Abstract
Human T-cell leukemia virus type 1 (HTLV-1) Tax regulates the expression of virally encoded genes, as well as various endogenous host genes in trans. Tax-mediated regulation of gene expression is important for the immortalization of normal human T lymphocytes and the transformation of fibroblast cells, such as Rat-1 cells. Tax has the ability to transactivate p21Waf1/Cip1/Sdi1, resulting in high expression levels in HTLV-1-immortalized cells. Since p21 expression is suppressed due to methylation of the promoter region in Rat-l cell line, p21 may not be critical for the transformation of this cell line by Tax. To further understand the role of p21 for the proliferation of Tax-transformed Rat-1 cells, we examined the effect of ectopic expression of p21 in these cells. Here, we observed that p21 expression enhanced the transformation of this cell line via at least two mechanisms: (i) the enhancement of NF-κB activation and/or CREB signaling and (ii) the excitation of antiapoptotic machinery. To analyze the role of p21 that is overexpressed in HTLV-1-immortalized lymphocytes, p21 expression was suppressed by using an antisense oligonucleotide specific for p21 mRNA; these cells then became sensitive to apoptotic induction. These results suggest that p21 plays an important role in the proliferation of Tax-expressing cells through the regulation of at least two independent mechanisms.
Human T-cell leukemia virus type 1 (HTLV-1) is a retrovirus causing adult T-cell leukemia and the neurological disorder HTLV-1-associated myelopathy/tropical spastic paraparesis (35). Tax, a 40-kDa protein encoded by this virus, activates expression of viral genes containing Tax-responsive elements, three 21-bp repeats in the long terminal repeat (LTR) of HTLV-1. Additionally, Tax also transactivates cellular genes driven by transcription factors such as CREB, NF-κB, and SRF (38). Tax contributes to virus-associated immortalization and transformation by transactivating such cellular genes. Previous studies demonstrated that the modulation of NF-κB signaling by Tax, but independent of the CREB pathway, is essential for Tax-mediated transformation of the rat fibroblast cell line, Rat-1, and for the immortalization of normal human T lymphocytes (2, 31, 37).
Tax also influences cellular responses to apoptotic signals. Tax facilitates apoptosis in a variety of cells, including rat fibroblasts and Jurkat lymphocytes, in response to serum deprivation, genotoxic agents, and UV irradiation (10, 20, 23, 26, 36). In contrast, additional studies reported that T cells derived from transgenic mice expressing the tax gene product are resistant to apoptosis triggered by Fas receptor ligation (21).
Tax alters the expression of cell cycle regulatory proteins, upregulating cyclin D2, cyclin E, CDK2, CDK4, CDK6, and p21Waf1/Cip1/Sdi1, and downregulating cyclin D3 and p18 (1, 4, 7, 18). Tax binds to p16INK4a to inhibit its negative regulation of CDK4/6, allowing the progression from G1 to S phase (33). Tax also binds to MAD1 to abolish its mitotic checkpoint function (19). Thus, Tax blocks the G1/S and M cell cycle check points, linking Tax expression to chromosomal abnormalities and abnormal cell division.
p21, a direct regulator of cyclin-dependent kinase (CDK) activity, is a mediator of a p53-induced growth arrest, whose expression is induced with cellular senescence. p21 is a member of the Cip/Kip family of CDK inhibitory proteins (CKIs), along with p27Kip1 and p57Kip2, which share significant sequence homology in the amino-terminal region. The amino-terminal domains of p21, p27, and p57 are both necessary and sufficient for cyclin/CDK inhibition. The unique carboxy-terminal domain of p21 associates with proliferating cell nuclear antigen (PCNA) to inhibit DNA replication (9, 11).
p21 expression is elevated at both the mRNA and protein levels in HTLV-1-infected T cells, as Tax transactivates the p21 promoter (7, 13). The function of p21 in HTLV-1-infected or Tax-immortalized cells remains unclear. To define the role of p21 in HTLV-1-infected cells, we explored the effect of p21 expression on the ability of Tax to transactivate gene expression, to transform cells, and to induce apoptosis. These analyses suggest that Tax capitalizes on p21 expression to stimulate Tax-mediated transactivation and transformation. We also demonstrate that p21 prevents apoptosis in HTLV-1-immortalized and Tax-transformed cells.
MATERIALS AND METHODS
Cell lines and cell culture.
The HTLV-1-infected T-cell lines, HUT102 and MT-2, were cultured at 37°C in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum. Rat-1, a rat fibroblast cell line, was cultured at 37°C in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum. Rat-1-derived cell lines Tax, 703, 410, and M22 were obtained by transfection of plasmids expressing wild-type Tax and tax mutants. A Tax mutant, 703, lacks the CREB pathway, 410 lacks both the NF-κB and CREB pathways, and M22 lacks the NF-κB pathway (2).
Plasmid construction.
pcDSRαΔ-sdi1 was the kind gift of A. Noda (Kobe University). pcDNA3-p21, p21N, and p21C were kindly provided by A. Dutta (Brigham and Women's Hospital, Boston, Mass.). Additional expression plasmids encoding wild-type and mutant p21 were created by PCR by using Pfu DNA polymerase (Promega). PCR products were engineered to contain a BamHI site upstream of the coding sequence with an XhoI site downstream of the sequence encoding the carboxyl terminus. These fragments were subsequently subcloned into pcDNA3 Flag expression vectors, which permits the addition of a Flag epitope tag at the N terminus. To construct expression plasmids encoding mutant Tax proteins, the DNAs encoding the mutant forms of Tax, excised from pLXEN-M22, 703, or 410 (2), were inserted into the pH2R vector.
Luciferase assay.
Appropriate plasmids were introduced into Rat-1 cells by using the FuGENE6 transfection reagent (Roche) according to the manufacturer's protocol. Luciferase assays were performed on cell lysates prepared 24 h after transfection. Transfections were equalized for the amount of total DNA by the addition of empty vector DNA when necessary. At least three assays were performed with lysates obtained by independent transfection.
Transfection and selection.
Rat-1 cells were transfected in 60-mm dishes with 2 to 4 μg of plasmid DNA by using FuGENE6 reagent. After 24 h, cells were treated with trypsin to allow culturing in seven 100-mm dishes. After a 24 h of incubation, cells were cultured in selection medium containing 1 mg of G418/ml. Each clone that appeared was grown in appropriate culture medium.
Colony formation in soft agar containing culture medium.
Rat-1 cells (5 × 103 cells) were cultured for 3 weeks in complete medium containing 0.33% agarose (SeaPlaque agarose; BMA). Colonies that grew to >125 μm in diameter were quantitated.
Western blotting analysis.
Cells were lysed in Tris-buffered saline (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 4 mM EDTA), containing 1% NP-40, 0.1% sodium dodecyl sulfate, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride. Lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by Western blotting analysis with anti-p21 (C-19; Santa Cruz), anti-Flag (M2; Sigma), or anti-Tax (MI73) antibodies.
Evaluation of cell death.
Cells were plated on 60-mm dishes 24 h before serum starvation. After two washes in phosphate-buffered saline, cells were incubated in Dulbecco minimal essential medium without serum. After a 4-h culture in serum-depleted medium, the amount of apoptosis that had occurred was examined by DNA fragmentation by using a cell death detection enzyme-linked immunosorbent assay (ELISA) kit (Roche). The level of fragmentation was measured by determining the ratio of cells transfected with the plasmids of interest to those transfected with control vector. These values are represented as the relative value of this ratio after the control signal was normalized to 1.0.
Antisense oligodeoxynucleotides.
Phosphorothioate oligonucleotides were purchased from Invitrogen. The p21 antisense sequence, 5′-AGCCGGTTCTGACATGGCGC-3′, is complementary to the sequence surrounding the initiation codon of the human p21 mRNA. The bases used in the p21 antisense sequence were shuffled to obtain a randomized control oligonucleotide, 5′-CAGGCCGCGGTGATCTACGT-3′. These oligonucleotides were added to culture medium at 30 μM for HUT102 and MT-2 cells, prior to performing the assay for apoptosis.
Materials.
G418 (Geneticin) was obtained from Nakalai Tesque (Kyoto, Japan). Pfu DNA polymerase was purchased from Promega. Actinomycin D (ActD) was acquired from Sigma. The restriction enzymes, BamHI and XhoI, were purchased from Roche and TaKaRa (Osaka, Japan), respectively. Anti-Tax (MI73) antibody was the kind gift of Y. Namba (Institute for Virus Research, Kyoto University).
RESULTS
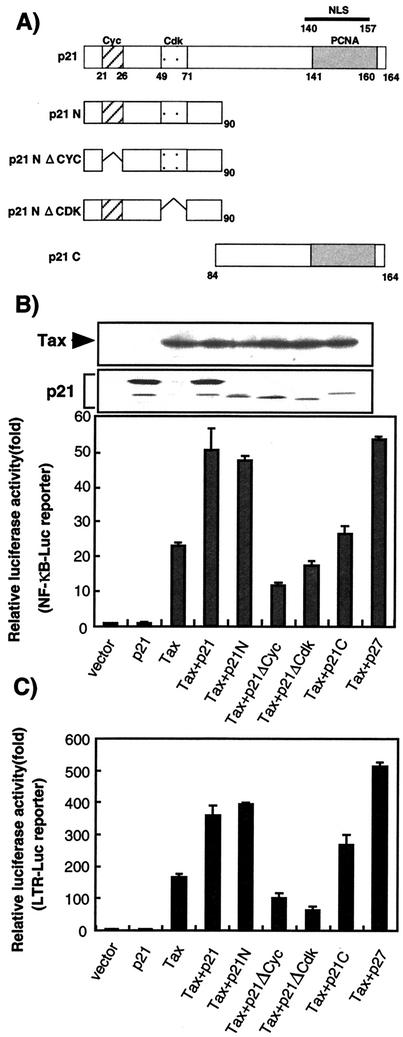
p21 stimulated the transactivational function of Tax through the amino-terminal Cyc1 and Cdk domains of p21.
HTLV-1 Tax activates the HTLV-1 LTR and NF-κB-dependent gene expression. p21 activates NF-κB transcriptional activity (29). p21 and Tax cooperatively stimulate transcription from the human immunodeficiency virus-LTR in a manner dependent on the NF-κB-binding domain (28). To determine the influence of p21 on the transactivational function of Tax, expression plasmids encoding p21 and Tax were cotransfected into Rat-1 cells. The Rat-1 cell line was chosen to analyze the functions of p21 because the cells do not express any endogenous p21 due to methylation of the p21 promoter region (3). Ectopic expression of p21 and its mutants did not affect the level of Tax (Fig. 1B). The presence of Tax stimulated reporter gene expression from a promoter containing Tax-responsive elements (HTLV-1 LTR) and from an NF-κB promoter in Rat-1 cells (Fig. 1B and C). Whereas p21 alone did not activate transcription, cotransfection of p21 and Tax expression plasmids resulted in a two- to threefold enhanced transcription of reporter gene containing NF-κB-binding site or HTLV-1 LTR. Similar results were obtained by using Jurkat T cells (data not shown). p21 has binding domains for both cyclin and CDK within the amino terminus and a PCNA-binding domain in the carboxyl terminus (Fig. 1A). To investigate the region of p21 involved in this enhancement, plasmid expressing either the amino-terminal (p21N) or carboxy-terminal (p21C) region (Fig. 1A) was cotransfected with Tax and the reporter gene expression plasmids. We observed the enhancement of luciferase gene expression from either the NF-κB promoter or the HTLV-1 LTR with p21N (Fig. 1B and C). p21C could not enhance transcription from the NF-κB promoter but slightly enhanced transcription from the HTLV-1 LTR (Fig. 1B and C). As p27 belongs to the Cip/Kip family of CKIs that share significant sequence homology in their amino-terminal regions (11), p27 was cotransfected with Tax and the reporter plasmid into Rat-1 cells, and the luciferase activity was measured. p27 enhanced the transactivation function of Tax in a similar manner to p21, suggesting that the N-terminal homologous sequence within the members of the Cip/Kip family is involved in the stimulation of Tax transactivational function. p21N contains two domains, the cyclin-binding domain 1 (Cyc1) and CDK-binding domain (Cdk). Plasmids expressing deletion mutants lacking either the Cyc1 or the CDK domain of p21, p21NΔCYC, and p21NΔCDK were constructed to allow the identification of the domain responsible for enhancement of Tax transactivation (Fig. 1A). p21NΔCYC or p21NΔCDK were cotransfected into Rat-1 cells with the plasmids expressing Tax and a reporter gene, prior to measurement of reporter gene expression. Neither mutant could stimulate the transactivational function of Tax for either reporter gene. This result suggested that both the Cyc1 and Cdk domains of p21N are necessary for the p21-mediated enhancement of Tax transactivation.
FIG. 1.
p21-mediated enhancement of Tax transactivational function through the action of the amino-terminal domain. (A) Schematic representation of the p21 mutants used in the present study. The cyclin(Cyc1), Cdk, and PCNA-binding domains and the nuclear localization signal (NLS) are indicated. p21N contains the amino-terminal domain of p21 (amino acids 1 to 90), while p21C contains the p21 carboxy-terminal domain (amino acids 84 to 164). p21NΔCYC comprises the amino-terminal domain lacking the Cyc1-binding domain (amino acids 1 to 21 and 26 to 90), while p21NΔCDK possesses the amino-terminal domain without the Cdk-binding domain (amino acids 1 to 49 and 71 to 90). (B) Effect of wild-type and mutant p21 on NF-κB promoter-mediated transcription and the protein level of Tax. Rat-1 cells were cotransfected with 0.1 μg of the reporter plasmid, pNF-κB-Luc, 0.1 μg of pH2R-Tax, and 0.1 μg of either the wild-type or mutant p21 expression plasmids (p21N, p21NΔCYC, p21NΔCDK, and p21C) by using FuGENE6 transfection reagent. Luciferase assays as well as Western blotting analysis with anti-Tax and anti-Flag antibodies were performed after 24 h of the transfection. Assays were performed with lysates obtained after three independent transfections. (C) Effect of wild-type and mutant p21 on HTLV-1 LTR promoter-driven transcription. Rat-1 cells were cotransfected with 0.1 μg of the reporter plasmid, pLTR-Luc, 0.1 μg of pH2R-Tax, and 0.1 μg of either wild-type or mutant p21 expression plasmids (p21N, p21NΔCYC, p21NΔCDK, and p21C) by using FuGENE6 transfection reagent. Luciferase assays were performed after 24 h of the transfection. Assays were performed with lysates obtained by three independent transfections.
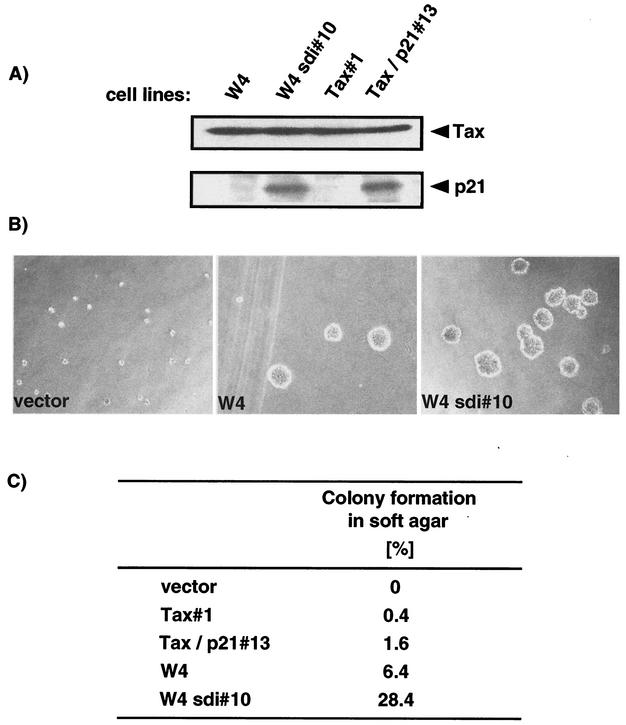
p21 stimulated Tax-mediated transformation.
Tax has the ability to transform Rat-1 fibroblast cells (34, 37). To examine the effect of p21 on Tax-mediated transformation, Rat-1 cells expressing either Tax alone or Tax with p21 were examined for growth properties by colony formation in soft agar. W4 cells, previously generated by the transfection of Rat-1 cells with the Tax-encoding plasmid, pH2R-Tax (37), and W4sdi#10 cells, generated by transfection of W4 with the pcDSRαΔ-sdi1 plasmid encoding p21 (27), were examined. In addition, Rat-1 cells were transfected with the Tax expression plasmid, pH2R-Tax, with or without a p21-encoding plasmid, pcDNA3-p21, to generate another cell lines. The resultant cell lines, Tax#1 or Tax/p21#13, constitutively expressing either Tax or Tax with p21, respectively, were also used in this work. The expression of Tax and p21 in each cell line was first examined by Western blotting (Fig. 2A). All cell lines expressed similar levels of Tax, whereas W4sdi#10 and Tax/p21#13 cells expressed p21 at nearly equivalent levels (Fig. 2A). The transforming activity of each cell line was analyzed by colony formation in soft agar (Fig. 2B and C). Rat-1 cells transfected with an empty vector did not grow in soft agar (Fig. 2B). Tax-expressing cells, however, exhibited a transformed morphology and acquired the ability to form colonies. W4sdi#10 cells also formed colonies in soft agar at higher numbers than W4 cells lacking p21. Quantitation of colonies greater than 125 μm in diameter revealed that four times as many W4sdi#10 cells formed colonies as W4 cells (Fig. 2C). The fold induction of colony formation after p21 expression in semisolid medium was similar between the cell lines examined, although the number of colonies varied between the cell lines (Fig. 2C and unpublished data). Thus, expression of p21 stimulated colony formation of Tax-transformed Rat-1 cells.
FIG. 2.
Stimulation of the Tax-mediated transformation of Rat-1 cells by p21. (A) Expression of Tax and p21 was examined by Western blotting with the C-19 and MI73 antibodies specific for p21 and Tax, respectively. W4 and Tax#1 are Tax-expressing Rat-1 cells, whereas W4sdi#10 and Tax/p21#13 are Rat-1 cells expressing both Tax and p21. (B) Cells (5 × 103 cells) were inoculated into complete medium containing 0.33% agarose. After a 3-week culture period, colonies were photographed. (C) The numbers of colonies greater than 125 μm in diameter were quantitated after cultivation for 3 weeks. Values indicate the percentages of colonies more than 125 μm in diameter.
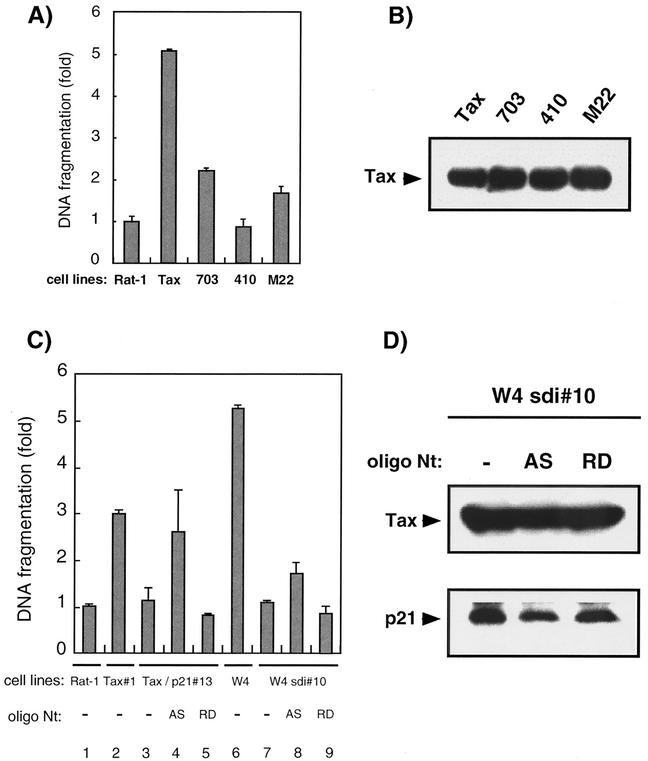
p21 protected Tax-expressing Rat-1 cells from apoptosis caused by serum deprivation.
HTLV-1 Tax induces programmed cell death in response to growth arrest signals evoked by UV irradiation, serum deprivation, or genotoxic agents in a subset of cells, including fibroblasts and the Jurkat T-cell line (10, 20, 23, 26, 36). We examined the sensitivity of parental and Tax-expressing Rat-1 cells to apoptosis after serum deprivation. The quantity of apoptosis evoked in each cell line was examined by a DNA fragmentation assay using a cell death detection ELISA kit. After 4 h of serum deprivation, parental Rat-1 cells exhibited little DNA fragmentation. Cell detachment and DNA fragmentation were easily observed in Rat-1 cells expressing wild-type Tax after serum deprivation (Fig. 3A). To examine whether the transactivational function of Tax affects Tax-mediated apoptosis, Rat-1-derived cell lines expressing Tax mutants were established. The expression of Tax in these cell lines was examined by Western blotting, and they expressed similar levels of Tax (Fig. 3B). Expression of either Tax mutant, M22 or 703, was less cytotoxic than the wild-type Tax. The 410 mutant exhibited almost no cytotoxicity, showing similar levels of DNA fragmentation to the parental Rat-1 cells upon serum deprivation. This pattern of cell death suggested that the ability of Tax not only to activate both HTLV-1 LTR and NF-κB signaling but also to activate either of these promoters promotes apoptosis in Rat-1 cells. To further examine the effect of p21 on Tax-induced cell death, Rat-1 cells expressing both p21 and Tax, Tax/p21#13 and W4sdi#10 cells, were incubated without serum for 4 h prior to an examination of DNA fragmentation. In these cell lines and others constitutively expressing Tax and p21, Tax-mediated apoptosis was inhibited (Fig. 3C, compare lanes 2 and 3 and lanes 6 and 7 [data not shown]), suggesting p21 blocks Tax-mediated apoptosis. To confirm the effect of p21 on apoptosis, Tax/p21#13 and W4sdi#10 cells were treated with an antisense oligonucleotide (AS) specific for p21 mRNA. We verified the suppression of p21 protein expression by the antisense oligonucleotide. After a 4-h treatment of W4sdi#10 cells and Tax/p21#13 with 10 μM antisense oligonucleotide, p21 protein levels were reduced in comparison with those in cells treated with the randomized control oligonucleotide (RD) (Fig. 3D and data not shown). As the production of Tax protein was not affected by this treatment, the reduction by the antisense oligonucleotide appears to be specific for p21 protein (Fig. 3D and data not shown). To examine the effect of p21 antisense oligonucleotide on apoptosis, Tax/p21#13 and W4sdi#10 cells were incubated for 4 h in serum-free medium supplemented with a 10 μM concentration of either the antisense or the randomized oligonucleotide prior to examination of DNA fragmentation by ELISA. Tax/p21#13 and W4sdi#10 cells exhibited an increase of DNA fragmentation upon treatment with the antisense oligonucleotide (Fig. 3C, lanes 3 to 5 and lanes 7 to 9), whereas Tax/p21#13 and W4sdi#10 cells treated with the randomized oligonucleotide showed DNA fragmentation levels similar to those seen in parental Rat-1 cells. These results suggested that p21 inhibits Tax-mediated apoptosis in Rat-1 cells.
FIG. 3.
p21 suppressed apoptosis of Tax-expressing Rat-1 cells caused by serum deprivation. (A) Parental, wild-type Tax (Tax)-expressing, and mutant Tax (M22, 703, or 410)-expressing Rat-1 cells were plated at 7 × 105 cells on 60-mm dishes 24 h prior to serum starvation. After 4 h of incubation in serum-free medium, DNA fragmentation in these cells was quantitated by using a cell death detection ELISA kit. The average of the relative ratio of the DNA fragmentation calculated for each of three independent experiments is shown. (B) The expression of Tax and its mutants was examined by Western blotting with the MI73 antibody. (C) Parental Rat-1 (Rat-1), Tax#1, Tax/p21#13, W4, or W4sdi#10 cells were plated at 7 × 105 cells on 60-mm dishes 24 h prior to serum starvation. These cells were then incubated in serum-free medium for 4 h (lanes 1 to 3, 6, and 7). Tax/p21#13 and W4sdi#10 cells were incubated in serum-free medium with 10 μM of the p21 antisense (AS) or the random oligonucleotide (RD) for 4 h (lanes 4 to 5 and lanes 8 to 9). DNA fragmentation was then quantitated by using a cell death detection ELISA kit. The average of the relative ratio calculated for each of three independent experiments is shown. (D) W4sdi#10 cells were cultured in medium containing either the antisense (AS) or the random oligonucleotide (RD) at 10 μM for 4 h. After the electrophoresis of total cell lysates, p21 and Tax were detected by Western blotting.
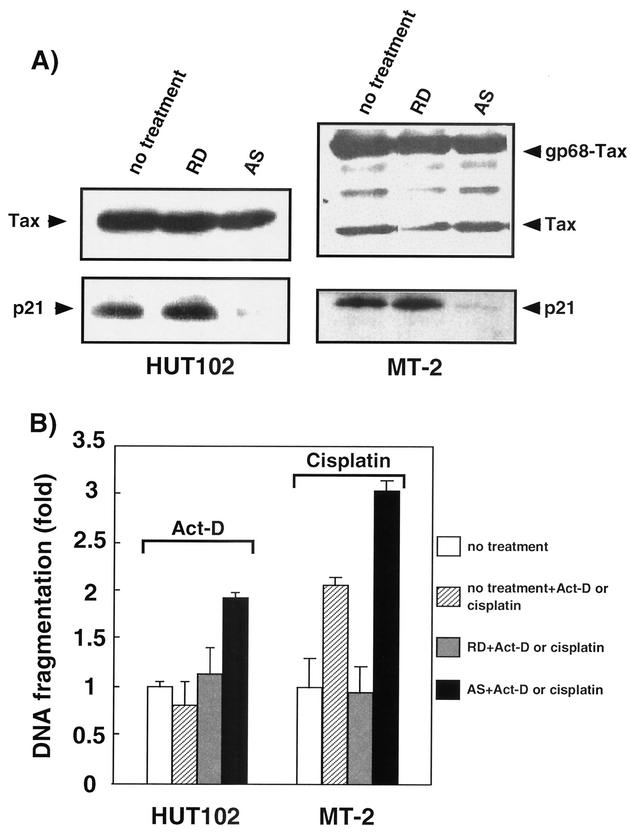
p21 protein reduced the sensitivity of HTLV-1-infected cells to apoptosis.
p21 expression is elevated at both the mRNA and protein levels in HTLV-1-infected T-cell lines (1, 7). Upon gamma irradiation of the HTLV-1-infected T-cell line, C81, p21 blocks the phosphorylation of retinoblastoma protein, Rb, to arrest cells in G1 phase (13). This result suggests that the function of p21 to arrest the cell cycle is not impaired in HTLV-1-infected T cells. Therefore, we investigated the contribution of p21 to the inhibition of apoptosis in HTLV-1-infected T cells. Addition of the antisense oligonucleotide specific for p21 mRNA to the culture medium of HUT102 and MT-2 cells reduced p21 protein levels, whereas the randomized control oligonucleotide did not alter the levels (Fig. 4A). After the reduction in p21 levels, the production of Tax protein was not affected (Fig. 4A). To examine the effect of downregulation of p21 by antisense on apoptosis, HUT102 cells were incubated in complete serum medium supplemented with 30 μM antisense oligonucleotide for 24 h prior to the addition of 0.5 μg of ActD/ml to induce apoptosis. After an additional 5 h incubation, DNA fragmentation was analyzed by ELISA assay. For the analysis of MT-2 cells, the cells were incubated in complete serum medium supplemented with 30 μM antisense oligonucleotide and 0.5 μg of cisplatin/ml for 24 h, and DNA fragmentation was analyzed. Both HUT102 and MT-2 cells treated with the randomized oligonucleotide showed only slight DNA fragmentation after ActD or cisplatin treatment (Fig. 4B). In contrast, treatment with the antisense oligonucleotide resulted in a >1.5-fold enhancement of DNA fragmentation (1.1 versus 1.9 and 0.96 versus 3.1 in HUT102 and MT-2 cells, respectively). The same enhancement of DNA fragmentation was also observed for MT-2 cells by serum deprivation (data not shown). We observed that MT-2 cells treated with the RD and cisplatin became more antiapoptotic than those treated with only cisplatin, the reason for which remained to be clarified. These results suggest that p21 plays an important role in repressing sensitivity to apoptosis in HTLV-1-infected T cells.
FIG. 4.
Expression of p21 decreased the sensitivity of HTLV-1-infected T cells to apoptosis. (A) The HTLV-1-infected T-cell lines, HUT102 and MT-2, were cultured in medium containing either the antisense (AS) or random oligonucleotide (RD) at 30 μM for 29 and 24 h, respectively. After the electrophoresis of total cell lysates, p21, Tax, and gp68, a viral Tax-Env fusion protein, were detected by Western blotting. (B) HUT102 cells were administered either the p21 antisense (AS) or the random oligonucleotide (RD) at 30 μM for 24 h. ActD was then added to this medium at 0.5 μg/ml. The relative ratios of DNA fragmentation were then measured by ELISA assay after 5 h of cultivation. MT-2 cells were administered either the AS or RD at 30 μM with 0.5 μg of cisplatin/ml. The relative ratios of DNA fragmentation were measured after 24 h of cultivation. Histograms represent the fold difference in DNA fragmentation after the levels in cells without oligonucleotide and ActD or cisplatin were normalized to 1.0. The average of the relative ratios of DNA fragmentation calculated for each of three independent experiments is shown.
DISCUSSION
HTLV-1 Tax induces the expression of many cellular genes by interacting with cellular transcription factors, transcriptional coactivators, and corepressors (12, 38). Activation of NF-κB signaling by Tax is important for the transformation of fibroblast cells, including the Rat-1 cells, and for the immortalization of normal T-lymphocytes (2, 31, 37). Despite the suppression of p53 by Tax (4, 7), the expression of the p21Waf1/Cip1/Sdi1 gene, a major downstream gene upregulated by p53, is enhanced in HTLV-1-infected cells. The activation of p21 gene expression is directly regulated by Tax (13). p21 expression, however, does not interfere with the proliferation of HTLV-1-infected cells. The Tax gene product employs multiple strategies to prevent p21 from causing cell cycle arrest, including increasing the transcription of the CDK2, CDK4, CDK6, cyclin D2, and cyclin E genes (1, 18) and the direct binding of Tax to CDK4, counteracting the inhibitory activity of p21 (17). Thus, the overexpression of p21 in Tax-immortalized cells plays an important, unclarified role(s) in cell proliferation independent of the induction of cell cycle arrest.
Rat-1 cells lack p21 expression due to the hypermethylation of the p21 promoter region (3). Since Tax expression is sufficient to transform Rat-1 fibroblasts, the transforming ability of Tax is independent of p21. We sought to examine the function of ectopically expressed p21 in the modulation of cell proliferation by Tax-transformed Rat-1 cells.
We analyzed the effect of p21 on the transformation efficiency of Tax in Rat-1 cells by counting the number of colonies grown in soft agar. To obtain Rat-1 cells expressing either Tax alone or Tax with p21, we used the previously established W4 cells and additional cell lines created by introducing Tax or Tax plus p21 into Rat-1 cells. Our analysis of colony formation (Fig. 2) demonstrated that no colony formation by Rat-1 cells occurred in the absence of Tax, which is consistent with the previous report (37). As reported earlier, exogenous Tax expression was sufficient to induce the appearance of colonies in soft agar. Interestingly, cells expressing both Tax and p21 exhibited an increased number of colonies. To quantitate the efficiency of Tax-mediated colony formation, we quantitated the numbers of colonies greater than 125 μm in diameter (Fig. 2C). Although the numbers of colonies differed slightly between the cell lines in each repetition of this experiment, the ratio of the number of colonies in Tax plus p21-expressing cells to that of Tax-expressing cells remained steady at approximately four. Since the expression levels of Tax and p21 in these cell lines were similar, it is unclear why W4 cells consistently produced more colonies. The W4 cell line, however, was established several years ago and may have developed an increasingly transformed phenotype during this passage period. It is also possible that integration of the Tax gene into the genome resulted in a more transformed phenotype.
To elucidate the reason for the enhanced transforming ability gained after p21 expression, we analyzed NF-κB signaling activity. We observed that NF-κB signaling was enhanced by expression of p21, the increase of which by Tax was shown to be important to the transformation of Rat-1 cells (37) (Fig. 1B). In addition, the CREB pathway, examined by transcription from the HTLV-1 LTR, was also enhanced (Fig. 1C). Through deletional studies, we determined that the region of p21 enhancing Tax transactivation is localized to the N terminus, requiring both the CDK and cyclin-binding sites. p21 binds to multiple regulatory proteins, in addition to cyclin/CDK and PCNA (11), which function to stimulate NF-κB-mediated transcription (28). This effect may be facilitated by activation of a transcriptional coactivator, CBP/p300 (29, 32). Since these coactivators are known to augment the expression of various host genes, it is likely that enhanced transcription from HTLV-1 LTR by p21 was mediated by activation of the coactivator. p21 also enhanced NF-κB activation by the Epstein-Barr virus protein LMP1, possibly acting through a similar mechanism (data not shown). To clarify the mechanism of coactivator enhancement by p21, we attempted to establish Rat-1 cell lines expressing Tax plus each of the p21 deletion mutants, p21N and p21C. This attempt, however, was unsuccessful as all of the cells died before a clone could be established.
Rat-1 cells activate antiapoptotic machinery during serum starvation (14, 36), whereas Tax-transduced Rat-1 cells become proapoptotic during treatment (14, 36). Rat-1 cells transduced with the Tax mutants, deficient in either NF-κB or CREB activation, respectively, exhibited reduced DNA fragmentation (Fig. 3A). These data indicate that the transactivation of Tax through both NF-κB and CREB signalings is involved in Tax-mediated apoptosis, a finding consistent with previous reports (26).
Upon serum starvation of Tax-transduced Rat-1 cells, we observed an ∼3-fold and an ∼5-fold increase in DNA fragmentation compared to the parental Rat-1 cells. In contrast, cells transduced with both Tax and p21 exhibited similar levels of DNA fragmentation to the parental Rat-1 cells (Fig. 3C). To confirm that the suppression of DNA fragmentation is associated with the production of p21, we designed a phosphorothioate antisense oligonucleotide targeting the region containing the initiation codon of p21 mRNA. After addition of this oligonucleotide to the culture medium, DNA fragmentation in these cells increased to levels similar to those observed in Tax-transduced Rat-1 cells. Cells treated with the randomized control oligonucleotide did not exhibit any changes in DNA fragmentation levels, suggesting that acquisition of resistance to apoptosis resulted from production of p21.
Both HTLV-1 infection and exogenous Tax expression result in the activation of p21 gene expression (1, 7, 13). The role of p21 production in HTLV-1-immortalized human lymphocytes, however, remains to be clarified. We treated such cells with an antisense oligonucleotide specific for p21 mRNA and analyzed the sensitivity of these cells to apoptosis after the addition of ActD or cisplatin. The levels of p21 decreased significantly (Fig. 4), concurrently with 1.7- and 3.2-fold increases in DNA fragmentation in HUT102 and MT-2 cells, respectively, compared to a given control oligonucleotide.
p21 is upregulated downstream of p53 signaling. p21 expression, however, is also induced by stress, including DNA damage, or by agents affecting DNA replication through p53-independent mechanisms. In cells in which the cell cycle is arrested by p53 activation, p21 inhibits cyclin/CDK activity, preventing the phosphorylation of RB. After treatment with apoptosis-inducing agents, however, caspase-mediated cleavage of p21 proceeds, promoting apoptosis (39). Human HCT116 cells, lacking p21 completely, exhibit drastic increases in the levels of apoptosis after treatment with radiation, possibly because these cells enter into mitosis prior to the repair of DNA damage (6). Since p21 knockout mice display reduced rates of radiation-induced tumorgenesis, p21 may play a significant role as an antiapoptotic factor (24). Thus, p21 protects cells from apoptosis, which may not require cell cycle progression (5, 15, 16, 30), suggesting that p21 possesses an antiapoptotic function, acting independently from its growth-inhibitory activity.
HTLV-1 Tax activates both NF-κB signaling and Akt, a gene product downstream of NF-κB (25). The Akt kinase phosphorylates p21 at positions 145 and 146. p21 phosphorylated at Ser146 possesses both a sustained half-life and an antiapoptotic function (22). We examined whether this scenario also occurs in HTLV-1-immortalized cells. HUT102 and MT-2 cells treated with an Akt inhibitor, LY294002, prior to the addition of ActD, exhibited reductions in DNA fragmentation exceeding those seen after treatment with the p21-specific antisense oligonucleotide (data not shown).
Recently, p21-induced cellular gene expression was analyzed by microarray analysis (8). This analysis revealed various genes involved in antiapoptotic and mitogenic functions, including connective tissue growth factor, epithelin, and galectin-3. Thus, p21 may exert an antiapoptotic function through an unclarified mechanism in HTLV-1-immortalized cells. Clarification of the mechanism underlying the antiapoptotic ability of p21 in HTLV-1-immortalized cells may eventually contribute to the creation of a therapeutic strategy for diseases caused by HTLV-1 infection.
Acknowledgments
We thank Y. Namba for supplying the anti-Tax antibody (MI73), A. Noda for pcDSRαΔ-sdi1, M. Hatanaka for W4 cells and pH2R-Tax, and Anindya Dutta for pcDNA3-p21, p21N, and p21C. We are grateful to B. Vogelstein for HCT116 p21+/+ and p21−/− cells and to S. Harada for pSGflagLMP1. We thank M. Hijikata, T. Ohshima, and members of the laboratory of our institute for helpful comments and discussions.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4, and p21Waf1/Cip1/Sdi1. Oncogene 12:1645-1652. [PubMed] [Google Scholar]
- 2.Akagi, T., H. Ono, H. Nyunoya, and K. Shimotohno. 1997. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene 14:2071-2078. [DOI] [PubMed] [Google Scholar]
- 3.Allan, L. A., T. Duhig, M. Read, and M. Fried. 2000. The p21WAF1/CIP1 promoter is methylated in Rat-1 cells: stable restoration of p53-dependent p21WAF1/CIP1 expression after transfection of a genomic clone containing the p21WAF1/CIP1 gene. Mol. Cell. Biol. 20:1291-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ariumi, Y., A. Kaida, J.-Y. Lin, M. Hirota, O. Masui, S. Yamaoka, Y. Taya, and K. Shimotohno. 2000. HTLV-1 Tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene 19:1491-1499. [DOI] [PubMed] [Google Scholar]
- 5.Asada, M., T. Yamada, H. Ichijo, D. Delia, K. Miyazono, K. Fukumuro, and S. Mizutani. 1999. Apoptosis inhibitory activity of cytoplasmic p21Cip1/WAF1 in monocytic differentiation. EMBO J. 18:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 7.Cereseto, A., F. Diella, J. C. Mulloy, A. Cara, P. Michieli, R. Grassmann, G. Franchini, and M. E. Klotman. 1996. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood 88:1551-1560. [PubMed] [Google Scholar]
- 8.Chang, B. D., K. Watanabe, E. V. Broude, J. Fang, J. C. Poole, T. V. Kalinichenko, and I. B. Roninson. 2000. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc. Natl. Acad. Sci. USA 97:4291-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Junjie., P. K. Jackson, M. W. Kirschner, and A. Dutta. 1995. Separatedomains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 374:386-388. [DOI] [PubMed] [Google Scholar]
- 10.Chlichlia, K., M. Busslinger, M. E. Peter, H. Walczak, P. H. Krammer, V. Schirrmacher, and K. Khazaie. 1997. ICE-proteases mediate HTLV-1 Tax-induced apoptotic T-cell death. Oncogene 14:2265-2272. [DOI] [PubMed] [Google Scholar]
- 11.Dotto, G. P. 2000. p21WAF1/Cip1: more than a break to the cell cycle? Biochem. Biophys. Acta 1471:M43-M56. [DOI] [PubMed] [Google Scholar]
- 12.Ego, T., Y. Ariumi, and K. Shimotohno. 2002. The interaction of HTLV-1 Tax with HDAC1 negatively regulates the viral gene expression. Oncogene 21:7241-7246. [DOI] [PubMed] [Google Scholar]
- 13.Fuente, C., F. Santiago, S. Y. Chong, L. Deng, T. Mayhood, P. Fu, D. Stein, T. Denny, F. Coffman, N. Azimi, R. Mahieux, and F. Kashanchi. 2000. Overexpression of p21waf1 in human T-cell lymphotropic virus type 1-infected cells and its association with cyclinA/cdk2. J. Virol. 74:7270-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita, M., and H. Shiku. 1995. Differences in sensitivity to induction of apoptosis among rat fibroblast cells transformed by HTLV-I tax gene or cellular nuclear oncogenes. Oncogene 11:15-20. [PubMed] [Google Scholar]
- 15.Glaser, T., B. Wagenknecht, and M. Weller. 2001. Identification of p21 as a target of cycloheximide-mediated facilitation of CD95-mediated apoptosis in human malignant glioma cells. Oncogene 20:4757-4767. [DOI] [PubMed] [Google Scholar]
- 16.Gorospe, M., C. Cirielli. X. Wang, P. Seth, M. C. Capogrossi, and N. J. Holbrook. 1997. p21Waf1/Cip1 protects against p53-mediated apoptosis of human melanoma cells. Oncogene 14:929-935. [DOI] [PubMed] [Google Scholar]
- 17.Haller, K., Y. Wu, E. Derow, I. Schmitt, K.-T. Jeang, and R. Grassmann. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulated the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 22:3327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwanaga, R., K. Ohtani, T. Hayashi, and M. Nakamura. 2001. Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 20:2055-2067. [DOI] [PubMed] [Google Scholar]
- 19.Jin, D.-Y., F. Spencer, and K.-T. Jeang. 1998. Human T-cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93:81-91. [DOI] [PubMed] [Google Scholar]
- 20.Kao, S.-Y., F. J. Lemoine, and S. J. Marriott. 2000. HTLV-1 Tax protein sensitizes cells to apoptotic cell death induced by DNA damaging agents. Oncogene 19:2240-2248. [DOI] [PubMed] [Google Scholar]
- 21.Kishi, S., S. Saijyo, M. Arai, S. Karasawa, S. Ueda, M. Kannagi, Y. Iwakura, M. Fujii, and S. Yonehara. 1997. Resistance to Fas-mediated apoptosis of peripheral T cells in human T lymphocyte virus type I (HTLV-I) transgenic mice with autoimmune arthropathy. J. Exp. Med. 186:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Ying., D. Dowbenko, and L. A. Lasky. 2002. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J. Biol. Chem. 277:11352-11361. [DOI] [PubMed] [Google Scholar]
- 23.Los, M., K. Khazaie, K. Schulze-Osthoff, P. A. Baeuerle, V. Schirrmacher, and K. Chlichlia. 1998. Human T-cell leukemia virus-I (HTLV-I) Tax-mediated apoptosis in activated T cells requires an enhanced intracellular prooxidant state. J. Immunol. 161:3050-3055. [PubMed] [Google Scholar]
- 24.Martin-Caballero, J., J. M. Flores, P. Garcia-Palencia, and M. Serrano. 2001. Tumor susceptibility of p21Waf1/Cip1-deficient mice. Cancer Res. 61:6234-6238. [PubMed] [Google Scholar]
- 25.Meng, F., L. Liu, P. C. Chin, and S. R. D'Mello. 2002. Akt is a downstream target of NF-κB. J. Biol. Chem. 277:29674-29680. [DOI] [PubMed] [Google Scholar]
- 26.Nicot, C., and R. Harrod. 2000. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 Tax protein. Mol. Cell. Biol. 20:8580-8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noda, A., Y. Ning, S. F. Venable, O. M. Pereira-Smith, and J. R. Smith. 1994. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211:90-98. [DOI] [PubMed] [Google Scholar]
- 28.Paeker, S. F., N. D. Perkins, S. D. Gitlin, and G. J. Nabel. 1996. A cooperative interaction of human T-cell leukemia virus type 1 Tax with p21 cyclin-dependent kinase inhibitor activates the human immunodeficiency virus type 1 enhancer. J. Virol. 70:5731-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins, N. D., L. K. Felzien, J. C. Betts, K. Leung, D. H. Beach, and G. J. Nabel. 1997. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science 275:523-527. [DOI] [PubMed] [Google Scholar]
- 30.Polyak, K., T. Waldman, T. C. He, K. W. Kinzler, and B. Vogelstein. 1996. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 10:1945-1952. [DOI] [PubMed] [Google Scholar]
- 31.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snowden, A. W., L. A. Anderson, G. A. Webster, and N. D. Perkins. 2000. A novel transcriptional repression domain mediates p21WAF1/CIP1 induction of p300 transactivation. Mol. Cell. Biol. 20:2676-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki, T., T. Narita, M. Uchida-Toita, and M. Yoshida. 1999. Downregulation of the INK4 family of cyclin-dependent kinase inhibitors by Tax protein of HTLV-1 through two distinct mechanisms. Virology 259:384-391. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 90:9528-9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchiyama, T. 1997. Human T-cell leukemia virus type-1 (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 36.Yamada, T., S. Yamaoka, T. Goto, M. Nakai, Y. Tsujimoto, and M. Hatanaka. 1994. The human T-cell leukemia virus type-1 Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J. Virol. 68:3374-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaoka, S., H. Inoue, M. Sakurai, T. Sugiyama, M. Hazama, T. Yamada, and M. Hatanaka. 1996. Constitutive activation of NF-κB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type 1 Tax protein. EMBO J. 15:873-887. [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida, M. 2001. Multiple virus strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19:475-496. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, Y., N. Fujita, and T. Tsuruo. 1999. Caspase-mediated cleavage of p21Waf1/Cip1 converts cancer cells from growth arrest to understanding apoptosis. Oncogene 18:1131-1138. [DOI] [PubMed] [Google Scholar]