Abstract
The Nrf1 transcription factor belongs to the CNC subfamily of basic leucine zipper proteins. Knockout of Nrf1 is lethal in mouse embryos, but nothing is known about the cell types that absolutely require its function during development. We show by chimera analysis that Nrf1 is essential for the hepatocyte lineage. Mouse embryonic stem cells lacking Nrf1 developed normally and contributed to most tissues in adult chimeras where Nrf1 is normally expressed. Nrf1-deficient cells contributed to fetal, but not adult, liver cells. Loss of Nrf1 function resulted in liver cell apoptosis in late-gestation chimeric fetuses. Fetal livers from mutant embryos exhibited increased oxidative stress and impaired expression of antioxidant genes, and primary cultures of nrf1−/− fetal hepatocytes were sensitive to tert-butyl hydroperoxide-induced cell death, suggesting that impaired antioxidant defense may be responsible for the apoptosis observed in the livers of chimeric mice. In addition, cells deficient in Nrf1 were sensitized to the cytotoxic effects of tumor necrosis factor (TNF). Our results provide in vivo evidence demonstrating an essential role of Nrf1 in the survival of hepatocytes during development. Our results also suggest that Nrf1 may promote cell survival by maintaining redox balance and protecting embryonic hepatocytes from TNF-mediated apoptosis during development.
Nrf1 is a basic leucine zipper protein (bZIP) in the Cap'n'Collar (CNC) transcription factor family characterized by the presence of a 45-amino-acid homology region referred to as the CNC domain (9). The primary structures of members of the CNC-bZIP subfamily are also well conserved in the basic DNA-binding and protein dimerization domains, and at this time in mice this family includes p45NFE2, Nrf1, Nrf2, Nrf3, Bach1, and Bach2 (3, 8, 9, 35, 50, 51). Some are expressed in a tissue-specific manner. For example, the expression of p45NFE2 is restricted to hematopoietic cells (3), and Bach2 is expressed in the brain and B-cell compartment (51). In contrast, Nrf1, Nrf2, and Nrf3 are expressed in a wide variety of cell lines and mouse tissues, and Bach1 transcripts are found in various tissues and expressed at high levels in the spleen and intestine (9, 35, 43, 51). CNC-bZIP factors control transcription from an extended AP-1-like site [TGCTGA(G/C)TCA(T/C)] called the NFE2 site and form heterodimers with other bZIP proteins (30, 33, 36). One class of bZIP proteins that dimerizes with CNC-bZIP factors is the small Maf proteins (7, 44).
Gene targeting experiments in embryonic stem (ES) cells to generate knockout mice have been performed to determine the biological requirements for CNC-bZIP proteins. Knockout mice revealed an important role for p45NFE2 in platelet production (60). Nrf2 knockout mice were found to be viable (11). While Nrf2 is dispensable for development, it is important in regulating antioxidant gene expression and expression of xenobiotic-metabolizing enzymes in multiple tissues (31, 32). In contrast, the biological function of Nrf1 is less well understood. While Nrf1 has been shown to play a role in the oxidative stress response pathway in fibroblasts, the importance of Nrf1 in this function in vivo compared to Nrf2 is not known (37). In our previous gene knockout study, we found that Nrf1 is required for development (10). Death at midgestation in nrf1−/− embryos is thought to be the result of anemia due to a presumed developmental arrest in fetal liver erythropoiesis. Because nrf1−/− erythropoietic cells grew normally in vitro and nrf1−/− ES cells contributed efficiently to erythroid cells of chimeric mice, it was suggested that the liver microenvironment in mutants failed to sustain erythropoiesis. Nrf1 is broadly expressed during development, but with the exception of anemia, discernible defects in other tissues were not readily apparent in mutants. Therefore, it was difficult to establish whether other tissue compartments were also affected by the loss of Nrf1 function in development. Moreover, the embryonic lethality precludes further analysis of the potential role of Nrf1 in fetal liver function and development.
To gain more insight into the function of Nrf1, we utilized nrf1−/− ES cells in an analysis of chimeric mice to determine in which cell type is Nrf1 important during development. We describe here rescue of embryonic lethality in chimeric mice generated with ES cells bearing two inactive nrf1 alleles. Characterization of these chimeric mice indicated that ES cells deficient in Nrf1 contributed to most tissues in adult animals, including the lung, kidney, muscle, and heart where Nrf1 is normally expressed at high levels. However, deficiency in Nrf1 is associated with impaired contribution of ES cells to adult, but not fetal, hepatocytes. While Nrf1 function is not required for normal fetal liver ontogeny, liver cells in chimeric embryos undergo apoptosis late in gestation. Our results also indicate that oxidative stress and impaired antioxidant gene expression in the fetal liver correlated with loss of Nrf1 function. Primary mutant fetal hepatocytes in cultures demonstrated increased sensitivity to tert-butyl hydroperoxide (tBHP) and died, indicating that the loss of Nrf1 sensitizes hepatocytes to oxidative stress. Mouse embryonic fibroblasts isolated from nrf1−/− embryos were very sensitive to tumor necrosis factor (TNF) cytotoxicity, suggesting that Nrf1 may be required to protect fetal liver cells from endogenous TNF-induced apoptosis. Together, these findings suggest that Nrf1 plays an important role in maintaining redox balance in the fetal liver cells and indicate a cell-specific and developmental stage-specific function of Nrf1 in protecting liver cells from apoptosis during development.
MATERIALS AND METHODS
Gene targeting in ES cells and generation of ES chimeras.
nrf-1 genomic clones were isolated from a 129/Sv mouse library described previously (10). A 10.6-kb EcoRI genomic DNA subfragment was used to generate the targeting vector. The 2.2-kb BamHI/EcoRI fragment containing the terminal exon of nrf1 was deleted and replaced with the internal ribosome entry site (IRES)-βGeo cassette that permits translation of two open reading frames from the same transcript (45). Therefore, the targeted allele directs the expression of β-galactosidase from the same transcript and allows identification of cells that express Nrf1. The phosphoglycerate kinase (PGK)-thymidine kinase (TK) gene cassette was inserted into the EcoRI/HindIII site 5′ to the nrf1 gene. The resulting vector has a 3.6-kb right arm and a 4.4-kb left arm flanking the βGeo cassette.
JM-1 ES cells were cultured on mitomycin C-treated primary mouse embryonic fibroblast feeders on gelatinized culture plates in Dulbecco modified Eagle medium (DMEM) culture medium supplemented with 15% fetal bovine serum (FBS) (HyClone), 0.3 mg of glutamine per ml, 0.1 mM nonessential amino acids, 0.055 mM β-mercaptoethanol, and 1,000 U of leukemia inhibitory factor (GIBCO-BRL). For transfection, 5 × 106 ES cells suspended in phosphate-buffered saline (PBS) was electroporated at 0.25 kV and 500 μF with 30 μg of NotI-linearized DNA. After electroporation, cells were chilled on ice for 20 min before they were plated on three gelatinized 100-mm-diameter culture dishes. After 48 h, cells were grown in selection medium containing G418 (200 μg/ml) and ganciclovir (2 μM). The medium was changed every 24 h until colony isolation at 1 week. Individual clones were picked and expanded in 96-well plates. Expanded clones were screened by Southern blotting of NsiI-digested DNA using a 5′ nrf1 external probe. The wild-type allele yields a 14-kb fragment, and the targeted allele yields a 16-kb band. Approximately 10 positive clones were identified from 100 clones screened. Chimeras were generated by injection of positive ES clones into blastocysts from C57BL/6J mice, which were then transferred into pseudopregnant C57BL/6J recipients, or by an aggregation method using eight-cell-stage embryos from CD-1 mice (65). ES cells aggregated with eight-cell-stage embryos were cultured overnight and then transferred into pseudopregnant B6D2 females.
GPI analysis.
Tissues were subjected to three cycles of freezing and thawing in sample buffer containing 50 mM Tris-HCl (pH 8) and 0.1% Triton X-100 to lyse the cells completely. Supernatants were cleared by centrifugation and electrophoresed on cellulose acetate membranes in 100 mM Tris-750 mM glycine (pH 8.5) running buffer for 45 min at 150 V. Glucose phosphate isomerase (GPI) activity was detected by overlaying the membrane with 10 ml of 0.4% agarose containing 80 mM Tris (pH 8.0), 5 mM magnesium acetate, 20 mg of fructose-6-phosphate, 0.25 mg of phenazine methosulfate, 2 mg of methylthiazolium tetrazolium, 2 mg of nicotinamide dinucleotide phosphate, and 1.4 U of glucose-6-phosphate. Incubation at room temperature was performed in the dark prior to detection of GPI isoenzyme bands. Band intensities were quantitated using an Alpha Innotech Gel Documentation system and software (San Leandro, Calif.).
Histology and staining for lacZ gene activity.
Tissue samples were fixed in 4% paraformaldehyde in 1× PBS for 60 min and then washed several times in rinse buffer (0.1 M NaPi [pH 7.4], 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% NP-40). Samples were then stained in 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) buffer {5 mM potassium ferrocyanide [K4Fe(CN)6], 5 mM potassium ferricyanide [K3Fe(CN)6], 1 mg of X-Gal per ml} for 24 h in an incubator set at 30°C. Stained samples were rinsed in 10% formalin and stored in 1× PBS prior to embedding and sectioning.
Quantitative reverse transcription-PCR (RT-PCR).
Total RNA was extracted using TRIZOL (Life Technologies) according to the manufacturer's instructions from individual or pooled fetal livers, and erythroid progenitors were generated as previously described (10) using embryonic day 13.5 (E13.5) fetal livers cultured for 3 days in methylcellulose containing interleukin-6, interleukin-3, stem cell factor, and erythropoietin. Contaminating DNA was removed from RNA samples by RQ1 DNase (Promega) digestion according to the manufacturer's instructions and by extraction using nonbuffered phenol. cDNAs were synthesized from 1 to 2 μg of total RNA in 100-μl reaction mixtures containing 1× reverse transcription buffer, 7.5 mM MgCl2, 1 mM deoxynucleoside triphosphates (dNTPs), 5 μM random hexamer, 40 U of RNase inhibitor, 250 U of Moloney murine leukemia virus reverse transcriptase. Reverse transcription reaction mixtures were incubated at 72°C for 5 min, 25°C for 10 min, and 45°C for 60 min, and aliquots of the reaction products were used in PCRs. Expression of transcripts encoding gamma-glutamylcysteine synthetase catalytic subunit (Gclc) and regulatory subunit (Gclm) was analyzed using the 5′ nuclease assay (real-time TaqMan RT-PCR) with the ABI PRISM 7700 instrument as described previously (20). PCR was conducted in triplicate with 50-μl reaction mixture volumes of 1× PCR buffer A (ABI), 5.5 mM MgCl2, 0.9 μM (each) primer, 200 μM dNTPs, 200 nM probe, and 0.025 U of Taq Gold (ABI) per μl. The sequences of the PCR primers and TaqMan probe for mouse Gclc follow: forward primer (exon 3), 5′-CAATATGAGGAAACGCCGGA-3′; reverse primer (exon 4), 5′-TGTTCTGGCAGTGTGAATCCA-3′; and TaqMan probe, 5′-FAM (6-carboxyfluorescein)-CCATCACTTCATTCCCCAGACTAGGCTGT-TAMRA (6-carboxytetramethylrhodamine)-3′ (Integrated DNA Technologies). For mouse Gclm, the sequences of the PCR primers and TaqMan probe were as follows: forward primer (exon 4), 5′-CATGTCCCATGCAGTGGAGA-3′; reverse primer (exon 5), 5′-GTCCAGCTGTGCAACTCCAA-3′; and TaqMan probe, 5′-FAM-CAGTTGACATGGCATGCTCCGTCCT-TAMRA-3′. PCR cycling conditions were the same for both cDNAs, consisting of 15 min at 95°C and 40 cycles of PCR, with 1 cycle consisting of 30 s at 95°C and 1 min at 60°C. Relative expression levels were calculated as detailed previously (21). Briefly, relative expression was calculated as follows: 2(Ct of the test gene − Ct of the GAPDH gene), where Ct is threshold cycle and the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an endogenous control gene. For SYBR green-based real-time PCR, aliquots of cDNA were amplified in an iCycler (Bio-Rad) using Quantitect PCR reagents (Qiagen). The sequences of the PCR primers were as follows: for mouse metallothionein 1 (MT-1), 5′-ATGGACCCCAACTGCTCCT-3′ for the forward primer and 5′-ACAGCCCTGGGCACATTT-3′for the reverse primer; for mouse MT-2, 5′-CCGATCTCTCGTCGATCTTCAACC-3′ for the forward primer and 5′-CAGGAGCAGCAGCTTTTCTTGCAG-3′ for the reverse primer; for mouse heme oxygenase 1, 5′-CACGCCAGCCACACAGCACTA-3′ for the forward primer and 5′-GGCTGTCGATGTTCGGGAAGG-3′ for the reverse primer; for mouse Gpx1, 5′-TGCTCATTGAGAATGTCGCGTCTC-3′ for the forward primer and 5′-AGGCATTCCGCAGGAAGGTAAAGA-3′ for the reverse primer. Expression levels of various mRNAs relative to GAPDH levels were calculated as 2(Ct of the test gene − Ct of the GAPDH gene).
Isolation of embryonic fibroblasts and hepatocytes and culture.
Isolation and culture of fibroblasts from embryos were described previously (37). Livers were dissected from E13 and E14 embryos and minced using a scalpel. The livers were then incubated briefly in liver perfusion medium containing EGTA (GIBCO-BRL) and dissociated further in liver digestion medium (GIBCO-BRL) or in 1× PBS containing 0.0625% trypsin. Tissue clumps were dispersed by gentle pipetting and filtered through a 70-μm-pore-size mesh (Falcon) to remove cell clumps. Cells were then collected, and their viability was assessed by trypan blue dye exclusion. Approximately 1 × 105 to 3 × 105 cells are recovered from each liver. The cells were then seeded in 60-mm-diameter collagen-coated dishes (Biocoat; Becton-Dickinson) in DMEM-F-12 supplemented with 20% heat-inactivated fetal calf serum. After 8 h, cultures were washed extensively with 1× PBS to remove nonadhering cells, and this process was repeated after overnight incubation to further remove leftover hematopoietic cells. The remaining adherent cells represent approximately half of the input cells.
To determine the cytotoxic effect of tumor necrosis factor-alpha (TNF-α) (R&D Technologies), approximately 2 × 105 mouse embryonic fibroblasts were plated in six-well dishes. Cells were treated with TNF alone or pretreated with 5 μg of cycloheximide per ml for 30 min prior to addition of TNF. After 12 h, cell viability was determined by exclusion of trypan blue dye. The percentage of surviving cells was calculated by taking the number of trypan blue-excluding cells after treatment, dividing this number by the number of untreated cells, and multiplying by 100. Pretreatment with antioxidant was done by culturing cells in 5 mM N-acetylcysteine (NAC) overnight prior to TNF induction. To determine the effects of oxidants on cell viability, hepatocytes were cultured with 0.25 mM tBHP for 8 h. Cell viability was determined by trypan blue dye exclusion, and apoptotic cells were visualized by fluorescent nuclear staining using 4′,6′-diamidino-2-phenylindole (DAPI).
Fluorescence measurement of ROS.
Fetal livers were dissected from embryos and disaggregated with 0.25% trypsin in 1× PBS to generate single-cell suspensions. Cells were either analyzed immediately or plated on collagen-coated dishes to remove nonadhering hematopoietic cells. To measure reactive oxygen species (ROS), cells were incubated with 10 μM DCFHDA (2′,7′-dichlorodihydrofluorescein diacetate; Molecular Probes) for 15 to 30 min in 1× PBS containing 2% fetal calf serum. Propidium iodide was added (1 μg/ml) prior to analysis. The oxidative conversion of DCFHDA to its fluorescent product, dichlorodihydrofluorescein diacetate (DCF), was assessed in live cells by flow cytometry.
GSH and GSSG assays.
An enzymatic method to measure glutathione (GSH) and glutathione disulfide (GSSG) was performed using a kit (Oxis International). Briefly, fetal livers were dissected from E13.5 embryos. Liver samples were weighed and homogenized in an ice-cold 5% metaphosphoric acid solution. For GSSG measurements, 1-methyl-2-vinyl-pyridium trifluoromethane sulfonate was added to scavenge intracellular GSH. Homogenates were then cleared by centrifugation at 4°C, and supernatants obtained were immediately used for assays according to the manufacturers' instructions. Detection of reaction products of DTNB [5,5′-dithiobis-(2-nitrobenzoic acid)] was monitored by a spectrophotometer with the wavelength set at 412 nm. Levels were calculated from standard curves of GSH and GSSG.
Caspase 3-like enzyme activity assay.
Cells were washed with cold 1× PBS and lysed in 500-μl volume of lysis buffer (10 mM Tris [pH 7.5], 10 mM NaH2PO4/NaHPO4 [pH 7.5], 130 mM NaCl, 1% Triton X-100, 10 mM NaPPi). Caspase 3-like activity was measured by mixing 50 μl of the lysate with 50 μl of reaction buffer (20 mM HEPES [pH 7.5], 10% glycerol, 2 mM DTT) containing 5 μg of Ac-DEVD-AMC (acetyl-Asp-Glu-Val-Asp-aminomethylcoumarin; BIOMOL). After 1 to 2 h of incubation at 37°C, products were determined using a fluorescence plate reader with an excitation wavelength of 360 nm and an emission wavelength of 460 nm for AMC substrates.
RESULTS
Nrf1-deficient ES cells do not contribute to adult hepatocytes in chimeric mice.
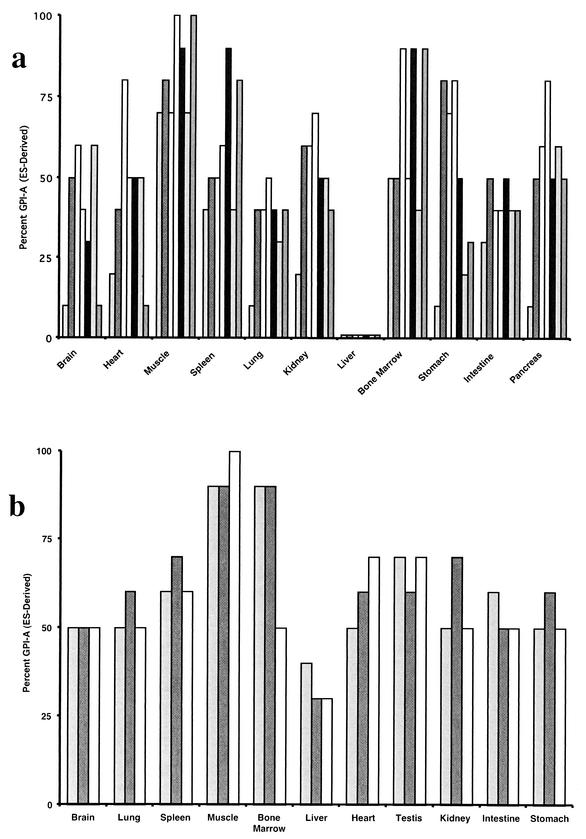
ES cells bearing mutations in both nrf1 alleles were generated as described previously by a two-step targeting approach (10). ES cells were injected into C57BL/6 blastocysts and transferred to pseudopregnant wild-type females. Several independently derived nrf1 homozygous mutant ES cells were used. Animals with a wide range of chimerism, as estimated from the extent of agouti coat color, were obtained. Chimeric mice were sacrificed and dissected at 8 to 16 weeks of age. The extent of contribution by ES cell clones to the formation of the different organs was determined by GPI isoenzyme analysis (29). ES cells express the GPI-A isoform, whereas C57BL/6 host embryos express the GPI-B isoform. A semiquantitative measure of chimerism in the various tissues was determined by cellulose acetate electrophoresis and staining of the two different isoenzymes. Analyses of multiple chimeric animals did not reveal a predominance of either wild-type or nrf1−/− ES cells in the various organs examined. In several examples, an equal ratio of wild-type to ES-specific bands was observed in various chimeric mice, and ES cell contribution was nearly 100% in several other tissue compartments (Fig. 1a). This demonstrates that Nrf1 function is not required for formation of these organs. In contrast, a comparison of the ratios of ES-derived GPI isoenzymes/blastocyst-derived GPI isoenzymes in all chimeric animals revealed selection against a contribution of nrf1−/− cells to the liver compartment. In liver samples, the GPI-A isoform was present in trace amounts in all animals (Fig. 1a). GPI analysis of chimeric animals generated using parental nrf1+/− ES cell clones that had been subjected to a similar selection procedure in culture did not eliminate ES cell contribution to the liver (Fig. 1b). Therefore, the lack of ES cell contribution to the liver is specific to the loss of Nrf1 function. These data demonstrate that Nrf1 function is not necessary for development of most organs except the liver.
FIG. 1.
Nrf1-deficient ES cells do not contribute to the livers of adult chimeric mice. The contributions of ES cells to tissues of nrf1−/− and nrf1+/− adult chimeric mice were estimated by GPI isoenzyme assay. The percentages of the GPI-A isoform (C57BL/6 host blastocyst-specific isoform) in tissues of chimeric mice are shown. Each bar is the value for one mouse. Seven nrf1−/− adult chimeric mice (a) and three nrf1+/− adult chimeric mice (b) were analyzed.
Nrf1 function is not required for early fetal liver development.
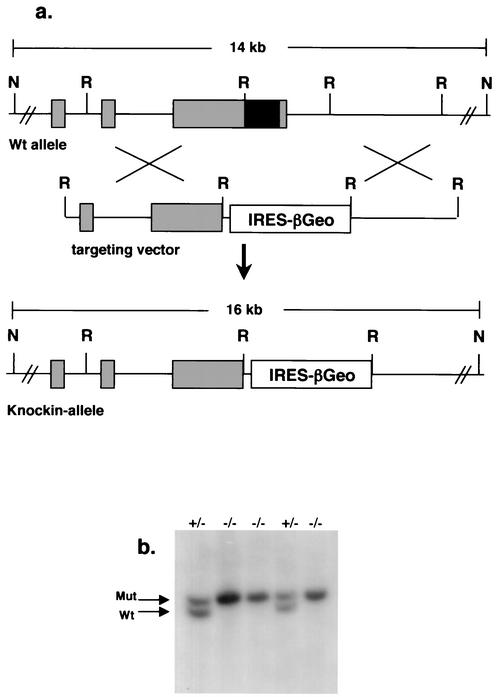
The cell types affected could not be discerned from GPI studies, as the liver is made up of multiple cell types including hepatocytes, endothelial cells, biliary epithelial cells, Kupffer cells, and hematopoietic cells during fetal development. To determine the requirement for Nrf1 at the cellular level, we examined chimeric animals derived from ES cells bearing a “knock-in” mutant allele of nrf1. The targeting vector used to disrupt the nrf1 gene is shown in Fig. 2. Successful targeting in ES cells using this vector results in replacement of the part of the nrf1 gene encoding the DNA-binding and dimerization domains with an IRES-βGeo cassette. Expression of the βGeo fusion protein simultaneously confers G418 resistance and lacZ activity upon cells (17). The IRES permits translation of two open reading frames from the same transcript (45), allowing cells expressing Nrf1 to be identified readily by expression of the linked βGeo gene. Therefore, in addition to knocking out nrf1 gene function, we also tagged it for expression studies. This allele is referred to herein as the nrf1lacZ allele (Fig. 2). JM-1 ES cells were transfected as described previously. Southern blotting identified clones containing the nrf1lacZ allele. The embryonic lethal phenotype of germ line-derived mice homozygous for the nrf1lacZ allele was similar to the phenotype of mice with our previous knockout allele (data not shown), thus confirming the predicted null function resulting from the nrf1lacZ allele. ES cells that are homozygous for the nrf1lacZ allele were then isolated by selection using high concentrations of G418.
FIG. 2.
Targeting strategy to generate the nrf1lacZ allele. (a) A partial genomic structure of the nrf1 gene and restriction map is shown at the top of the figure. The targeting vector and the targeted allele are shown at the bottom. Exons of the nrf1 gene (shaded boxes) and the DNA-binding and leucine zipper domain that was replaced with the IRES-βGeo cassette (black box) are indicated. A probe external to the targeting vector was used for Southern blotting, and the sizes of NsiI (N) and EcoRI (R) fragments of the targeted and wild-type (Wt) loci are indicated. (b) Southern blot analysis of genomic DNAs from ES cell clones. The nrf1 genotypes of ES cell clones are indicated above the lanes. The positions of the 16-kb mutant (Mut) and 14-kb wild-type (Wt) bands are indicated by arrows.
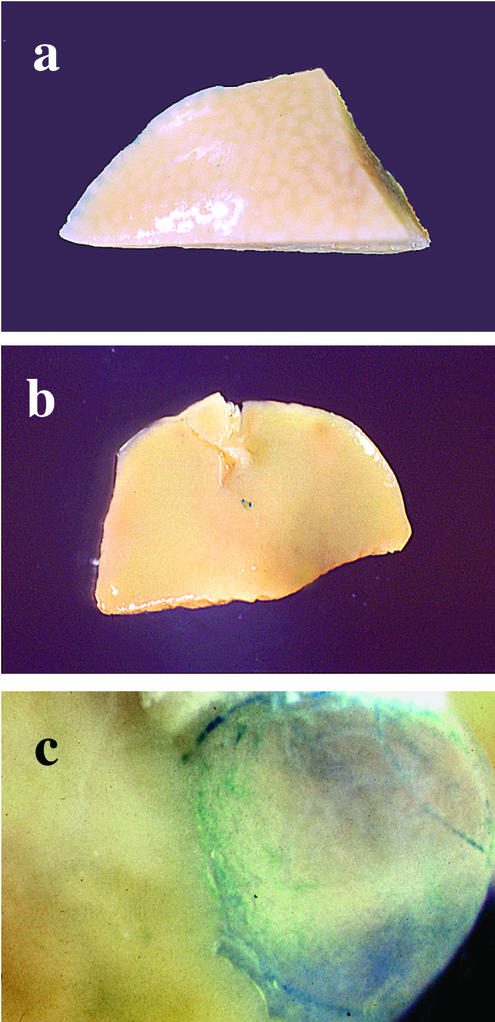
Chimeras were generated using heterozygous and homozygous nrf1lacZ ES cells, and X-Gal staining was used to determine the contributions of ES cells to various tissues. In adult chimeric mice generated from homozygous nrf1lacZ ES cells, we were not able to detect X-Gal staining in hepatocytes (Fig. 3a and b). These results were confirmed in adult chimeras derived from another homozygous knock-in ES clone (data not shown). In contrast, substantial X-Gal staining was detected in the cells lining the gall bladder, as well as cells in sinusoids of adult livers (Fig. 3c and data not shown). In adult chimeras derived from heterozygous nrf1lacZ ES cells, lacZ-positive cells were readily detected throughout the liver parenchyma (data not shown). Therefore, the defect appears to be restricted to hepatocytes.
FIG. 3.
X-Gal staining shows the absence of nrf1lacZ/lacZ ES cells in the hepatic parenchyma of adult chimeric mice. (a and b) Whole-mount X-Gal staining of livers from two representative adult mice, both of which have extensive chimerism determined from coat color and X-Gal staining of other tissues. Note the absence of blue staining in the hepatic parenchyma. (c) Gall bladder showing intense blue staining.
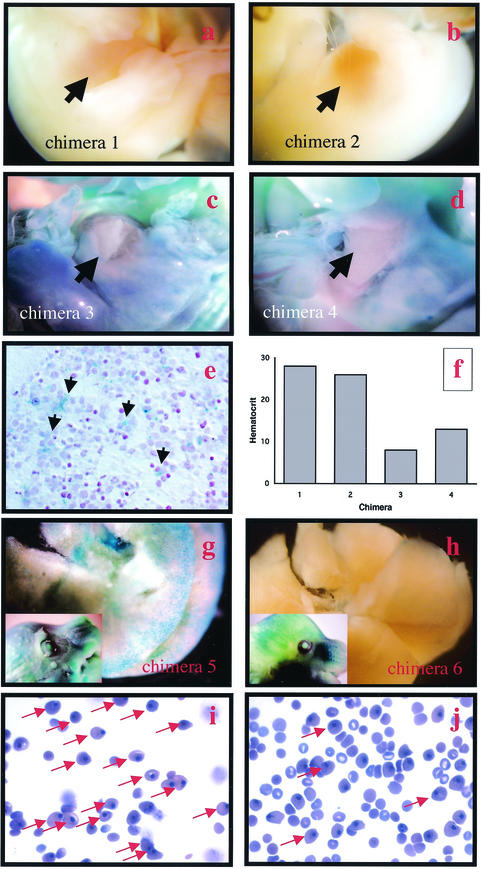
The extent of ES cell contribution to the liver during fetal development was also analyzed by examining E14.5 chimeric embryos derived from homozygous nrf1lacZ ES cells. The whole embryo and sections stained with X-Gal revealed extensive contribution of null ES cells in several chimeric embryos, including their livers (Fig. 4c, d, and e). Therefore, Nrf1-deficient ES cells are able to compete efficiently with wild-type cells in forming the fetal liver in chimeric animals. The absence of Nrf1-deficient hepatocytes in adult livers suggests that either they have a growth disadvantage or they do not survive and are replaced by wild-type cells as development proceeds. Another aspect that is noteworthy was that anemia was observed in several of the chimeric embryos that exhibited prominent X-Gal staining in their livers (Fig. 4). This anemia occurred prior to detection of cell death in the liver parenchyma (see below). In contrast, chimeric embryos that exhibited little or no X-Gal staining in the liver compartment were normal (Fig. 4, compare panels a, b, f, h, and j to panels c, d, f, g, and i). It appears then that anemia was present when the livers of chimeric embryos were largely derived from mutant ES cells. These findings strengthen our previous conclusion that the anemia observed in mutant fetuses is secondary to failure in the liver microenvironment to sustain hematopoiesis. However, because anemia occurred prior to the onset of significant liver degeneration, a specific defect in supporting erythropoiesis versus a generalized defect cannot be ruled out.
FIG.4.
Chimeric embryos with livers with large contributions of ES cells are anemic. (a to d) Four E14.5 nrf1lacZ/lacZ chimeric littermates whole mount stained with X-Gal. (e) Cross section of an E14.5 chimeric fetal liver stained with X-Gal showing intense blue staining of hepatocytes. (f) Hematocrits of the chimeric mice shown in panels a to d. Note that chimeras 3 and 4 (c and d), which showed blue staining in their livers (arrows), have low hematocrits. (g and h) E16.5 nrf1lacZ/lacZ chimeric littermates whole mount stained with X-Gal. (i and j) Blood smears of E16.5 nrf1lacZ/lacZ chimeric littermates. (i) Note that the blood of chimera 5 (g), which showed intense blue staining in the liver, contains large number of yolk sac-derived nucleated red cells (arrows) and few nonnucleated red cells of fetal liver origin. (j) Blood of chimera 6 (h), which showed intense X-Gal staining in the embryo (inset) but not the liver, contains both nucleated erythrocytes and nonnucleated red cells.
Apoptosis and degeneration of chimeric mouse livers derived from nrf1−/− ES cells.
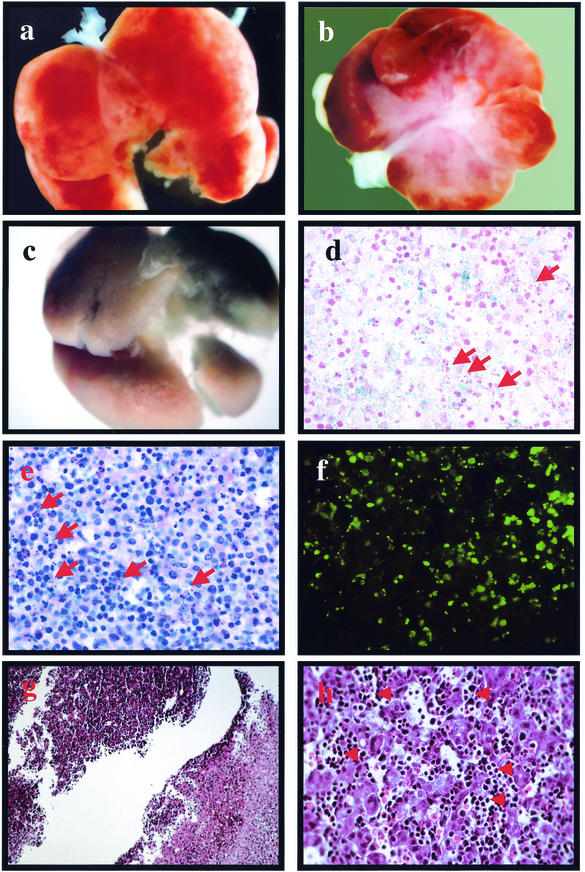
The findings described above are consistent with the idea that Nrf1 function is not required for formation of the liver, but its function is crucial at later stages of liver development. Our previous analysis in germ line mutants revealed no apparent differences in development or morphology between wild-type and nrf1−/− fetuses until midgestation. At E14.5 to E15.5, almost all nrf1−/− fetuses that were viable have disproportionately smaller livers, and most mutants do not live beyond this stage of development. In contrast to our findings in germ line mutants, the livers of chimeric fetuses harvested between E13.5 and E16.5 appeared normal. At E17.5 to E18.5, however, the livers of several fetuses looked abnormal; they had patches of pale tissues, and they were very fragile (Fig. 5a and b). These abnormal livers were extensively chimeric as determined by X-Gal staining (Fig. 5c and d). Histologic exams showed large areas of cells with pyknotic and fragmented nuclei indicating apoptosis (Fig. 5e and h), which was confirmed by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) (Fig. 5f). Large areas of necrosis were also seen bordering areas of apparently healthy tissues (Fig. 5g). Necrosis appeared to be confined to hepatocytes, as many nucleated erythrocytes were present (Fig. 5h). Therefore, erythroid cells appear to be unaffected, which is consistent with our previous analysis that failed to detect abnormalities in cultures of erythroid progenitors from the livers of nrf1−/− embryos (10). These data provide evidence that Nrf1 is not required for liver formation. Instead, Nrf1 serves an important role in the survival of liver cells during late stages of embryonic development, and the increased rate of apoptosis observed could explain the absence of nrf1−/− hepatocytes in adult chimeric animals.
FIG.5.
Late-gestation fetal livers of nrf1lacZ/lacZ chimeric mice show increased apoptosis and necrosis. (a and b) Livers of E18.5 chimeric fetuses showing patches of necrotic tissue. (c) Liver shown in panel b that was whole mount stained with X-Gal. (d) Cross section of E18.5 liver showing X-Gal staining in hepatocytes. (e) Cross section of E18.5 liver stained with hematoxylin and eosin. Note the presence of pkynotic and fragmented nuclei (red arrows in panels d and e). (f) TUNEL showing apoptotic cells. (g) Section of an E18.5 chimeric liver showing the border between healthy (upper left) and necrotic (lower right) tissues. (h) Higher-magnification photomicrograph of an E18.5 fetal liver section showing clusters of nucleated erythroid cells (red arrows) scattered between necrotic tissue.
Increased oxidative stress in fetal livers derived from nrf1−/− embryos.
To determine the molecular basis of increased apoptosis, we examined the expression of Bcl-2 family members. Immunoblot analysis did not reveal significant differences in BclxL, Bclw, and Bax levels in fetal liver samples from E13 and E14 wild-type and nrf1−/− embryos (data not shown). Therefore, increased cell death is not caused by impaired expression of antiapoptotic Bcl-2 proteins or induction of Bax. It has been suggested that oxidative stress induces apoptosis (26, 62). CNC-bZIP factors, particularly Nrf2, have been implicated in antioxidant defense, and loss of redox balance could cause increased apoptosis in mutant liver cells. Therefore, we were interested in determining whether ROS levels were increased in Nrf1 mutant livers.
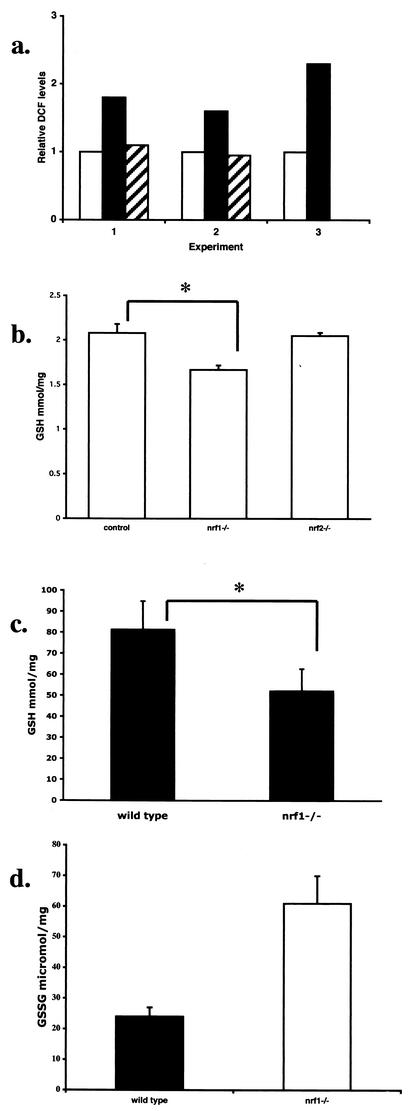
We assessed intracellular accumulation of reactive oxygen intermediates using DCF. DCF becomes fluorescent upon oxidation, which allows quantitation of intracellular ROS by flow cytometry. Pooled samples of fetal liver cells from E13.5 embryos were analyzed. The level of fluorescent DCF was twofold higher in nrf1−/− fetal liver samples than in control samples (Fig. 6a). Fetal liver samples from nrf2−/− mice were also analyzed, and they did not show an elevation of ROS, indicating that the increase in ROS is specific to the nrf1 mutation. As we have previously shown that GSH levels in fibroblasts derived from Nrf1 mutants were reduced, we were interested in determining whether GSH levels were also affected in mutant fetal livers. GSH levels in nrf1−/− livers were slightly lower (by 20%, which was statistically significant [P < 0.05]) than those in the control livers (Fig. 6b). Although Nrf2 has also been shown to regulate glutathione expression (64), it is interesting that GSH levels in nrf2−/− fetal livers were not altered from the levels in wild-type livers (Fig. 6b). Therefore, the decrease in GSH levels in fetal liver correlated with a deficiency in Nrf1, but not Nrf2.
FIG.6.
Nrf1-deficient fetal livers exhibit increased oxidative stress. (a) ROS levels of E13.5 liver samples from wild-type (white bars), nrf1−/− (black bars), and nrf2−/− (hatched bars) mice as determined by flow cytometric analysis of intracellular DCF fluorescence. Results from three experiments are shown. Levels are expressed relative to the signal for the wild-type sample in each experiment, which was arbitrarily assigned a value of one. In experiment 3, primary hepatocytes were used; fetal liver cells were cultured overnight on collagen-coated plates to remove nonadhering hematopoietic cells. (b) GSH levels in the livers of wild-type and nrf1+/− (control) (n = 8), nrf1−/− (n = 4), and nrf2−/− (n = 4) mice. (c) GSH levels of primary hepatocytes from E13.5 control (n = 5) and nrf1−/− (n = 3) fetuses. (d) GSSG levels of wild-type and nrf1−/− livers. Mean values ± standard deviations (error bars) are shown. Values that were statistically significantly different (P < 0.05 by Student's t test) are indicated by an asterisk.
To determine the cell type that was affected by oxidative stress, analysis was also performed on fetal liver cells cultured briefly in collagen-coated dishes to remove nonadhering hematopoietic cells and enrich for hepatocytes. The reduction in GSH was slightly greater (40% versus 20%) in primary cultures of fetal hepatocytes than in whole fetal liver samples (Fig. 6, compare panels c and b). However, GSH levels in hematopoietic cells (as represented by the nonadhering fraction) were not different in control and mutant mice (data not shown). Therefore, GSH levels were primarily affected in hepatocytes. Similarly, the difference in the DCF levels of control and nrf1−/− hepatocytes was also enhanced when blood cells were removed from the analysis (Fig. 6a, experiment 3). We also compared GSSG, which is generated as GSH is oxidized during the reduction of peroxides catalyzed by antioxidant enzymes (24), in fetal livers as a measure of oxidative stress. GSSG levels in mutant livers were 2.5-fold higher than in wild-type liver samples (Fig. 6d). Taken together, the accumulation of GSSG and increased fluorescent DCFH levels indicate that oxidative stress is a feature of Nrf1 mutant livers.
Impaired antioxidant gene expression in nrf1−/− fetal liver.
We screened the expression of several antioxidant genes to determine the basis of oxidative stress in Nrf1 mutant liver cells. We chose E13.5 and E14.5 embryos for our analysis to minimize changes that might be related to a degenerating liver at late stages of gestation. RNA was extracted from individual or pooled samples of fetal liver and analyzed by quantitative RT-PCR. Because of the limited sample size, we used a real-time PCR technique to compare the transcript levels in control and mutant liver samples. Consistent with the reduced GSH levels described above, the steady-state levels of transcripts encoding the rate-limiting enzyme, glutamate-cysteine ligase, involved in GSH biosynthesis were significantly reduced in Nrf1 mutant liver samples. The levels of transcripts of Gclc and Gclm were reduced by at least threefold in mutant livers from the levels in wild-type livers (Table 1), and Nrf1-mediated transactivation of Gclc and Gclm genes has been previously demonstrated in cell culture systems. Therefore, Gclc and Gclm are likely to represent direct targets of Nrf1 in liver cells as well (37, 49). Transcripts of glutathione peroxidase 1 (Gpx-1) and heme oxygenase 1, as well as MT-1 and MT-2, were also reduced (Table 1). Apart from Gpx-1, the regulatory sequences of these genes are known to contain antioxidant response elements (ARE). However, transcripts of other genes that also have ARE-containing regulatory sequences, such as ferritin H, ferritin L, and catalase, were expressed equally in wild-type and mutant liver and cell samples (data not shown). Therefore, it appears that not all ARE-containing antioxidant genes are down regulated. RT-PCR quantitation also did not reveal alteration in the mRNA levels of alpha-fetoprotein, C-reactive protein, α1 antitrypsin, and apoprotein A1 (or other antioxidants, such as glutaredoxin, superoxide dismutase 1 and superoxide dismutase 2), indicating that the defect is a specific, rather than global, reduction in expression of genes in nrf1−/− livers. We also examined Gclc, Gclm, and MT-1 expression in hematopoietic progenitors derived from Nrf1 mutant livers. No significant differences in expression in mutant and control livers were seen (Table 1). Therefore, we infer that the defect is specific to hepatocytes. Together, the combined decrease in antioxidant provides a potential basis for the increased oxidative stress that is observed in mutant livers.
TABLE 1.
Changes in gene expression identified by quantitative RT-PCR in nrf1−/− fetal livers and erythroid progenitorsa
| Gene | Fold changeb (mean ± SD)
|
|
|---|---|---|
| Fetal liver | CFU-E from ES cells | |
| Gclc | −2.9 ± 1.1 | 0.3 ± 0.4 |
| Gclm | −3.7 ± 1.2 | −0.3 ± 0.5 |
| Gpx-1 | −5.1 ± 1.7 | ND |
| MT-1 | −4.0 ± 2.0 | 0.3 ± 0.3 |
| MT-2 | −3.0 ± 2.0 | ND |
| HO-1c | −2.1 ± 1.3 | NT |
The levels of expression of genes in each sample were quantitated relative to endogenous GAPDH levels as an internal reference. Expression levels relative to GAPDH levels were calculated as follows: 2(Ct of the test gene − Ct of the GAPDH gene).
The change in expression of the various genes was determined from the difference between the average expression levels of wild-type and Nrf1 mutant samples. Decreases are indicated by minus signs before the values. For fetal liver comparisons, the livers of five wild-type and six nrf1−/− mice were used. For comparisons of CFU of erythroid cells (CFU-E) from ES cells, cells from two wild-type and three nrf1−/− mice were used. ND, not determined; NT, not detected.
HO-1, heme oxygenase 1.
Primary nrf1−/− fetal hepatocytes are sensitive to tBHP-induced apoptosis.
We wished to determine whether hepatocytes deficient in Nrf1 function are sensitive to oxidative stress. To study this, E13 and E14 fetal liver cells enriched for hepatocytes were tested for sensitivity to oxidant treatment in culture. Primary hepatocytes treated with tBHP for 8 h were harvested, stained with trypan blue dye, and counted. No difference in viability was detected between untreated control and mutant samples. However, tBHP treatment caused a significant decrease in the viability of primary nrf1−/− hepatocytes compared to that of control hepatocytes (Fig. 7a). The proportion of viable cells was 83% in control samples compared to 51% in nrf1−/− samples. In nrf1−/− hepatocyte cultures, numerous cells showed morphological features consistent with apoptosis, including condensed and fragmented nuclei (Fig. 7, compare panels b and c). These results indicate that Nrf1-deficient hepatocytes are sensitized to oxidative stress-induced apoptosis.
FIG. 7.
Nrf1-deficient primary hepatocytes are sensitive to tBHP-induced cell death. (a) Percent survival of control (white bars) and nrf1−/− (shaded bars) hepatocytes that were treated with 0.25 mM tBHP (+ TBHP) or not treated with tBHP (− TBHP). (b and c) Detection of apoptosis (arrows) by DAPI staining of hepatocytes treated with tBHP.
Loss of Nrf1 sensitizes cells to TNF-α-induced cell death.
To begin to determine the mechanistic basis of what might be inducing hepatic cell death in nrf1−/− chimeric mice during development, we examined whether Nrf1-deficient cells are sensitized to TNF-induced apoptosis. TNF is a cytokine with a wide variety of functions in many cell types. It mediates acute-phase response in the liver and can initiate cell proliferation and cause cell death. Hepatocytes are normally resistant to the lethal effects of TNF, but exposure to toxins or GSH depletion and oxidative stress have been shown to sensitize hepatocytes to TNF-induced apoptosis (34). TNF treatment of nrf1−/− fibroblasts resulted in a dose-dependent decrease in cell viability as determined by trypan blue dye exclusion (Fig. 8a). Pretreatment of cells with cycloheximide further sensitized nrf1−/− cells to the cytotoxic effect of TNF (Fig. 8b). A 50% decrease in viability was observed at a TNF concentration of 2 ng/ml, which had no effect on wild-type cells. The decrease in viability was associated with increased caspase 3 activity, suggesting an apoptotic mechanism (Fig. 8c). To determine whether oxidative stress was involved in the sensitization to TNF-induced toxicity, nrf1−/− fibroblasts were treated with NAC as an antioxidant prior to TNF induction. Pretreatment with NAC rescued nrf1−/− cells from TNF-induced toxicity, suggesting that sensitization in mutant cells is linked to oxidative stress (Fig. 8b). Together, the data indicate that cells deficient in Nrf1 are sensitized to TNF-induced apoptosis and suggest that Nrf1 is required to protect liver cells from TNF toxicity during development.
FIG. 8.
Nrf1-deficient cells are sensitized to TNF-induced cell death. (a) Percent survival of wild-type and nrf1−/− fibroblasts treated with various concentrations of TNF. (b) Percent survival of wild-type and nrf1−/− fibroblasts treated with cycloheximide and various concentrations of TNF. (c) Caspase 3-like enzyme activity of wild-type and nrf1−/− fibroblasts after treatment with cycloheximide and TNF. Changes in caspase 3-like activity are expressed relative to the activity in untreated cells. Wild-type cells (black bars), nrf1−/− cells (gray bars), and NAC-pretreated nrf1−/− cells (hatched, light gray bars in panel b) were studied. Mean values ± standard deviations (error bars) are shown.
DISCUSSION
We previously found that loss of Nrf1 function in mice leads to anemia and they die progressively from E13.5. While the analysis of germ line nrf1−/− embryos suggested an abnormal fetal liver environment as a cause of anemia, the cell types primarily affected by loss of Nrf1 function during development have not been established. Here, we analyzed chimeric mice to examine the requirements of Nrf1 in different cell lineages during development. The results demonstrate that Nrf1-deficient cells participate in the normal development of almost all the tissues examined in chimeric mice. This implies that the loss of Nrf1 function in these compartments imposes no significant consequences to cell differentiation and function. Alternatively, it is possible that the presence of surrounding healthy cells is sufficient to rescue defects in mutant cells or that the loss of Nrf1 function is compensated for by other transcription factors such as Nrf2. ES cells deficient in Nrf1 colonized the livers of chimeric fetuses efficiently. Therefore, Nrf1 is not needed for liver formation during early development. However, we found that nrf1 homozygous mutant ES cells do not contribute to adult livers. While we previously found that the livers in nrf1−/− embryos were hypoplastic, we did not see this feature in fetuses with highly chimeric livers in this study. We also did not see this feature in chimeric fetuses that were anemic, arguing against a decrease in hematopoiesis as a basis for small livers that are typical of germ line mutants. Instead, our findings indicate that liver cells do not survive beyond the later stages of development in the absence of Nrf1 function. The apoptosis observed in chimeric livers, which increased progressively in older fetuses, suggests that Nrf1 plays a crucial role in the maintenance of liver cells and/or liver development. Therefore, nrf1−/− hepatocytes are lost continuously as the liver develops.
The precise role of Nrf1 in promoting survival of liver cells is not known. Members of the CNC-bZIP transcription factor family have been linked to antioxidant gene expression. In particular, Nrf2 has been shown to play a major role in this function by regulating a battery of genes encoding antioxidant enzymes, as well as xenobiotic-metabolizing enzymes. Although Nrf1 has also been implicated in antioxidant defense, its role in this function during development and its contribution (compared to that of Nrf2's contribution) are not clear. One possible explanation for the increased cell death observed in the livers of chimeric mice is that cell death is caused by the disruption of redox balance resulting from impaired antioxidant gene expression. Primary mutant hepatocytes were more sensitive to oxidant-induced cell death, and the results showed that GSH levels are reduced in mutant fetal liver cells that correlated with decreased expression of transcript levels encoding Gclc and Gclm. Both Gclc and Gclm are expressed abundantly in the liver during development (13), and Nrf1 and Nrf2 have both been shown to directly regulate the promoter function of these genes in vitro (37, 49, 64). GSH is important for establishing and maintaining the reducing environment in cells, and it is crucial for normal liver development (4, 54, 59). In addition, GSH may also regulate apoptosis independent of its role as an antioxidant (18, 19). Pharmacologic inhibition of GSH synthesis leads to growth retardation and malformation of rat embryos, and mice deficient in GSH exhibit increased apoptosis and die at E7.5 (28, 42, 61). Our results also showed that expression of several important cellular stress response genes was reduced in Nrf1 mutant livers than in wild-type livers. The expression of heme oxygenase 1, as well as MT1 and MT2, is modulated primarily at the transcriptional level by a variety of external stimuli. Consistent with a potential role of Nrf1 in their expression, ARE are present in their regulatory sequences (1, 2). Expression of Gpx-1, a selenium-dependent enzyme that catalyzes the breakdown of hydrogen peroxide, was also decreased in mutant livers. Gpx-1 has been shown to play a crucial role in hydrogen peroxide detoxification in the liver (15). Mice deficient in MTs, Gpx-1, and heme oxygenase 1 are developmentally normal (15, 41, 53). Therefore, deficiency in these individual genes is not likely to result in the defects observed in Nrf1-deficient livers. We speculate that additional genes involved in oxidative stress response are also down regulated in mutant livers, and our limited search has identified only a subset of these Nrf1 targets. It is likely that the reduction of these antioxidant enzymes, in combination with the reduction in GSH levels, result in oxidative stress in nrf1−/− livers. Given the rapid rate of hepatocyte growth during the perinatal period, an excess of ROS generated from increased energy need would not be unexpected. The ability to maintain redox balance is thought to be crucial during liver growth (16). In this regard, expression of antioxidants is up regulated during the fetal-neonatal transition period suggesting that protection from oxidative stress becomes increasingly important as development proceeds (12, 48, 55).
It is interesting that our results are similar to features of mice with deletions of genes that are in some way related to processing of cellular oxidants. For example, mice deficient in c-Jun also have small livers, and most of these mice die between E12.5 and E15.5 (27). ES cells deficient in c-Jun do not contribute to adult livers, and chimeric fetuses have increased apoptosis in their livers (14). Therefore, c-Jun also plays a critical role in the survival of liver cells during development. The similarities in the liver phenotype between Nrf1 and c-Jun mutants suggest that Nrf1 and c-Jun may exert similar functions in the liver during development. Fibroblasts deficient in c-Jun have been reported to have decreased levels of GSH as well, and the regulatory sequences of Gclc and Gclm genes have been shown to contain both ARE and Ap1 binding sites (46, 47, 58). Unlike c-Jun−/− fibroblasts, however, Nrf1-deficient fibroblasts do not exhibit reduced proliferation rates (unpublished data). As nrf1−/− livers did not show altered levels of c-Jun mRNA (data not shown), it is unlikely that Nrf1 works upstream of c-Jun. Moreover, lethality in c-Jun−/− animals occurred earlier in gestation. It would be interesting to know whether Nrf1 and c-Jun exert their function as bZIP partners in liver cells. The phenotype of Nrf1-deficient embryos is also similar to that of MTF-1-deficient mice. MTF-1 is a zinc finger protein that regulates transcription of genes in response to heavy metal stress, and MTF-1 deficiency causes liver degeneration and embryonic lethality at E14 (25). Gclc, MT-1, and MT-2 have been shown to be under MTF-1 control as well, but increased oxidative stress in the livers of MTF-1 knockout mice has not been reported. It is also notable that anemia was not observed in the degenerating livers of MTF-1 knockout mice prior to their death, arguing that abnormal erythropoiesis in nrf1−/− embryos may be due to a specific defect in hepatic parenchymal cells as opposed to a generalized failure of the liver microenvironment. However, a mechanism for this observation remains to be determined.
TNF-α has been shown to mediate hepatocyte killing after exposure of cells to toxins, virus, and injury from various insults. Therefore, TNF-α has a prominent role in the pathophysiology of liver disorders (34). A role for TNF-α-mediated killing of hepatocytes during development has also been demonstrated through the finding that apoptosis and degeneration of livers in mice deficient in the NF-κB signaling pathway are rescued by inactivation of the TNF receptor gene (40, 56). Sensitization is not limited to hepatocytes, as fibroblasts derived from mice lacking the RelA component of NF-κB are sensitive to TNF-α killing as well (5). Our results show that cells deficient in Nrf1 function are also sensitized to TNF-α-induced apoptosis. We propose that cell death observed in the livers of Nrf1 chimeric animals is related to the effects of TNF during liver development. The mechanism of TNF toxicity is complex and may include alterations in the cellular redox potential (22, 39, 57). In liver regeneration models, the period of rapid growth after a partial hepatectomy is associated with increased production of oxidants that are linked to TNF-α (38). The availability of GSH and other antioxidants has been shown to influence whether TNF-α induces growth or apoptosis in hepatocytes (52). ROS production induced by TNF presumably occurs in the mitochondria and is scavenged by GSH (23). Therefore, in this scenario, the absence of Nrf1 does not directly lead to liver cell death, but its deficiency leads to sensitivity to TNF-mediated apoptotic signals. Generating mice deficient in both Nrf1 and TNF receptor is one way this possibility could be tested.
Apoptosis is a highly regulated process, and this form of cell death serves a critical function during development. Many important genes in this pathway have been identified. The possibility that the increased cell death also occurs as a result of induction of a pathway independent of oxidative stress cannot be excluded. For example, Nrf1 may have an antiapoptotic role in mature hepatocytes by controlling the transcription of specific survival genes. Such a role for NF-κB is supported by the fact that mice with disrupted RelA and IKK2 genes have hypoplastic livers that are characterized by elevated apoptosis and by the fact that antiapoptotic genes including TRAF and IAP are regulated by NF-κB (6, 40, 63). Although our preliminary results do not indicate altered levels of genes (such as BclxL and IAP genes) in nrf1−/− fetal livers (data not shown), a potential role for Nrf1 in other antiapoptotic genes will need further exploration. Alternatively, Nrf1 may negatively regulate proapoptotic genes. Nrf1 is likely to be involved in multiple pathways in the cell, and other abnormalities beside the liver defect may also contribute to the lethal phenotype. Irrespective of the actual explanation for the observed lethality in germ line Nrf1 knockout mice, the observations here indicate that Nrf1 is essential for the survival of liver cells. Understanding the precise role of Nrf1 in determining death versus survival of hepatocytes beyond development would be of interest, as apoptosis is an important component in the pathogenesis of liver diseases. This result also raises an interesting question as to why the defect is limited to hepatocytes, given that Nrf1 is widely expressed. The results of our study suggest that Nrf1 plays an important role in maintaining redox balance in liver cells. The finding that defective GSH expression correlated with loss of Nrf1, but not Nrf2, suggests that Nrf1 may be more important than Nrf2 in protecting fetal liver cells from oxidative stress. Therefore, another important aspect that warrants further investigation is to delineate the mechanistic basis of the apparently separate roles of Nrf1 and Nrf2 in mediating transcription of antioxidant genes in liver cells. As oxidative stress is thought to play an important role in promoting cell death in response to various stimuli, these findings draw attention to the importance of the Nrf1-antioxidant pathway in various pathological conditions.
Acknowledgments
We thank Paul Dazin (HHMI-Flow Core Facility, UCSF) for assistance with fluorescence-activated cell sorting analysis.
This work was supported in part by grants from the NIH (DK02603 and DK063757) and by the American Society of Hematology Junior Faculty award to J.Y.C.
REFERENCES
- 1.Alam, J., D. Stewart, C. Touchard, S. Boinapally, A. M. Choi, and J. L. Cook. 1999. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 274:26071-26078. [DOI] [PubMed] [Google Scholar]
- 2.Andrews, G. K. 2000. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 59:95-104. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, N. C., B. H. Erdjument, M. B. Davidson, P. Tempst, and S. H. Orkin. 1993. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature 362:722-728. [DOI] [PubMed] [Google Scholar]
- 4.Arrigo, A. P. 1999. Gene expression and the thiol redox state. Free Radic. Biol. Med. 27:936-944. [DOI] [PubMed] [Google Scholar]
- 5.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 6.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. [DOI] [PubMed] [Google Scholar]
- 7.Blank, V., and N. C. Andrews. 1997. The Maf transcription factors: regulators of differentiation. Trends Biochem. Sci. 22:437-441. [DOI] [PubMed] [Google Scholar]
- 8.Caterina, J. J., D. Donze, C. W. Sun, D. J. Ciavatta, and T. M. Townes. 1994. Cloning and functional characterization of LCR-F1: a bZIP transcription factor that activates erythroid-specific, human globin gene expression. Nucleic Acids Res. 22:2383-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan, J. Y., X. L. Han, and Y. W. Kan. 1993. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc. Natl. Acad. Sci. USA 90:11371-11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, J. Y., M. Kwong, R. H. Hua, J. Chang, T. S. B. Yen, and Y. W. Kan. 1998. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 17:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan, K., R. Lu, J. C. Chang, and Y. W. Kan. 1996. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. USA 93:13943-13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Haan, J. B., M. J. Tymms, F. Cristiano, and I. Kola. 1994. Expression of copper/zinc superoxide dismutase and glutathione peroxidase in organs of developing mouse embryos, fetuses, and neonates. Pediatr. Res. 35:188-196. [DOI] [PubMed] [Google Scholar]
- 13.Diaz, D., C. M. Krejsa, and T. J. Kavanagh. 2002. Expression of glutamate-cysteine ligase during mouse development. Mol. Reprod. Dev. 62:83-91. [DOI] [PubMed] [Google Scholar]
- 14.Eferl, R., M. Sibilia, F. Hilberg, A. Fuchsbichler, I. Kufferath, B. Guertl, R. Zenz, E. F. Wagner, and K. Zatloukal. 1999. Functions of c-Jun in liver and heart development. J. Cell Biol. 145:1049-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito, L. A., J. E. Kokoszka, K. G. Waymire, B. Cottrell, G. R. MacGregor, and D. C. Wallace. 2000. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic. Biol. Med. 28:754-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fausto, N. 2000. Liver regeneration. J. Hepatol. 32:19-31. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich, G., and P. Soriano. 1991. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5:1513-1523. [DOI] [PubMed] [Google Scholar]
- 18.Ghibelli, L., S. Coppola, G. Rotilio, E. Lafavia, V. Maresca, and M. R. Ciriolo. 1995. Non-oxidative loss of glutathione in apoptosis via GSH extrusion. Biochem. Biophys. Res. Commun. 216:313-320. [DOI] [PubMed] [Google Scholar]
- 19.Ghibelli, L., C. Fanelli, G. Rotilio, E. Lafavia, S. Coppola, C. Colussi, P. Civitareale, and M. R. Ciriolo. 1998. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J. 12:479-486. [DOI] [PubMed] [Google Scholar]
- 20.Ginzinger, D. G. 2002. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp. Hematol. 30:503-512. [DOI] [PubMed] [Google Scholar]
- 21.Ginzinger, D. G., T. E. Godfrey, J. Nigro, D. H. Moore II, S. Suzuki, M. G. Pallavicini, J. W. Gray, and R. H. Jensen. 2000. Measurement of DNA copy number at microsatellite loci using quantitative PCR analysis. Cancer Res. 60:5405-5409. [PubMed] [Google Scholar]
- 22.Goossens, V., K. De Vos, D. Vercammen, M. Steemans, K. Vancompernolle, W. Fiers, P. Vandenabeele, and J. Grooten. 1999. Redox regulation of TNF signaling. Biofactors 10:145-156. [DOI] [PubMed] [Google Scholar]
- 23.Goossens, V., J. Grooten, K. De Vos, and W. Fiers. 1995. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc. Natl. Acad. Sci. USA 92:8115-8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith, O. W. 1997. Glutathione, 2nd ed., vol. 4. Academic Press, San Diego, Calif.
- 25.Günes, C., R. Heuchel, O. Georgiev, K. H. Müller, P. Lichtlen, H. Blüthmann, S. Marino, A. Aguzzi, and W. Schaffner. 1998. Embryonic lethality and liver degeneration in mice lacking the metal-responsive transcriptional activator MTF-1. EMBO J. 17:2846-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hampton, M. B., and S. Orrenius. 1998. Redox regulation of apoptotic cell death. Biofactors 8:1-5. [DOI] [PubMed] [Google Scholar]
- 27.Hilberg, F., A. Aguzzi, N. Howells, and E. F. Wagner. 1993. c-Jun is essential for normal mouse development and hepatogenesis. Nature 365:179-181. [DOI] [PubMed] [Google Scholar]
- 28.Hiranruengchok, R., and C. Harris. 1995. Formation of protein-glutathione mixed disulfides in the developing rat conceptus following diamide treatment in vitro. Teratology 52:196-204. [DOI] [PubMed] [Google Scholar]
- 29.Hogan, B., R. Beddington, F. Constantini, and E. Lacy. 1994. Manipulating the mouse embryo. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 30.Igarashi, K., K. Itoh, H. Motohashi, N. Hayashi, Y. Matuzaki, H. Nakauchi, M. Nishizawa, and M. Yamamoto. 1995. Activity and expression of murine small Maf family protein MafK. J. Biol. Chem. 270:7615-7624. [DOI] [PubMed] [Google Scholar]
- 31.Ishii, T., K. Itoh, S. Takahashi, H. Sato, T. Yanagawa, Y. Katoh, S. Bannai, and M. Yamamoto. 2000. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 275:16023-16029. [DOI] [PubMed] [Google Scholar]
- 32.Itoh, K., T. Chiba, S. Takahashi, T. Ishii, K. Igarashi, Y. Katoh, T. Oyake, N. Hayashi, K. Satoh, I. Hatayama, M. Yamamoto, and Y. Nabeshima. 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236:313-322. [DOI] [PubMed] [Google Scholar]
- 33.Johnsen, O., P. Murphy, H. Prydz, and A. B. Kolsto. 1998. Interaction of the CNC-bZIP factor TCF11/LCR-F1/Nrf1 with MafG: binding-site selection and regulation of transcription. Nucleic Acids Res. 26:512-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplowitz, N. 2002. Biochemical and cellular mechanisms of toxic liver injury. Semin. Liver Dis. 22:137-144. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi, A., E. Ito, T. Toki, K. Kogame, S. Takahashi, K. Igarashi, N. Hayashi, and M. Yamamoto. 1999. Molecular cloning and functional characterization of a new Cap'n'collar family transcription factor Nrf3. J. Biol. Chem. 274:6443-6452. [DOI] [PubMed] [Google Scholar]
- 36.Kotkow, K. J., and S. H. Orkin. 1996. Complexity of the erythroid transcription factor NF-E2 as revealed by gene targeting of the mouse p18 NF-E2 locus. Proc. Natl. Acad. Sci. USA 93:3514-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong, M., Y. W. Kan, and J. Y. Chan. 1999. The CNC basic leucine zipper factor, Nrf1, is essential for cell survival in response to oxidative stress-inducing agents. Role for Nrf1 in gamma-gcs(l) and gss expression in mouse fibroblasts. J. Biol. Chem. 274:37491-37498. [DOI] [PubMed] [Google Scholar]
- 38.Lee, F. Y., Y. Li, H. Zhu, S. Yang, H. Z. Lin, M. Trush, and A. M. Diehl. 1999. Tumor necrosis factor increases mitochondrial oxidant production and induces expression of uncoupling protein-2 in the regenerating mice [correction of rat] liver. Hepatology 29:677-687. [DOI] [PubMed] [Google Scholar]
- 39.Leist, M., F. Gantner, I. Bohlinger, P. G. Germann, G. Tiegs, and A. Wendel. 1994. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J. Immunol. 153:1778-1788. [PubMed] [Google Scholar]
- 40.Li, Q., D. Van Antwerp, F. Mercurio, K. F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 41.Masters, B. A., E. J. Kelly, C. J. Quaife, R. L. Brinster, and R. D. Palmiter. 1994. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. USA 91:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meister, A., and A. Larsson. 1995. Glutathione synthetase deficiency and other disorders of the gamma-glutamyl cycle, p. 1461-1477. In C. Scriver, A. Beaudet, W. Sly, and D. Valle (ed.), Metabolic and molecular basis of inherited disease, vol. 3. McGraw-Hill Inc., New York, N.Y.
- 43.Moi, P., K. Chan, I. Asunis, A. Cao, and Y. W. Kan. 1994. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA 91:9926-9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motohashi, H., J. A. Shavit, K. Igarashi, M. Yamamoto, and J. D. Engel. 1997. The world according to Maf. Nucleic Acids Res. 25:2953-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mountford, P., B. Zevnik, A. Düwel, J. Nichols, M. Li, C. Dani, M. Robertson, I. Chambers, and A. Smith. 1994. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc. Natl. Acad. Sci. USA 91:4303-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulcahy, R. T., and J. J. Gipp. 1995. Identification of a putative antioxidant response element in the 5′-flanking region of the human gamma-glutamylcysteine synthetase heavy subunit gene. Biochem. Biophys. Res. Commun. 209:227-233. [DOI] [PubMed] [Google Scholar]
- 47.Mulcahy, R. T., M. A. Wartman, H. H. Bailey, and J. J. Gipp. 1997. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J. Biol. Chem. 272:7445-7454. [DOI] [PubMed] [Google Scholar]
- 48.Muniz, P., M. J. Garcia Barchino, A. Iradi, E. Mahiques, V. Marco, M. R. Oliva, and G. T. Saez. 2000. Age-related changes of liver antioxidant enzymes and 8-hydroxy-2′-deoxyguanosine during fetal-neonate transition and early rat development. IUBMB Life 49:497-500. [DOI] [PubMed] [Google Scholar]
- 49.Myhrstad, M. C., C. Husberg, P. Murphy, O. Nordstrom, R. Blomhoff, J. O. Moskaug, and A. B. Kolsto. 2001. TCF11/Nrf1 overexpression increases the intracellular glutathione level and can transactivate the gamma-glutamylcysteine synthetase (GCS) heavy subunit promoter. Biochim. Biophys. Acta 1517:212-219. [DOI] [PubMed] [Google Scholar]
- 50.Ney, P. A., N. C. Andrews, S. M. Jane, B. Safer, M. E. Purucker, S. Weremowicz, C. C. Morton, S. C. Goff, S. H. Orkin, and A. W. Nienhuis. 1993. Purification of the human NF-E2 complex: cDNA cloning of the hematopoietic cell-specific subunit and evidence for an associated partner. Mol. Cell. Biol. 13:5604-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oyake, T., K. Itoh, H. Motohashi, N. Hayashi, H. Hoshino, M. Nishizawa, M. Yamamoto, and K. Igarashi. 1996. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol. Cell. Biol. 16:6083-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierce, R. H., J. S. Campbell, A. B. Stephenson, C. C. Franklin, M. Chaisson, M. Poot, T. J. Kavanagh, P. S. Rabinovitch, and N. Fausto. 2000. Disruption of redox homeostasis in tumor necrosis factor-induced apoptosis in a murine hepatocyte cell line. Am. J. Pathol. 157:221-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poss, K. D., and S. Tonegawa. 1997. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 94:10925-10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Powis, G., M. Briehl, and J. Oblong. 1995. Redox signalling and the control of cell growth and death. Pharmacol. Ther. 68:149-173. [DOI] [PubMed] [Google Scholar]
- 55.Rickett, G. M., and F. J. Kelly. 1990. Developmental expression of antioxidant enzymes in guinea pig lung and liver. Development 108:331-336. [DOI] [PubMed] [Google Scholar]
- 56.Rosenfeld, M. E., L. Prichard, N. Shiojiri, and N. Fausto. 2000. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am. J. Pathol. 156:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulze-Osthoff, K., R. Beyaert, V. Vandevoorde, G. Haegeman, and W. Fiers. 1993. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 12:3095-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sekhar, K. R., M. J. Meredith, L. D. Kerr, S. R. Soltaninassab, D. R. Spitz, Z. Q. Xu, and M. L. Freeman. 1997. Expression of glutathione and gamma-glutamylcysteine synthetase mRNA is Jun dependent. Biochem. Biophys. Res. Commun. 234:588-593. [DOI] [PubMed] [Google Scholar]
- 59.Shi, Z. Z., J. Osei-Frimpong, G. Kala, S. V. Kala, R. J. Barrios, G. M. Habib, D. J. Lukin, C. M. Danney, M. M. Matzuk, and M. W. Lieberman. 2000. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc. Natl. Acad. Sci. USA 97:5101-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shivdasani, R. A., M. F. Rosenblatt, D. Zucker-Franklin, C. W. Jackson, P. Hunt, C. J. Saris, and S. H. Orkin. 1995. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell 81:695-704. [DOI] [PubMed] [Google Scholar]
- 61.Slott, V. L., and B. F. Hales. 1987. Effect of glutathione depletion by buthionine sulfoximine on rat embryonic development in vitro. Biochem. Pharmacol. 36:683-688. [DOI] [PubMed] [Google Scholar]
- 62.Ueda, S., H. Masutani, H. Nakamura, T. Tanaka, M. Ueno, and J. Yodoi. 2002. Redox control of cell death. Antioxid. Redox Signal 4:405-414. [DOI] [PubMed] [Google Scholar]
- 63.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 64.Wild, A. C., H. R. Moinova, and R. T. Mulcahy. 1999. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 274:33627-33636. [DOI] [PubMed] [Google Scholar]
- 65.Wood, S. A., N. D. Allen, J. Rossant, A. Auerbach, and A. Nagy. 1993. Non-injection methods for the production of embryonic stem cell-embryo chimaeras. Nature 365:87-89. [DOI] [PubMed] [Google Scholar]