHV68, and because there is some controversy in the field over the choice of the route of infection, we also studied latency in mice after i.n. inoculation. We examined splenic latency after i.n. inoculation of B6 mice with doses of γHV68 ranging from 0.4 PFU to 4 × 105 PFU in 40 μl of Dulbecco modified Eagle medium. We selected 4 × 105 PFU as the largest dose because this is a standard protocol in the field (21, 22).
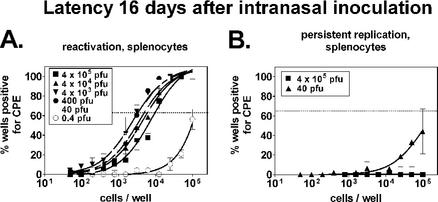
We first examined ex vivo reactivation from latency at 16 days postinfection (Fig. 3A). At 4 × 105 PFU, the frequency of cells reactivating virus from latency was 1 in 9,800, a value nearly identical to the frequency of reactivation displayed by splenocytes from mice infected i.p. (1 in 9,700). All of the i.n. doses tested over a 104-fold range (4 × 105 to 40 PFU) yielded comparable results, with frequencies differing, at most, by threefold. In contrast, mice infected with 0.4 PFU displayed significantly lower levels of reactivation. As with the 0.01-PFU i.p. infection, this could reflect infection of less than 100% of the mice or establishment of a lower level of latency in all of the mice. Similar to the results obtained by i.p. infection, low levels of preformed infectious virus (corresponding to ≤1 in 500,000 cells productively infected; Fig. 3B, legend) could be detected in splenocytes at the 40-PFU dose (Fig. 3B), but this was not substantial enough to make a significant contribution to the detection of reactivation from latency (1 in 5,000). Thus, maximal levels of latency were achieved over a 104-fold range of doses starting at 40 PFU.
FIG. 3.
Establishment of latency (16 days) following i.n. infection is independent of the infectious dose. B6 mice were infected i.n. with the indicated doses of γHV68, and 16 days later, splenocytes were evaluated. Cells from five mice were pooled and analyzed for each experiment (n = 2). (A) Ex vivo reactivation from latency. A limiting-dilution ex vivo reactivation assay was used to determine the frequency of peritoneal cells and splenocytes reactivating from latency, as described in the legend to Fig. 1A. (B) Preformed infectious virus. Duplicate samples from the reactivation assay were mechanically disrupted to test for the presence of preformed virus, as described in the legend to Fig. 1B. The presence of preformed virus in splenocytes from the 40-PFU sample group corresponded to the productive infection of, at most, 1 in 500,000 cells (0.2 PFU at 100,000 cells = 1 PFU in 500,000 cells). CPE, cytopathic effect.
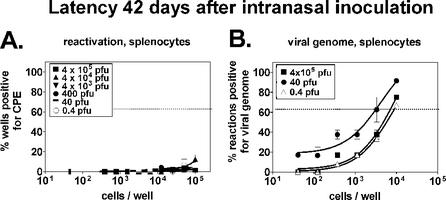
At 42 days postinoculation, ex vivo reactivation from latency decreased to a level below the 63.2% Poisson distribution line (Fig. 4A) and therefore could not be calculated. The frequency of cells positive for viral genome was 1 in 7,600 in mice infected with 4 × 105 PFU (Fig. 4B). It is unclear why the frequency of cells harboring viral genome was slightly increased in mice infected with 40 PFU, but this represented a less-than-fourfold difference compared to the other samples. The detection of high levels of viral genome in the 0.4-PFU sample (1 in 9,800) is interesting considering that ex vivo reactivation was low at 16 days postinfection in the same sample. Although this represents the only case, for either route of infection, in which the presence of viral genome could be uncoupled from ex vivo reactivation from latency, this finding suggests that the processes of harboring viral genome and reactivation from latency are independently regulated. This is consistent with previous data demonstrating immune control of one process but not the other (25). Alternatively, it is formally possible that these results represent a delay in the establishment of latency at 0.4 PFU. Thus, on the basis of ex vivo reactivation and viral genome assays, at doses equal to and greater than 40 PFU, the level of latency following i.n. infection was not dependent on the infectious dose.
FIG. 4.
Maintenance of latency (42 days) following i.n. infection is independent of the infectious dose. B6 mice were infected for 42 days and harvested as described in the legend to Fig. 3. (A) Ex vivo reactivation from latency. A limiting-dilution ex vivo reactivation assay was used to determine the frequency of peritoneal cells and splenocytes reactivating from latency as described in the legend to Fig. 1A. (B) Presence of viral genome in latently infected cells. A limiting-dilution nested PCR assay specific for γHV68 gene 72 was used to determine the frequencies of peritoneal cells and splenocytes containing viral genome as described in the legend to Fig. 2B. CPE, cytopathic effect.
Acute infection following i.p. or i.n. inoculation.
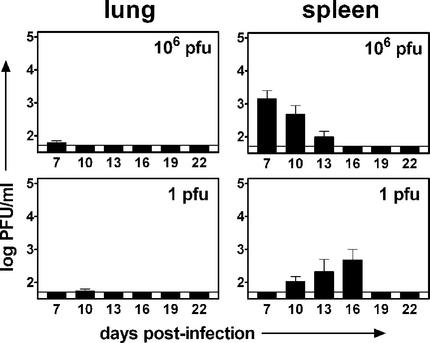
It was surprising that small doses of γHV68, whether administered systemically or mucosally, revealed low levels of preformed infectious virus as late as 16 days postinfection. This led us to speculate that at smaller doses of virus, the peak level or kinetics of acute infection may be altered. To determine the relationship of the infectious dose to acute-phase replication, we infected mice i.p. or i.n. with γHV68 and monitored acute-phase replication in the lungs and spleen. Acute-phase titers were determined with a standard plaque assay as previously described (6, 27). For the experiments, we selected the largest dose (1 × 106 PFU for i.p. inoculation, 4 × 105 PFU for i.n. inoculation) and one of the smallest doses that resulted in a maximal level of latency (1 PFU for i.p. inoculation, 40 PFU for i.n. inoculation). Although most previous studies have focused on acute-phase replication at 4 to 9 days, we chose to monitor acute-phase titers over the first 22 days of infection to provide a more complete view of acute-phase replication in the context of vastly different challenge doses. γHV68 replication in the lungs following i.p. infection was undetectable in most of the mice (Fig. 5). In contrast, replication in the spleen was readily detectable, but the day of peak replication differed significantly depending on the infectious dose used. Following i.p. infection with 1 × 106 PFU of γHV68, the peak of acute-phase replication (1.5 × 103 PFU/ml) occurred at 7 days postinfection. This is similar to other published work demonstrating peak splenic replication at 7 to 10 days postinfection (6, 22, 28). In contrast, peak replication following infection with 1 PFU occurred at day 16 (4.9 × 102 PFU/ml; threefold lower than the peak value for 1 × 106 PFU), which is consistent with data indicating the presence of low levels of preformed infectious virus in the 1-PFU sample at 16 days post i.p. infection (Fig. 1B).
FIG. 5.
The kinetics of acute-phase replication following i.p. infection are dependent on the dose. B6 mice were infected with 106 or 1 PFU of γHV68. At 7 to 22 days postinfection, lungs and spleens were harvested and the viral titer was determined by plaque assay. Columns represent the mean log titer determined for seven or eight individual mice ± the standard error of the mean. The solid line indicates the detection level of the plaque assay (50 PFU). (A) γHV68 titer following i.p. infection with 106 PFU of γHV68. (B) γHV68 titer following i.p. infection with 1 PFU of γHV68.
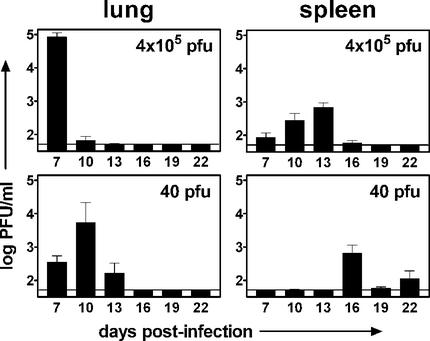
A similar difference in acute-phase replication kinetics was seen following i.n. infection (Fig. 6). The titer in the lungs following infection with 4 × 105 PFU was highest 7 days postinfection, achieving a level of 1.0 × 105 PFU/ml. Although the peak of replication following infection with 40 PFU occurred 3 days later (10 days postinfection), the peak titer was nearly as high (7.1 × 104 PFU/ml; 1.4-fold lower than the peak value for 4 × 105 PFU). Similarly, peak replication in the spleen occurred at day 13 following infection with 4 × 105 PFU (7.0 × 102 PFU/ml) but at day 16 after infection with 40 PFU (6.5 × 102 PFU/ml). Thus, the peak day, but not the peak titer, of acute-phase replication differed depending on the infectious dose.
FIG. 6.
The kinetics of acute-phase replication following i.n. infection are dependent on the dose. B6 mice were infected with 4 × 105 or 40 PFU of γHV68. At 7 to 22 days postinfection, lungs and spleens were harvested and the viral titer was determined by plaque assay. Columns represent the mean log titer determined for seven or eight individual mice ± the standard error of the mean. The solid line indicates the detection level of the plaque assay (50 PFU). (A) γHV68 titer following i.n. infection with 4 × 105 PFU of γHV68. (B) γHV68 titer following i.n. infection with 40 PFU of γHV68.
Discussion.
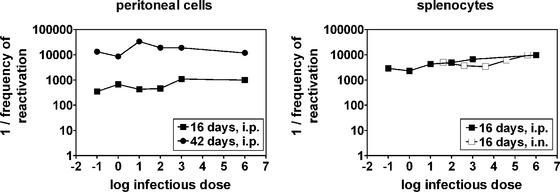
In this report, we have demonstrated that the level of latent infection with a gammaherpesvirus is independent of the infectious dose over at least a 104-fold range, regardless of whether inoculation occurs via a systemic or a mucosal route. Accordingly, the frequency of ex vivo reactivation from latency did not differ over a wide range of doses (Fig. 7A and B) and, notably, was nearly identical for i.p. and i.n. inoculations (Fig. 7B). Thus, the level of γHV68 latency is not dependent on the infectious dose or the route of infection. The experiments described here were performed only with B6 mice; thus, it remains possible that the results of similar experiments with other strains will differ. However, because γHV68 is a natural pathogen of mice (2, 3) and because our observations were consistent between mucosal and systemic routes of infection, we speculate that the conclusions drawn here will extend both to other strains of mice and to other gammaherpesviruses in their natural hosts.
FIG. 7.
The frequency of ex vivo reactivation from latency is independent of the infectious dose. Summary of frequencies of reactivation derived from Poisson distribution of data from Fig. 1A, 2A, 3A, and 4A. Frequencies of reactivation from splenocytes at 42 days postinfection (Fig. 2A and 4A) could not be calculated because the curves fell below the 63.2% Poisson distribution line.
The conclusions drawn here for a gammaherpesvirus differ from those for betaherpesviruses. In a study of murine CMV infection with neonatal versus adult mice, the authors concluded that the extent of virus replication and dissemination determines the overall burden of latent CMV DNA in organs and, consequently, the risk for recurrence of viral disease (15). That is, a higher level of acute-phase replication in multiple organs resulted in a higher level of latency. Data consistent with this have also been obtained with a vaccination system (14). On the other hand, the authors of a detailed study of alphaherpesvirus (HSV-1) replication and latency in an ocular-disease model determined that an infectious dose that generated acute-phase replication above a threshold level was sufficient to result in maximal levels of latency (9). At greater than 2,000 PFU, acute-phase replication in the eyes and latency in the trigeminal ganglia were similar over a range of doses. At 200 or 20 PFU, replication and latency were undetectable in 9 out of 10 mice. Interestingly, the single mouse that did become infected replicated virus and established latency at normal levels, suggesting that any HSV-1 infectious event is sufficient to result in maximal latency. On the basis of our results with γHV68, this may also be the case for gammaherpesviruses, although we cannot conclude this with certainty since our γHV68 latency assays used pools of cells from five mice per group. However, the facts that (i) doses as small as 0.1 PFU i.p. and 40 PFU i.n. induced maximal levels of both acute-phase replication and latency and (ii) doses over a 107-fold range for i.p. inoculation and over a 104-fold range for i.n. inoculation yielded similar levels of latency strongly suggest that establishment of gammaherpesvirus latency is independent of the infectious dose above a level that infects 100% of the mice.
It is notable that although the peak level of acute-phase replication did not differ significantly with the infectious dose, the peak day of acute-phase replication was substantially delayed following small-dose inoculation. This is an important consideration for the interpretation of gammaherpesvirus immunity and pathogenesis experiments that use small doses of virus or for evaluation of the efficacy of strategies to reduce acute-phase replication and latency. For example, vaccination protocols that purport to reduce acute-phase gammaherpesvirus replication may only delay the expansion of the virus for a few days. In fact, the ability of gammaherpesviruses to replicate and expand at extremely low particle numbers may be the key reason why vaccination protocols that reduce acute infection at early times have failed to alter long-term latency (13, 19).
The acute-phase titer data generated here indicate that, regardless of the dose or route of infection, initiation of γHV68 infection leads to viral replication and expansion at a similar level. That the delayed acute-phase replication peak following administration of small doses of γHV68 does not impact the establishment of latency suggests that either (i) acute-phase replication is not required for establishment of latency or (ii) any infectious event will lead to acute-phase replication and viral expansion and consequent establishment of a latent infection. The former hypothesis can be tested by using γHV68 mutants that are defective in the ability to replicate, as has been described for HSV mutants (reviewed in reference 16). The latter supposition would be consistent with findings for alphaherpesviruses (see above). For γHV68, this would require that latency be established at the same time as or prior to peak acute-phase replication since, at small doses, peak replication in the spleen does not occur until 16 days postinfection.
It is unclear what determines the latency set point for each cell population, but this likely involves both viral and host factors. One hypothesis is that for the virus to have a symbiotic relationship with the host but maintain itself at a high level, it must maintain an overall latency state that is periodically interrupted by episodes of reactivation and reseeding of the susceptible cell population. When balanced against the finite lives of host cells, this would allow the virus to sustain a relatively constant level, a latency “set point.” It follows, then, that the virus would encode proteins that act to maintain an optimum level of in vivo reactivation. Consistent with this hypothesis, γHV68 carries both genes that restrict reactivation from latency, such as M1 (6), and genes that enhance reactivation from latency, such as M11 and gene 72 (8).
Host factors that may contribute to determination of the latency set point include activation of the immune system and availability of infectible cells. For example, the frequency of cells latently infected with EBV is significantly increased in immunosuppressed patients (1). Similarly, with the γHV68 system, multiple components of the immune system have been implicated in alteration of latency, including CD8 T cells (20, 25, 28), CD4 T cells (5, 20, 26), gamma interferon (25), perforin (25), B cells, and antibody (7, 12, 29). For example, we have previously demonstrated that CD8 T cells can alter both the number of latently infected cells and the ability of those cells to reactivate from latency (25). Furthermore, the levels of reactivation from latency and viral genome-positive cells are significantly altered in mice deficient in B cells, and this relates to both the loss of a major reservoir for latency and an immunoregulatory role for B cells (29). Moreover, immune recognition of infected cells can alter long-term infection, as we have recently demonstrated in studies of vaccination against γHV68 with a live attenuated virus (24). Alternatively, it is possible that the latency set point is determined simply by the availability of latency-permissive cells, such that any degree of infection will result in maximal acute-phase replication and consequent latent infection of all permissive cells. In the future, it will be important to determine which viral and host factors contribute to the stabilization of a latency set point and whether the presence of these factors can be directly exploited to alter or eliminate chronic gammaherpesvirus infection.
Acknowledgments
The first two authors contributed equally to this work.
H.W.V. and S.H.S. were supported by National Institutes of Health grant CA74730. S.A.T. was supported by grant 5 T32 CA09547-14 from the National Institutes of Health and is a Leukemia & Lymphoma Society fellow (5609-01). J.S.M. and S.B.K. were supported by predoctoral grants from the Cancer Research Institute. V.V.B. was supported by National Institutes of Health grant GM-07200.
We thank members of the Speck and Virgin laboratories, as well as members of the laboratories of David Leib, Lynda Morrison, and Paul Olivo, for helpful discussions.
REFERENCES
- 1.Babcock, G. J., L. L. Decker, R. B. Freeman, and D. A. Thorley-Lawson. 1999. Epstein-Barr virus-infected resting memory B cells, not proliferating lymphoblasts, accumulate in the peripheral blood of immunosuppressed patients. J. Exp. Med. 190:567-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasdell, K., C. McCracken, A. Morris, A. A. Nash, M. Begon, M. Bennett, and J. P. Stewart. 2003. The wood mouse is a natural host for murid herpesvirus 4. J. Gen. Virol. 84:111-113. [DOI] [PubMed] [Google Scholar]
- 3.Blaskovic, D., M. Stancekova, J. Svobodova, and J. Mistrikova. 1980. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468. [PubMed] [Google Scholar]
- 4.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, J. P., R. D. Cardin, K. C. Branum, and P. C. Doherty. 1999. CD4+ T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-γ. Proc. Natl. Acad. Sci. USA 96:5135-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clambey, E. T., H. W. Virgin IV, and S. H. Speck. 2000. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J. Virol. 74:1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangappa, S., S. B. Kapadia, S. H. Speck, and H. W. Virgin IV. 2002. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J. Virol. 76:11460-11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangappa, S., L. F. Van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halford, W. P., and P. A. Schaffer. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J. Virol. 74:5957-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby, M. A., H. W. Virgin IV, and S. H. Speck. 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not i.p., inoculation. J. Virol. 76:1790-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan, G., E. M. Miyashita, B. Yang, G. J. Babcock, and D. A. Thorley-Lawson. 1996. Is EBV persistence in vivo a model for B cell homeostasis? Immunity 5:173-179. [DOI] [PubMed] [Google Scholar]
- 12.Kim, I. J., E. Flano, D. L. Woodland, and M. A. Blackman. 2002. Antibody-mediated control of persistent γ-herpesvirus infection. J. Immunol. 168:3958-3964. [DOI] [PubMed] [Google Scholar]
- 13.Liu, L., E. J. Usherwood, M. A. Blackman, and D. L. Woodland. 1999. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 73:9849-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald, M. R., X.-Y. Li, R. M. Stenberg, A. E. Campbell, and H. W. Virgin IV. 1998. Mucosal and parenteral vaccination against acute and latent murine cytomegalovirus (MCMV) infection by using an attenuated MCMV mutant. J. Virol. 72:442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddehase, M. J., M. Balthesen, M. Rapp, S. Jonjic, I. Pavic, and U. H. Koszinowski. 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 179:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roizman, B. 1996. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc. Natl. Acad. Sci. USA 93:11307-11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawtell, N. M. 1998. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J. Virol. 72:6888-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson, P. G., G. T. Belz, M. R. Castrucci, J. D. Altman, and P. C. Doherty. 1999. A gamma-herpesvirus sneaks through a CD8+ T cell response primed to a lytic-phase epitope. Proc. Natl. Acad. Sci. USA 96:9281-9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson, P. G., R. D. Cardin, J. P. Christensen, and P. C. Doherty. 1999. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J. Gen. Virol. 80(Pt. 2):477-483. [DOI] [PubMed] [Google Scholar]
- 21.Stewart, J. P., N. Micali, E. J. Usherwood, L. Bonina, and A. A. Nash. 1999. Murine gamma-herpesvirus 68 glycoprotein 150 protects against virus-induced mononucleosis: a model system for gamma-herpesvirus vaccination. Vaccine 17:152-157. [DOI] [PubMed] [Google Scholar]
- 22.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gammaherpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 23.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 24.Tibbetts, S. A., J. S. McClellan, S. Gangappa, S. H. Speck, and H. W. Virgin IV. 2003. Effective vaccination against gammaherpesvirus latency. J. Virol. 77:2522-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tibbetts, S. A., L. van Dyk, S. H. Speck, and H. W. Virgin IV. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Usherwood, E. J., A. J. Ross, D. J. Allen, and A. A. Nash. 1996. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J. Gen. Virol. 77:627-630. [DOI] [PubMed] [Google Scholar]
- 27.Van Dyk, L. F., H. W. Virgin IV, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weck, K. E., M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin IV. 1996. Mature B cells are required for acute splenic infection, but not for establishment of latency, by murine gammaherpesvirus 68. J. Virol. 70:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. B cells regulate murine gammaherpesvirus 68 latency. J. Virol. 73:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]