Abstract
In eukaryotes, the Src homology domain 3 (SH3) is a very important motif in signal transduction. SH3 domains recognize poly-proline-rich peptides and are involved in protein-protein interactions. Until now, the existence of SH3 domains has not been demonstrated in prokaryotes. However, the structure of the C-terminal domain of DtxR clearly shows that the fold of this domain is very similar to that of the SH3 domain. In addition, there is evidence that the C-terminal domain of DtxR binds to poly-proline-rich regions. Other bacterial proteins have domains that are structurally similar to the SH3 domain but whose functions are unknown or differ from that of the SH3 domain. The observed similarities between the structures of the C-terminal domain of DtxR and the SH3 domain constitute a perfect system to gain insight into their function and information about their evolution. Our results show that the C-terminal domain of DtxR shares a number of conserved key hydrophobic positions not recognizable from sequence comparison that might be responsible for the integrity of the SH3-like fold. Structural alignment of an ensemble of such domains from unrelated proteins shows a common structural core that seems to be conserved despite the lack of sequence similarity. This core constitutes the minimal requirements of protein architecture for the SH3-like fold.
The Src homology domain 3 (SH3) is a ubiquitous small protein domain that typically spans about 55 to 70 residues and is found as a modular entity in a variety of eukaryotic and viral proteins (9, 31). The SH3 domain was originally discovered as a homologous module through sequence comparisons of several tyrosine kinases. It has been demonstrated that SH3 domains participate in a number of signal transduction mechanisms and cell-cell communication by binding to poly-proline-rich peptides that are folded in a left-handed poly-proline helix II (PPII) conformation, with the consensus general form P-X-X-P, where P is proline and X is any amino acid (8, 9, 31). Recognition of the target motif mediates protein-protein interactions by forming and maintaining macromolecular aggregates either inter- or intramolecularly.
The three-dimensional structure of the SH3 domain is also known. The first structures of SH3 domains determined were those of spectrin and Src by X-ray crystallography and nuclear magnetic resonance (NMR), respectively (27, 48). The SH3 fold contains five β-strands forming two orthogonal anti-parallel β-sheets of three strands each, with the third strand shared by the two β-sheets. This β-sandwich arrangement defines the hydrophobic core of the fold. The connectivity of the fold is provided by three loops that define the regions of higher residue variability in the sequence. Two of these three loops contribute to peptide binding. In the SH3 domains, the RT-Src loop (which connects strands 1 and 2) and the N-Src loop (which connects strands 2 and 3) participate in ligand binding. The other connecting loop, known as the distal loop because it is found opposite to the other two loops, connects strands 3 and 4.
Although very similar in most cases, the amino acid sequences of SH3 domains can show limited but recognizable degrees of residue conservation. Some key hydrophobic positions are well conserved to preserve the protein core and participate in ligand binding (22). This degree of residue conservation allows identification of SH3 domains by using sequence analysis techniques. A striking observation is that, regardless of the residue similarity, the general topology of the SH3 domain fold is very well conserved, and structural alignment of SH3 domains yields very low root mean square deviations (RMSDs), usually below 2 Å (22). It has been hypothesized that SH3 domains may not be found in prokaryotes, because the pathways in which they are normally implicated are not present in bacteria. However, recent sequence searches using the sequence of the SH3 domain of the tyrosine kinase from Pacific electric ray as a probe have identified the N-terminal domain of the protein P60 from the prokaryote Listeria grayi among the matches, although with a low probability score (E = 0.95). Nevertheless, despite the fact that the overall residue conservation between P60 and SH3 domains is very low, several key positions in the sequence are well conserved. Profile-based threading techniques and model building predict an SH3-like fold for the N-terminal domain of this protein (45).
An early structure determination of diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae showed a C-terminal domain with an SH3-like fold. A subsequent NMR structure of the C-terminal domain alone confirmed that initial observation (35, 44). This repressor is the best characterized of a superfamily of metal ion-dependent transcriptional regulators found in a variety of gram-positive bacteria that include, among others, several pathogenic species (4, 13, 14, 17, 20, 29, 34, 37, 43). DtxR is a 226-residue protein containing two independent structural domains that are connected by a short unstructured linker of approximately 26 residues. The N-terminal domain contains the helix-turn-helix motif responsible for DNA binding as well as two metal ion binding sites (6, 36, 41). One of these, the primary metal ion binding site, has been shown to be primarily responsible for the structural transition that allows tight binding of the repressor to its operator DNA (10).
The other domain, the C-terminal domain, accounts for approximately the last 76 (149 to 226) residues of the sequence. X-ray data generally give poor electron density for the C-terminal region, and it was initially believed to be highly flexible and disordered (35, 36, 41). The role of the C-terminal domain has not yet been established. A few crystallographic experiments have now shown an ordered structure for this domain, both for that from DtxR and for that of the structural and functional homologue from Mycobacterium tuberculosis, IdeR. Recent structures of both DtxR and IdeR have shown that the C-terminal domain contributes two ligands to one of the metal ion binding sites, albeit not the one essential for DNA binding (12, 32).
The SH3-like fold for the C-terminal domain contains five β-strands that form a similar β-sandwich arrangement as that found in the classical SH3 fold. There are five β-strands forming two orthogonal anti-parallel β-sheets of two and three β-strands each. The overall fold, however, is only minimally affected by these changes, even though the residue similarity between the C-terminal domain of DtxR and that of the SH3 domain of the eukaryotic spectrin is only 7% (35). The original NMR structure shows that the C-terminal domain of DtxR has regions of high stability that form the hydrophobic core and more mobile loops that could easily undergo conformational change, presumably associated with domain function.
Sequence analysis techniques fail to identify SH3 domains when the sequence of the C-terminal domain of DtxR is used as a probe. Consequently, structural alignments may be the only way to identify any relationship between them. In this paper we present the results of such an alignment and we show that some key hydrophobic residues are responsible for maintaining the fold in both types of domains. Our results show that, although the sequences are not identical, the pattern of conservation of hydrophobic residues is very similar for both.
MATERIALS AND METHODS
Sample preparation and crystallization.
The mutant DtxR(H79A) was overexpressed and purified as previously described (42). Protein samples were incubated with 3 mM NiCl2, concentrated to 10 mg/ml, and crystallized by vapor diffusion using the hanging drop method at room temperature. Protein solution (5 μl) was mixed with an equal volume of the well solution consisting of 1.8 M (NH4)2SO4, 0.1 M Tris (pH 8.0). After 2 weeks, large trigonal crystals suitable for crystallographic analysis appeared in the drops. The crystals had an approximate size of 0.2 by 0.2 by 0.35 mm.
Data collection.
The data for the DtxR(H79A) mutant were collected on a Rigaku Rotaflex 2000 series generator operated at 36 kV and 26 mA and equipped with an R-Axis IV area detector. The data were collected to a maximal resolution of 2.1 Å. In agreement with previous structures of DtxR, the crystals were in space group P3121 with cell dimensions of a = b = 63.39, c = 109.87, α = β = 90, γ = 120, similar to those observed for previous structures of DtxR. The data were scaled and integrated using the programs DENZO and SCALEPACK of the HKL suite. Statistics for the data collection are presented in Table 1 (30).
TABLE 1.
Data collection and refinement statistics of DtxR(H79A)
| Parameter | DtxR(H79A) value(s) |
|---|---|
| Data collection | |
| Space group | P3121 |
| Unit cell parameters | |
| a = b, c | 63.39, 109.87 |
| α = β, γ | 90, 120 |
| Resolution (Å) | 30-2.1 |
| Observed reflections | 34,271 |
| Unique reflections | 15,278 |
| Signal to noise I/σ(I)(I) | 12.9 |
| Rmerge | 0.078 |
| Temperature (K) | 277 |
| Light source | Rotating anode |
| Refinement | |
| Resolution (Å) | 30-2.1 |
| Total no. of reflections | 14,509 |
| Completeness (%) | 98.8 |
| R factor | 0.24 |
| Rfree | 0.28 |
| R factor (highest resolution) | 0.29 |
| Model | |
| Non-hydrogen atoms | 1,791 |
| Metal ions | 0 |
| RMSD from ideal geometry | |
| Bonds (Å) | 0.007 |
| Angles (degrees) | 2.02 |
Structure determination and refinement.
The structure of the mutant DtxR(H79A) was solved by molecular replacement with the program EPMR using the coordinates 1BYM for the C-terminal domain and 2TDX for the N-terminal domain as search models (10, 19, 44). After a round of rigid body refinement, several rounds of manual building and molecular refinement were performed, using the programs O and CNS (7, 18). During the refinement process, the quality of the geometry was monitored using Procheck (23). After every round of refinement, new difference Fourier electron density maps with the coefficients 2Fo-Fc and Fo-Fc were generated. Simulated annealing omit maps were generated to help the interpretation of the electron density and reduce bias introduced by initial placement of the model (15). Both structures showed only four nonglycine residues outside the traditionally allowed regions of the Ramachandran plot (38). Recent studies have shown that the conformations of such residues, although not allowed based on traditional geometrical constraints, are justified and often observed due to important enthalpic interactions that further stabilize them (26). These residues have been consistently found in similar conformations in previous structures of DtxR, mutants and homologues (46). The structure of DtxR(H79A) was refined, including all data between 2.1 to 30 Å to a final R factor of 24% and an Rfree of 27%. Complete statistics for the refinement are shown in Table 1. No metal ions were found in either of the metal ion binding sites of the mutant DtxR(H79A).
Structural alignment.
Structural alignments were produced with the program STAMP by using a combination of the rough alignment and scan options and DALI (distance matrix alignment) (16, 39). A large pool of sequences for SH3-like domains was identified using BLAST and profile-based threading searches (1, 5; N. N. Alexandrov, R. Nussinov, and R. M. Zimmer, Pacific Symp. Biocomput. '96, p. 53-72, 1995). Those whose three-dimensional structures are known were used to produce structural alignments. To test the validity of the results obtained with the software, manual alignments were also performed. The result was used to produce structure-based sequence alignments in each case. Molecular coordinates that did not give a good fit over a significant number of residues were eliminated from the final comparison. The final 11 structures that met all the requirements for structural alignment are listed in Table 2.
TABLE 2.
Ensemble of structures used in the structure-based sequence analysis
| PDBa code | Protein | Organism |
|---|---|---|
| Prokaryote | ||
| 1P92 | H79A mutant of DtxR | C. diphtheria |
| 1bia, 1bib | Biotin holoenzyme synthetase/biorepressor (BirA) and complex | E. coli |
| 1bxy | Ribosomal protein L30 | Thermus thermophilus |
| 1ee8 | Mutm (Fpg) protein | T. thermophilus Hb8 |
| 1h9j | Molybdate-binding protein (Modg) | Azotobacter vinelandii |
| Eukaryote | ||
| 1abo, 1bbz | Abl tyrosine kinase and complex with a designed high-affinity peptide ligand | Mus musculus |
| 1cka | SH3 domain of c-Crk | M. musculus |
| 1fmk | Tyrosine-protein kinase c-Src | Human |
| 1gri | Growth factor-bound protein 2 | Human |
| 1jo8 | Actin binding protein Abp1 | Saccharomyces cerevisiae |
| 1lck | Sh3 domain of P56-Lck tyrosine kinase | Human |
| 1ycs | P53-53Bp2 complex | Human |
PDB, Protein Data Bank.
The average displacements of the atoms of the main chain of all the members of the ensemble were produced using an in-house program written by Mark Wilson and Timothy Fenn and plotted using POVScript+ (12a). The results were used as a test of the validity of the structural alignment and to estimate the degree of structural conservation within the ensemble.
RESULTS
Structure of the mutant DtxR(H79A).
The electron density for the protein model is continuous and smooth throughout the whole structure, with the exception of part of the flexible linker between the two structural domains. It was possible to build the C-terminal domain into the electron density and the final model accounts for all the residues from 1 to 139 and 148 to 226. Up until now, all the structures of DtxR determined in our laboratory have shown only the N-terminal domain of the protein. The overall structure of the mutant DtxR(H79A) seems to be unaffected by the mutation except for the vicinity of the side chain of residue 79.
C-terminal domain.
The C-terminal domain of DtxR(H79A) contains all the general features observed in the SH3 domain fold (27, 48). A ribbon diagram of the DtxR(H79A) dimer showing the spatial relationships between the N-terminal and C-terminal domains is shown in Fig. 1. The general structure of the C-terminal domain of DtxR has been previously described (35). For this study we have used the coordinates of the C-terminal domain of the mutant DtxR(H79A) as a structural probe to try to understand the common features that define the SH3-like fold.
FIG. 1.
Spatial relationship between the N-terminal and C-terminal domains of the mutant DtxR(H79A) hypothetical dimer. The N-terminal domain of monomer 1, shown in red, is attached to the C-terminal domain (green) through a flexible linker. The N terminal of monomer 2, shown in yellow, is attached to the C-terminal domain (blue). The flexible linker is not seen in the electron density. All figures were created using Molscript and POVScript+ (12a, 21).
Structural alignment.
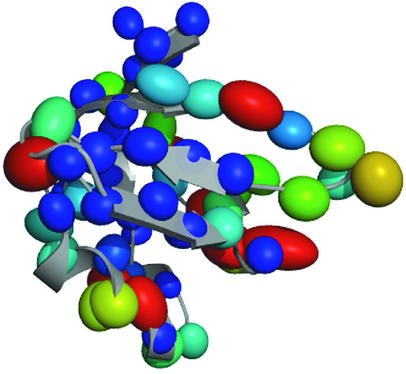
The structural alignment of the C-terminal domain of the mutant DtxR(H79A) with 10 different structure files corresponding to SH3 and SH3-like domains from organisms ranging from bacteria to human (Table 2) yielded good results. The structures were used in the alignment only if more than 50% of the residues contributed to the fit. The RMSDs of the final pairwise aligned members of the ensemble with the C-terminal domain of the mutant DtxR(H79A) ranged from 1.2 to 2.4 Å. This result is remarkable considering the extremely low sequence similarity and biodispersity among the test set. The residue identity is lower than 10% in the best case. Structural alignments of the SH3 family have shown pairwise RMSDs of 0.47 to 1.72 Å (22). Figure 2 shows the extent of deviation among α-carbons for the 11 structures. The ellipsoids represent averaged deviations of the α-carbon positions of any residue with respect to the equivalent residue of the C-terminal domain of the mutant DtxR(H79A). The color of the ellipsoid also corresponds to the magnitude of the average displacement, where blue represents the smallest differences and red the largest. The smallest displacements were observed within the β-sandwich, suggesting fold conservation despite the lack of absolute sequence conservation. Loops and other exposed areas of the structure showed larger deviations, partially due to differences in sequence length. This pattern of loop variability is also seen in the structure determined by NMR. In that case, such variability was interpreted as indicative of the relative flexibility of a particular region (44).
FIG. 2.
Graphical representation of the deviation of the positions of the α-carbons for the overlay of the C-terminal domain of DtxR with 10 SH3-like structures. The size of the ellipsoid represents averaged deviations with respect to the equivalent position in the C-terminal domain of the mutant DtxR(H79A). The color of the ellipsoid also corresponds to the magnitude of such differences, where blue represents the smallest differences and red represents the largest.
In order to illustrate the roles of conserved residues in the protein fold, the structure of the SH3 domain from tyrosine kinase Abl was compared in detail with that of the C-terminal domain of DtxR. Figure 3 shows both of those structures individually and Fig. 4 shows the aligned and overlaid structures of the two. The alignment of these two structures yielded an RMSD of 1.5 Å over 30 residues. Figure 4A shows a diagram of the structural alignment highlighting hydrophobic positions that are conserved in both the SH3 and the DtxR families. A few of the conserved hydrophobic positions were slightly shifted relative to one another, possibly due to the differences in length of the two sequences. Figure 4B illustrates a structure-based sequence alignment of the two structures, showing residue conservation between the two sequences. There are five regions where strands of five or more consecutive residues overlap. The sequence gaps observed indicate regions where the alignment was less than perfect. The sequence of the C-terminal domain of DtxR is 10 residues longer than that of the SH3 domain of the Abl tyrosine kinase with most of the insertions located in the first and last β-strand, where they are found forming short helices. The effect of these insertions is to slightly distort the fold. The highest degree of sequence variability in the SH3 domain is generally found around the N-Src and distal loops (Fig. 3, top) (22). During the course of this investigation, it was noticed that there is some similarity in sequence between the turn in the N-Src loop and its equivalent in DtxR. A sequence pattern of hydrophobic-polar-basic-polar-glycine is maintained. Similarly, a sequence pattern was observed in the turn of the distal loop and its equivalent in DtxR, where the pattern polar-basic-polar-glycine was observed.
FIG. 3.
Structures of the SH3 domain from tyrosine kinase Abl and the C-terminal domain of DtxR(H79A). (Top) The structure of the SH3 domain from tyrosine kinase Abl. The structure shows two orthogonal β-sheets formed by five β-strands designated by different colors. The locations of the three connecting loops are indicated. (Bottom) The structure of the C-terminal domain of the mutant DtxR(H79A). The structure shows a similar overall fold to that of the SH3 domain from tyrosine kinase Abl (top). Note that the helical regions that are present in this structure slightly distort the fold.
FIG. 4.
(A) Overlay of the structures of the SH3 domain from tyrosine kinase Abl in blue and the C-terminal domain of the mutant DtxR(H79A) in yellow. Conserved hydrophobic positions for the two families are also shown: SH3 (magenta), DtxR (red). (B) Structure-based sequence alignment of tyrosine kinase Abl and the C-terminal domain of DtxR. Purple boxes show conserved hydrophobic positions. Red boxes show regions of five or more consecutive residues that spatially overlap, forming a conserved core.
DISCUSSION
The C-terminal domain of DtxR, as well as other similar folds found in prokaryotes, has often been referred to as SH3-like. This distinction indicates a fold that closely resembles the SH3 domain but cannot be traced to classical SH3 domains in terms of function or sequence similarity. Unlike most of these bacterial proteins, the C-terminal domain of DtxR shares functional similarity with that of the SH3 domain. It has been shown that the C-terminal domain of DtxR also binds poly-proline-rich regions (44). This fact separates this domain from other bacterial domains that present the same general fold. It has been suggested that the GW domain, a bacterial fold that also resembles the SH3 domain, is not likely to mimic the function of SH3 domains because its equivalent binding sites are blocked or destroyed (24). The C-terminal domain of DtxR constitutes a unique example of a bacterial protein that shares function in addition to structural similarity with its SH3 counterpart.
The sequence of the SH3 domain from Abl tyrosine kinase fails to identify bacterial SH3-like domains either by sequence comparison or profile-based threading techniques. In fact, such searches report primarily other SH3 domains. However, in a recent study a potential bacterial SH3 domain was identified in the organism L. grayi with such strategies, although using the SH3 domain of the tyrosine kinase from Pacific electric ray as a probe (45). We have found several examples of SH3-like domains by using sequence comparison and the sequence of the C-terminal domain of DtxR as a probe. In cases where the three-dimensional structure is available, comparisons with the C-terminal domain of DtxR yield RMSDs of less than 3 Å. Because a wide number of these bacterial domains have been structurally characterized in recent years, the task of identifying SH3-like domains based on sequence may become increasingly easier (2, 3, 11, 24, 25, 28, 40, 47).
The similarity between the three-dimensional structures of the C-terminal domain of DtxR and SH3 domains is not obvious from sequence comparison using the available tools. Even the use of sequence comparison coupled with profile-based threading techniques with the sequence of the C-terminal domain of DtxR as a probe does not identify members of the SH3 family. The apparent conservation of hydrophobic residues in key positions observed between the DtxR and SH3 families, which constitutes the only tangible structural feature shared at the sequence level, is not enough to allow identification of a member of one family by using a member from the other. Far from outlining the weaknesses of such methods, these results reflect the inherent difficulties of the task at hand. It is reasonable to expect that possible bacterial homologues of the SH3 domain could share much lower similarity with them, even if they were evolutionarily related, than other members of the SH3 family (22). A single ancient horizontal transfer followed by independent evolution has been suggested as a plausible explanation for such lack of sequence similarity (33). Another possible explanation is that these domains originally evolved in bacteria and were transferred to eukaryotes as a result of mitochondrial endosymbiosis (33). Despite not being able to identify members of the SH3 family, sequence comparison and profile-based threading using the C-terminal domain of DtxR as a probe are able to identify SH3-like sequences from a variety of organisms ranging from bacteria to humans, and those whose structure are known show an SH3-like fold. The ubiquity of the SH3-like fold might be derived from the simplicity of the determinants of the fold. Conservation of a few key hydrophobic positions seems to be the only requirement for maintaining the fold without significant loss of modularity, making it perfect for adapting to a wide range of sequences.
Acknowledgments
This work was supported by a grant from the NIH (AI21628).
We are grateful to Timothy Fenn and Mark Wilson for their help and support.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. H. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth, P., P. Savarin, B. Gilquin, B. Lagoutte, and F. Ochsenbein. 2002. Solution NMR structure and backbone dynamics of the PsaE subunit of photosystem I from Synechocystis sp. PCC 6803. Biochemistry 41:13902-13914. [DOI] [PubMed] [Google Scholar]
- 3.Bilwes, A. M., L. A. Alex, B. R. Crane, and M. I. Simon. 1999. Structure of CheA, a signal-transducing histidine kinase. Cell 96:131-141. [DOI] [PubMed] [Google Scholar]
- 4.Boland, C. A., and W. G. Meijer. 2000. The iron dependent regulatory protein IdeR (DtxR) of Rhodococcus equi. FEMS Microbiol. Lett. 191:1-5. [DOI] [PubMed] [Google Scholar]
- 5.Bowie, J. U., R. Luthy, and D. Eisenberg. 1991. A method to identify protein sequences that fold into a known 3-dimensional structure. Science 253:164-170. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, R. G., and B. W. Matthews. 1989. The helix-turn-helix DNA-binding motif. J. Biol. Chem. 264:1903-1906. [PubMed] [Google Scholar]
- 7.Brunger, A. T., P. D. Adams, G. M. Clore, W. L. DeLano, P. Gros, R. W. Grosse-Kunstleve, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54:905-921. [DOI] [PubMed] [Google Scholar]
- 8.Cicchetti, P., B. J. Mayer, G. Thiel, and D. Baltimore. 1992. Identification of a protein that binds to the Sh3 region of Abi and is similar to Bcr and Gap-Rho. Science 257:803-806. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, G. B., R. B. Ren, and D. Baltimore. 1995. Modular binding domains in signal-transduction proteins. Cell 80:237-248. [DOI] [PubMed] [Google Scholar]
- 10.Ding, X., H. Zeng, N. Schiering, D. Ringe, and J. R. Murphy. 1996. Identification of the primary metal ion-activation sites of the diphtheria tox repressor by X-ray crystallography and site-directed mutational analysis. Nat. Struct. Biol. 3:382-387. [DOI] [PubMed] [Google Scholar]
- 11.Falzone, C. J., Y. H. Kao, J. D. Zhao, D. A. Bryant, and J. T. J. Lecomte. 1994. 3-Dimensional solution structure of Psae from the Cyanobacterium synechococcus sp. strain Pcc-7002, a photosystem-I protein that shows structural homology with SH3 domains. Biochemistry 33:6052-6062. [DOI] [PubMed] [Google Scholar]
- 12.Feese, M. D., B. P. Ingason, J. Goranson-Siekierke, R. K. Holmes, and W. G. J. Hol. 2001. Crystal structure of the iron-dependent regulator from Mycobacterium tuberculosis at 2.0-angstrom resolution reveals the Src homology domain 3-like fold and metal binding function of the third domain. J. Biol. Chem. 276:5959-5966. [DOI] [PubMed] [Google Scholar]
- 12a.Fenn, T. D., D. Ringe, and G. A. Petsko. 2003. POVScript+: a program for model and data visualization using persistence of vision ray-tracing. J. Appl. Crystallogr. 36:944-947. [Google Scholar]
- 13.Gunter, K., C. Toupet, and T. Schupp. 1993. Characterization of an iron-regulated promoter involved in desferrioxamine B synthesis in Streptomyces pilosus: repressor-binding site and homology to the diphtheria toxin gene promoter. J. Bacteriol. 175:3295-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill, P. J., A. Cockayne, P. Landers, J. A. Morrissey, C. M. Sims, and P. Williams. 1998. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect. Immun. 66:4123-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodel, A., S. H. Kim, and A. T. Brunger. 1992. Model bias in macromolecular crystal structures. Acta Crystallogr. A 48:851-858. [Google Scholar]
- 16.Holm, L., and C. Sander. 1993. Protein-structure comparison by alignment of distance matrices. J. Mol. Biol. 233:123-138. [DOI] [PubMed] [Google Scholar]
- 17.Jakubovics, N. S., A. W. Smith, and H. F. Jenkinson. 2000. Expression of the virulence-related Sea (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR. Mol. Microbiol. 38:140-153. [DOI] [PubMed] [Google Scholar]
- 18.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron- density maps and the location of errors in these models. Acta Crystallogr. A 47:110-119. [DOI] [PubMed] [Google Scholar]
- 19.Kissinger, C. R., D. K. Gehlhaar, and D. B. Fogel. 1999. Rapid automated molecular replacement by evolutionary search. Acta Crystallogr. D 55:484-491. [DOI] [PubMed] [Google Scholar]
- 20.Kitten, T., C. L. Munro, S. M. Michalek, and F. L. Macrina. 2000. Genetic characterization of a Streptococcus mutans LraI family operon and its role in virulence. Infect. Immun. 68:4441-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kraulis, P. J. 1991. Molscript—a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24:946-950. [Google Scholar]
- 22.Larson, S. M., and A. R. Davidson. 2000. The identification of conserved interactions within the SH3 domain by alignment of sequences and structures. Protein Sci. 9:2170-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laskowski, R. A., M. W. Macarthur, D. S. Moss, and J. M. Thornton. 1993. Procheck—a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 24.Marino, M., M. Banerjee, R. Jonquieres, P. Cossart, and P. Ghosh. 2002. GW domains of the Listeria monocytogenes invasion protein InlB are SH3-like and mediate binding to host ligands. EMBO J. 21:5623-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayer, K. L., G. Z. Shen, D. A. Bryant, J. T. J. Lecomte, and C. J. Falzone. 1999. The solution structure of photosystem I accessory protein E from the cyanobacterium Nostoc sp. strain PCC 8009. Biochemistry 38:13736-13746. [DOI] [PubMed] [Google Scholar]
- 26.McCaldon, P., and P. Argos. 1988. Oligopeptide biases in protein sequences and their use in predicting protein coding regions in nucleotide sequences. Proteins Struct. Funct. Genet. 4:99-122. [DOI] [PubMed] [Google Scholar]
- 27.Musacchio, A., M. Noble, R. Pauptit, R. Wierenga, and M. Saraste. 1992. Crystal structure of a Src homology 3 (Sh3) domain. Nature 359:851-855. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa, A., T. Nakashima, M. Taniguchi, H. Hosaka, M. Kimura, and I. Tanaka. 1999. The three-dimensional structure of the RNA-binding domain of ribosomal protein L2; a protein at the peptidyl transferase center of the ribosome. EMBO J. 18:1459-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oguiza, J. A., X. Tao, A. T. Marcos, M. Malumbres, J. F. Martin, and J. R. Murphy. 1995. Molecular cloning, DNA sequence analysis, and characterization of the Corynebacterium diphtheriae Dtxr homolog from Brevibacterium lactofermentum. J. Bacteriol. 177:465-467. [DOI] [PMC free article] [PubMed]
- 30.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307-326. [DOI] [PubMed] [Google Scholar]
- 31.Pawson, T. 1995. Protein modules and signaling networks. Nature 373:573-580. [DOI] [PubMed] [Google Scholar]
- 32.Pohl, E., R. K. Holmes, and W. G. J. Hol. 1999. Crystal structure of a cobalt-activated diphtheria toxin repressor-DNA complex reveals a metal-binding SH3-like domain. J. Mol. Biol. 292:653-667. [DOI] [PubMed] [Google Scholar]
- 33.Ponting, C. P., L. Aravind, J. Schultz, P. Bork, and E. V. Koonin. 1999. Eukaryotic signalling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J. Mol. Biol. 289:729-745. [DOI] [PubMed] [Google Scholar]
- 34.Posey, J. E., J. M. Hardham, S. J. Norris, and F. C. Gherardini. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc. Natl. Acad. Sci. USA 96:10887-10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu, X. Y., E. Pohl, R. K. Holmes, and W. G. J. Hol. 1996. High-resolution structure of the diphtheria toxin repressor complexed with cobalt and manganese reveals an SH3-like third domain and suggests a possible role of phosphate as co-corepressor. Biochemistry 35:12292-12302. [DOI] [PubMed] [Google Scholar]
- 36.Qiu, X. Y., C. Verlinde, S. P. Zhang, M. P. Schmitt, R. K. Holmes, and W. G. J. Hol. 1995. 3-Dimensional structure of the diphtheria toxin repressor in complex with divalent cation co-repressors. Structure 3:87-100. [DOI] [PubMed] [Google Scholar]
- 37.Que, Q., and J. D. Helmann. 2000. Manganese homeostasis in Bacillus subtilis is regulated by MntR, a bifunctional regulator related to the diphtheria toxin repressor family of proteins. Mol. Microbiol. 35:1454-1468. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran, G. N., C. Ramakrishnan, and V. Saisekharan. 1963. Stereochemistry of polypeptide chain configuration. J. Mol. Biol. 7:95-99. [DOI] [PubMed] [Google Scholar]
- 39.Russell, R. B., and G. J. Barton. 1992. Multiple protein sequence alignment from tertiary structure comparison—assignment of global and residue confidence levels. Proteins Struct. Funct. Genet. 14:309-323. [DOI] [PubMed] [Google Scholar]
- 40.Safro, M., and L. Mosyak. 1995. Structural similarities in the noncatalytic domains of phenylalanyl transfer RNA and biotin synthetases. Protein Sci. 4:2429-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiering, N., X. Tao, H. Y. Zeng, J. R. Murphy, G. A. Petsko, and D. Ringe. 1995. Structures of the Apo-activated and the metal ion-activated forms of the diphtheria Tox repressor from Corynebacterium diphtheriae. Proc. Natl. Acad. Sci. USA 92:9843-9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt, M. P., and R. K. Holmes. 1993. Analysis of diphtheria toxin repressor-operator interactions and characterization of a mutant repressor with decreased binding activity for divalent metals. Mol. Microbiol. 9:173-181. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt, M. P., M. Predich, L. Doukhan, I. Smith, and R. K. Holmes. 1995. Characterization of an iron-dependent regulatory protein (IdeR) of Mycobacterium tuberculosis as a functional homolog of the diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae. Infect. Immun. 63:4284-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, G., G. P. Wylie, P. D. Twigg, D. L. D. Caspar, J. R. Murphy, and T. M. Logan. 1999. Solution structure and peptide binding studies of the C-terminal Src homology 3-like domain of the diphtheria toxin repressor protein. Proc. Natl. Acad. Sci. USA 96:6119-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whisstock, J. C., and A. M. Lesk. 1999. SH3 domains in prokaryotes. Trends Biochem. Sci. 24:132-133. [DOI] [PubMed] [Google Scholar]
- 46.White, A., X. C. Ding, J. C. vanderSpek, J. R. Murphy, and D. Ringe. 1998. Structure of the metal-ion-activated diphtheria toxin repressor tox operator complex. Nature 394:502-506. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, K. P., L. M. Shewchuk, R. G. Brennan, A. J. Otsuka, and B. W. Matthews. 1992. Escherichia coli biotin holoenzyme synthetase biorepressor crystal structure delineates the biotin binding and DNA-binding domains. Proc. Natl. Acad. Sci. USA 89:9257-9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, H. T., M. K. Rosen, T. B. Shin, C. Seideldugan, J. S. Brugge, and S. L. Schreiber. 1992. Solution structure of the Sh3 domain of Src and identification of its ligand-binding site. Science 258:1665-1668. [DOI] [PubMed] [Google Scholar]