Abstract
We previously reported that autoantibodies (autoAbs) to the main epitope on CD69 reacted to its homologous amino acid sequence in low-density-lipoprotein-receptor-related protein 2 (LPR2), a multiligand receptor for protein reabsorption. In this study, we have investigated the prevalence, autoepitope distribution, and clinical significance of the autoAbs to LRP2 in patients with systemic autoimmune diseases. Using six recombinant proteins (F2–F7) for LRP2 and one for CD69, we detected autoAbs to LRP2 in sera of patients with rheumatoid arthritis (RA), systemic lupus erythematosus, Behçet's disease, systemic sclerosis, and osteoarthritis and then mapped autoepitopes by Western blotting. The autoAbs to LRP2 were detected in 87% of the patients with rheumatoid arthritis, 40% of those with systemic lupus erythematosus, 35% of those with systemic sclerosis, 15% of those with osteoarthritis, and 3% of those with Behçet's disease. Multiple epitopes on LRP2 were recognized by most of the anti-LRP2+ serum samples. All of the tested anti-CD69 autoAb+ samples reacted to LRP2-F3 containing the homologous sequence to the main epitope of CD69; however, only 38% of the anti-LRP2-F3+ samples reacted to CD69. Clinically, the existence of the autoAbs to LRP2-F4, -F5, and -F6 correlated with the presence of proteinuria in RA. This study revealed that LRP2 is a major autoantigen in RA. The autoAbs to LRP2 are probably produced by the antigen-driven mechanism and the autoimmunity to LRP2 may spread to include CD69. The anti-LRP2 autoAbs may play pathological roles by inhibiting the reabsorbing function of LRP2.
Keywords: autoantibody, CD69, LRP2, proteinuria
Introduction
Autoantibodies (autoAbs) to cell-surface molecules, including antilymphocyte antibodies, are often detected in the sera of patients with systemic autoimmune diseases such as systemic lupus erythematosus (SLE). Although the presence of antilymphocyte antibodies has been correlated with disease activity [1], lymphocyte subset distortions, and functional abnormalities [2,3], the detailed roles of these antibodies remain to be elucidated, as do the roles of autoAbs to surface molecules on other types of cell. One of the main factors hampering the analysis of autoAbs to surface molecules is that only a few target antigens have been identified, such as CD45 [4]. In this regard, we recently reported that CD28, CTLA-4, and CD69 were among the targets of antilymphocyte antibodies [5,6]. In our study on the autoAbs to CD69 [6], most of the tested serum samples recognized only one epitope on CD69. Interestingly, the amino acid sequence of this main epitope (EKNLYWI) is highly homologous to a part (EKRLYWI) of low-density-lipoprotein-receptor-related protein 2 (LRP2). In that study, we showed that autoAbs to the main epitope on CD69 cross-reacted with the homologous epitope in LRP2 [6]. Therefore, the generation of the anti-CD69 autoAbs may be related to that of the anti-LRP2 autoAbs.
LRP2 (also designated as megalin or gp330) is one of the superfamily of low-density-lipoprotein receptors (LDLRs) [7,8]. It is a huge molecule, with a molecular weight of approximately 600 kDa, and contains four LDLR domains. LRP2 is expressed in a variety of epithelia, such as renal proximal tubule, epididymis, and thyroid cells. Because mice lacking the gene for LRP2 exhibit a deficiency of proximal tubule reabsorption and a significant reduction of the number and size of organelles associated with endocytosis in the proximal tubule [9], LRP2 is thought to play central roles in reabsorption of proteins and endocytosis. More than 30 ligands for LRP2 have been reported so far, including vitamin-binding proteins, apolipoproteins, hormones, and other low-molecular-weight peptides, as reviewed in [8].
LRP2 was originally identified as a pathogenic autoantigen in a rat experimental model of human membranous glomerulonephritis (Heymann's nephritis) [10], although no pathological role has been shown in humans. Recently, autoAbs to LRP2 have been reported in patients with autoimmune thyroiditis [11]. However, no other data have been available on the autoimmunity to LRP2 in humans. Therefore, we have investigated the autoimmunity to LRP2 in systemic autoimmune diseases, focusing on prevalence, autoepitope distribution, clinical significance, and antigenic relationships with the anti-CD69 autoAbs.
Materials and methods
Human sera
Serum samples were obtained from a total of 147 patients with systemic autoimmune diseases, including 47 with rheumatoid arthritis (RA) (35 females,12 males; mean age 57.2 years, range 22–79), 30 with SLE (28 females, 2 males; mean age 42.7 years, range 20–72), 30 with Behçet's disease (20 females, 10 males; mean age 50.9 years, range 24–78), 20 with osteoarthritis (OA) (14 females, 6 males; mean age 62.9 years, range 55–78) and 20 with systemic sclerosis (SSc) (17 females, 3 males; mean age 52.9 years, range 29–71). Each patient was diagnosed according to the standard criteria for the disease in question [12-16]. Serum samples from 75 healthy donors (58 females, 17 males; mean age 49.7 years, range 22–82), were used as age- and sex-matched control samples.
Plasmid construction for the expression of recombinant LRP2 and CD69 molecules
Six cDNA fragments (cDNAF2 to cDNAF7), each encoding a part of the LRP2 molecule, were amplified by PCR from cDNA prepared from thyroid cells. DNA primers were designed based on the nucleotide (N) sequence of LRP2 (European Molecular Biology Laboratory U04441). The sequences of the primers are as follows (the lower-case letters indicate the restriction enzyme sites):
5'-TTTgaattcCTGATGCACCTGTGCCACACC-3' and 5'-TTTgtcgacAAAAATGAGATAGGGTTCGATGTTA-3' for cDNAF2 (N9294–9713),
5'-TTTgaattcAACAGTAACATCGAACCCTATCTC-3' and 5'-TTTgtcgacATTGTTGGTACCACAGGGATTGC-3' for cDNAF3 (N9684–10523),
5'-TTTggatccAATCCCTGTGGTACCAACAATGGT-3' and 5'-TTTgtcgacACAGCCTTGCTCATCACTGTTGTC-3' for cDNAF4 (N10494–11180),
5'-TTTgaattcGAATTCAGCTGCAAAACAAATTAC-3' and 5'-TTTgtcgacCTCTGTCTCATCAGTTCCATCTCC-3' for cDNAF5 (N11157–11855),
5'-TTTggatccACTGATGAGACAGAGGAGCACTGT-3' and 5'-TTTgtcgacATCAACAGCTTGGATATATTCCTCATC-3' for cDNAF6 (N11826–12374),
5'-TTTgaattcCGAAAATATAATCTCTCATCT-3' and 5'-TTTgtcgacCTGTTTGCAAAGGTTGGGCACTGA-3' for cDNAF7 (N12345–13112).
These cDNA fragments covered approximately one-third (N9294–13112) of the entire LRP2 molecule (N74–14072, 13999 bp), with some overlaps. Each of the six cDNA fragments was cloned into the EcoRI/SalI or BamHI/SalI sites of the pMAL-eHis, a derivative of the pMAL-c expression vector (New England Biolabs, Beverly, MA, USA), designated as pMAL-eHis-LRP2F2 to pMAL-eHis-LRP2F7, respectively. The inserted cDNA fragments were expressed as a hybrid protein with maltose-binding protein (MBP). Recombinant CD69 as an MBP fusion protein was similarly prepared, as we described earlier [6]. Purification of the recombinant fusion proteins were performed using Ni-chelated columns (HiTrap Chelating, Amersham Biosciences, Piscataway, NJ, USA), in accordance with the manufacturer's instructions.
Western blotting
Western blotting was performed as described previously [6]. Briefly, 5 μg of each purified protein was separated by 12.5% SDS–PAGE and then transferred onto a nitrocellulose membrane. After blocking with PBS containing 1% BSA and 0.1% Tween 20 (PBS-BT) for 1 hour, the membranes were washed in PBS with 0.1% Tween 20 for 30 min. Then each membrane was incubated for 1 hour with each serum sample, which had been previously diluted 1:100 with PBS-BT and preincubated with 20 μg/ml of bacterial lysate containing nonrecombinant pMAL™-c product for 2 hours at room temperature. The membrane was washed as before, and the bound antibodies were put to react with horseradish-peroxidase-conjugated goat antihuman IgG (Zymed Laboratories, San Francisco, CA, USA) diluted 1:2000 with PBS-BT for 30 min. The bound antibodies were visualized with diaminobenzidine.
Laboratory findings and statistical analysis
Laboratory findings are expressed as the mean ± standard error of the mean. The Mann–Whitney U test and Fisher's exact test were used to test the significance of differences between the laboratory findings for the patients with and without anti-LRP2 autoAbs. P values of less than 0.05 were considered to be statistically significant. The proteinuria was measured semiquantitatively by Urieflet S (Arkray, Inc, Osaka, Japan), in which the degree of proteinuria of more than approximately 30, 50, 70, and 100 mg/dl was expressed as 1+, 2+, 3+ and 4+, respectively. The degrees of proteinuria were 1+ in four, 2+ in two, and 3+ in two of the eight proteinuria-positive patients with RA.
Results
Expression of the recombinant LRP2 molecules
To investigate autoepitopes of LRP2, we prepared recombinant LRP2 proteins. Since LRP2 is such a huge molecule, it was difficult to search all autoepitopes on the entire molecule in Escherichia coli. Therefore, we focused on approximately one-third of the entire region, which corresponded to the proximal end of its extracellular region. This region contains the CD69-homologous amino acid sequence and one of the LDLR domains, which were thought to bind various ligands such as apolipoprotein Eβ very-low-density lipoproteins, lactoferrin, and aprotinin [17] (Fig. 1). We divided the region with 1273 amino acid residues into six regions, F2–F7, and amplified cDNA for each region by RT-PCR (cDNAF2–cDNAF7). The nucleotide sequences of each amplified DNA fragment were confirmed to be identical to those reported previously [18]. They were expressed as an MBP fusion protein, recombinant LRP2 (rLRP2). The produced and purified proteins of rLRP2-F2, -F3, and -F7, and rCD69 showed their expected molecular weights on staining with Ponceau S after SDS–PAGE separation and transfer to the membrane (Fig. 2, upper panel). In the case of rLRP2-F4, -F5, and -F6, the molecular weights of the produced fusion proteins were approximately 10 kDa heavier than expected. This slowed mobility in the SDS gels is possibly due to aberrant binding of SDS or additional translation of the LacZ-α gene downstream of the multiple cloning sites. However, full expression of each of the rLRP2-F2 to -F7 regions was confirmed by positive staining of its C-terminal histidine tag with horseradish-peroxidase-conjugated nickel triacetoacid on Western blotting (data not shown). Therefore, we judged that they were good enough to be used to investigate the reactivity of serum samples.
Figure 1.
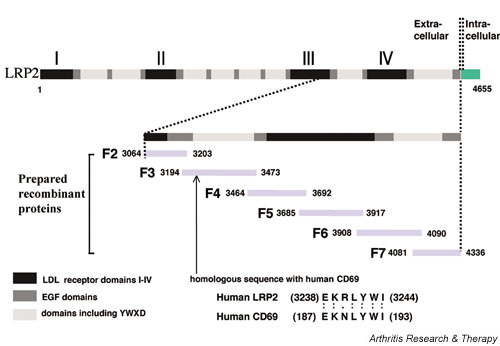
Map of low-density-lipoprotein-receptor-related protein 2 (LRP2). Six gene fragments that, with overlaps, covered approximately one-third of the extracellular region of human LRP2 were obtained by RT-PCR. They were then expressed as a maltose-binding protein (MBP) fusion protein. EGF, epidermal growth factor; LDL, low-density lipoprotein.
Figure 2.
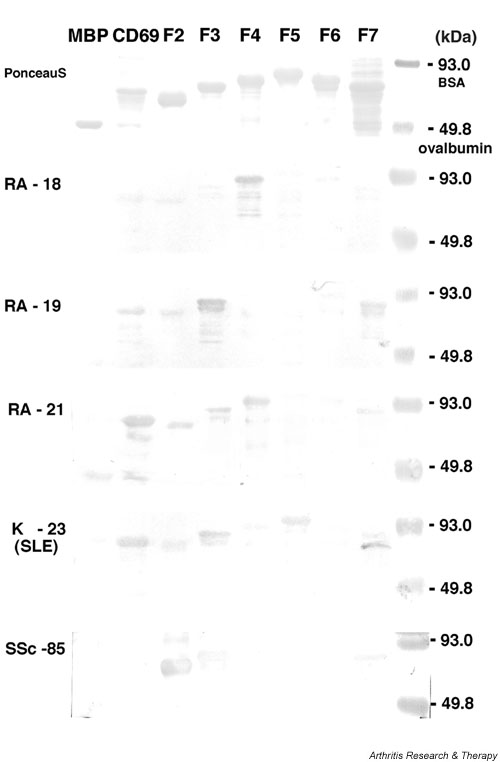
Recombinant proteins and representative results of Western blotting. Recombinant LRP2 F2–F7 fragments, CD69, and maltose-binding protein (MBP) were separated by 12.5% SDS–PAGE and were transferred onto nitrocellulose membranes. The membranes were stained with Ponceau S (top panel) or reacted with serum samples diluted at 1:100 (lower five panels). Representative results from patients with RA (RA-18, RA-19 and RA-21), SLE (K-23), and SSc (SSc-85) are shown. RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Reactivity of the sera of patients with systemic autoimmune diseases to the rLRP2 proteins
Using the rLRP2 prepared as above, we used Western blotting to investigate whether autoAbs to rLRP2 were present in sera of patients with systemic autoimmune diseases. We detected IgG-type autoAbs to at least one fragment of the rLRP2 molecules in 41 (87%) of the 47 patients with RA, 12 (40%) of the 30 patients with SLE, 7 (35%) of the 20 patients with SSc, 3 (15%) of the 20 patients with OA, 1 (3%) of the 30 patients with Behçet's disease, and 4 (5%) of the 75 healthy donors. Representative results of the Western blotting in RA, SLE, and SSc are shown in Fig. 2.
The reactivities of the positive serum samples are summarized in Table 1. One serum sample recognized 2.7 fragments on average and the epitope(s) in the F3 region were recognized most frequently. The F3 region of LRP2 contains continuous seven amino acid residues homologous to CD69, as shown in Fig. 1, and in our previous study the residues were found to be dominant autoepitopes of CD69 in RA [6]. We therefore investigated whether autoAbs to rLRP2-F3 were directed toward the CD69-homologous amino acid sequence by investigating reactivity to the rCD69 molecule in Western blotting. As shown in Table 1, all of the 14 anti-CD69 autoAb+ serum samples reacted to rLRP2-F3; however, 23 (62%) of the 37 anti-rLRP2-F3 autoAb+ samples did not react to rCD69 in RA. Including all the disease categories and healthy donors tested, 62% of the anti-rLRP2-F3 autoAb+ samples did not react with rCD69. This indicates that there are multiple autoepitopes on the F3 region, one of which is the CD69-homologous epitope, as mentioned above.
Table 1.
Reactivity of serum samples to the CD69 and to fragments of LRP2
| Recombinant protein | ||||||||
| LRP2 | ||||||||
| Disease | No. of sera | CD69 | F2 | F3 | F4 | F5 | F6 | F7 |
| RA (n = 47) | ||||||||
| 6 | - | - | + | - | - | - | + | |
| 6 | - | - | + | - | - | - | - | |
| 4 | + | + | + | + | + | + | + | |
| 3 | + | - | + | + | - | + | + | |
| 3 | + | - | + | - | - | - | + | |
| 2 | - | + | + | - | - | + | + | |
| 2 | + | + | + | - | - | - | - | |
| 2 | - | - | + | - | + | - | + | |
| 2 | - | - | + | + | - | - | - | |
| 2 | - | - | + | - | - | + | - | |
| 2 | - | - | - | + | - | - | - | |
| 1 | + | - | + | + | + | + | + | |
| 1 | + | - | + | - | + | - | + | |
| 1 | - | - | + | - | + | + | + | |
| 1 | - | - | + | + | - | + | - | |
| 1 | - | - | - | + | + | + | - | |
| 1 | - | - | - | - | + | + | + | |
| 1 | - | - | + | - | + | - | - | |
| Total sera | 41 | |||||||
| Positive reactions [No. (%)] | 14 (34) | 8 (20) | 37 (90) | 14 (34) | 12 (29) | 16 (39) | 24 (58) | |
| SLE (n = 30) | ||||||||
| 6 | - | - | - | - | + | - | - | |
| 2 | - | - | - | - | + | + | - | |
| 2 | - | - | - | + | - | - | - | |
| 1 | + | + | + | - | + | - | + | |
| 1 | - | - | - | - | - | - | + | |
| Total sera | 12 | |||||||
| Positive reactions [No. (%)] | 1 (8) | 1 (8) | 1 (8) | 2 (17) | 9 (75) | 2 (17) | 2 (17) | |
| SSc (n = 20) | ||||||||
| 2 | - | + | - | - | - | - | - | |
| 2 | - | - | + | - | - | - | - | |
| 2 | - | + | + | - | - | - | - | |
| 1 | - | - | + | - | - | - | + | |
| Total sera | 7 | |||||||
| Positive reactions [No. (%)] | 0 (0) | 4 (57) | 5 (71) | 0 (0) | 0 (0) | 0 (0) | 1 (14) | |
| OA (n = 20) | ||||||||
| 1 | + | + | + | - | - | - | + | |
| 1 | - | - | + | - | - | - | + | |
| 1 | - | - | - | - | - | + | - | |
| Total sera | 3 | |||||||
| Positive reactions [No. (%)] | 1 (33) | 1 (33) | 2 (67) | 0 (0) | 0 (0) | 1 (33) | 2 (67) | |
| None (healthy controls) (n = 75) | ||||||||
| 2 | - | - | + | - | - | - | + | |
| 2 | - | - | - | - | + | - | - | |
| Total sera | 4 | |||||||
| Positive reactions [No. (%)] | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 2 (50) | 0 (0) | 2 (50) | |
LRP2 = low-density-lipoprotein-receptor-related protein 2; OA = osteoarthritis; RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; SSc = systemic sclerosis.
In the patients with SLE, the F3 region was not the dominant epitope region. Instead, rLRP2-F5 was recognized by 9 (75%) of the 12 anti-rLRP2 autoAb+ serum samples, and recognition of other fragments was not more than 25% (Table 1). One serum sample recognized 1.5 fragments on average. Thus the distribution and numbers of the recognized autoepitopes appeared different from those in RA. In the patients with SSc and those with OA, the main epitope appeared to be located in the F3 region, but the percentage of serum samples that recognized rLRP2-F3 was lower than in RA.
Laboratory findings for anti-rLRP2 autoAb+ and autoAb- patients with RA
Since anti-rLRP2 autoAbs were detected most frequently in the sera of patients with RA, we first compared the laboratory findings for the anti-rLRP2 autoAb+ and autoAb- patients with RA. Two values of clinical findings differed significantly between the two groups. First, the anti-rLRP2 autoAb+ patients displayed proteinuria more frequently. Within the anti-rLRP2 autoAb+ population, patients who had positive tests for anti-rLRP2-F4, -F5, or -F6 had proteinuria more frequently (Table 2). Second, the serum levels of IgA were significantly higher in the anti-rLRP2-F6 autoAb+ patients than in the autoAb- ones (373.6 ± 170.6 mg/dl vs 244.2 ± 105.6 mg/dl, P = 0.007). The peripheral lymphocyte counts, white blood cell (WBC) counts, platelet counts, rheumatoid factors (RFs), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and serum levels of IgG and IgM did not differ between the two groups (data not shown).
Table 2.
Correlation between the presence of anti-LRP2 autoantibodies and of proteinuria in patients with rheumatoid arthritis
| Proteinuria in patients | ||
| Autoantibody | Absent (n = 39) [No. (%)] | Present (n = 8) [No. (%)] |
| Anti-F2 (-) | 33 (85) | 6 (75) |
| (+) | 6 (15) | 2 (25) |
| Anti-F3 (-) | 9 (23) | 1 (12) |
| (+) | 30 (77) | 7 (88) |
| Anti-F4 (-) | 31 (80) | 2 (25) |
| (+) | 8 (20) | 6 (75)a |
| Anti-F5 (-) | 32 (82) | 3 (38) |
| (+) | 7 (18) | 5 (62)b |
| Anti-F6 (-) | 29 (74) | 2 (25) |
| (+) | 10 (26) | 6 (75)c |
| Anti-F7 (-) | 19 (49) | 4 (50) |
| (+) | 20 (51) | 4 (50) |
aP = 0.005, bP = 0.018, cP = 0.013 (Fisher's exact probability test). LRP2, low-density-lipoprotein-receptor-related protein 2.
Similarly, we compared the laboratory findings for the anti-rLRP2 autoAb+ and autoAb- patients in SLE and SSc (in SLE, positivity for proteinuria; titers of anti-dsDNA autoAbs, anti-Sm autoAbs, and RF; WBC, lymphocyte, and platelet counts; serum levels of CRP, IgG, and IgM; and ESR; and, in SSc, positivity of proteinuria; titers of anti-nuclear antibodies, anti-Scl-70 autoAbs, and RF; WBC, lymphocyte, and platelet counts; serum levels of CRP, IgG, and IgM; and ESR). However, no significant difference was found (data not shown).
Discussion
Our results can be summarized as follows:
1. Anti-rLRP2 autoAbs were detected in 87% of the RA patients tested, less frequently in SLE, SSc, and OA, and only rarely in Behçet's disease and healthy donors.
2. Multiple autoepitopes were identified on the LRP2 molecule.
3. Almost all the anti-LRP-2+ RA serum samples reacted to the F3 fragment, which contains an amino acid sequence homologous to CD69, and 71% of the anti-LRP2+ SSc serum samples reacted to the F3 fragment, but only 8% of the anti-LRP-2+ SLE serum samples reacted to the F3 fragment.
4. All of the tested anti-CD69+ serum samples reacted to F3, but only 38% of the anti-F3+ serum samples reacted to the rCD69.
5. Anti-F4+, anti-F5+, and anti-F6+ patients with RA had proteinuria more frequently than those with negative results for these antibodies, and the serum IgA level was significantly higher in anti- F6+ patients than in the negative group in RA.
Regarding the first point, we had already shown that the anti-CD69 autoAbs reacted to LRP2 [6]. Further, autoAbs to LRP2 were detected in 50% of patients with autoimmune thyroiditis [11]. These findings encouraged us to investigate autoAbs to LRP-2 in systemic autoimmune diseases. We found that 87% of the tested RA serum samples reacted to at least one fragment of LRP2. Since our recombinants covered only about one-third of the LRP-2 molecule and would not express conformational epitopes, the frequency of the autoAb to LRP2 might be found to be higher than 87% if the whole and/or native molecule of LRP2 were available. In the comparison according to disease category, the anti-LRP2 autoAbs were detected most frequently in RA, less frequently in SLE, SSc, and OA, and only rarely in Behçet's disease. The high prevalence of the anti-LRP2 autoAbs in RA indicates that this autoAb is probably a marker for RA, even though it is not highly specific for RA. In addition, autoAbs to LRP2 were found more frequently in patients with OA (15%) than in healthy donors (5%). Although more patients with OA should be studied, it seems likely that autoimmunity may be involved in the pathogenesis of OA, since various autoAbs have been reported in OA recently [19,20].
Regarding points 2 to 4, the recognition of multiple epitopes indicates that the anti-LRP2 autoimmunity is antigen-driven. Previously, we showed that about 30% of the serum samples from RA patients carried autoAbs to CD69 and that almost all of the positive samples reacted to the epitope shared by CD69 and LRP2 (EKNLYWI in CD69 and EKRLYWI in LRP2) [6]. The epitope in LRP2 is located in the F3 region. In our study, in RA, 37 (90%) of the 41 serum samples tested reacted to rLRP2-F3, showing that the F3 region contains dominant epitopes of LRP2. However, only 14 of the 37 anti-rLRP2-F3+ samples reacted to CD69 and all 14 of the anti-CD69+ samples reacted to rLRP2-F3. These two observations taken together indicate that there are at least two epitopes within the F3 region and that one of them is the shared epitope of EKRLYWI. Considering that most of the anti-CD69 autoAb+ serum samples reacted exclusively to the shared epitope and that LRP2 has multiple epitopes, we think that the anti-CD69 autoAb may be among the anti-LRP-2 autoAbs in RA.
Regarding point 5, we found that possession of the anti-LRP2 autoAbs, in particular anti-rLRP2-F4, -F5, or -F6 autoAbs, was associated with the presence of proteinuria in RA. The patients with positive proteinuria had no past history of renal diseases before the onset of RA. Further, the use of antirheumatic drugs, including nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, and steroids, were similar and produced no significant difference between proteinuria-positive and -negative patients (data not shown). We deduce that the proteinuria is related to the disease process of RA. Interestingly, LRP2 is a pathogenic autoantigen that causes experimental glomerulonephritis in rats, Heymann's nephritis [10]. Further, the F4, F5, and F6 regions corresponded to the entire fourth LDLR domain of LRP2, which is thought to be a ligand-binding region (see Fig. 1). This suggests that the autoimmunity to LRP2 may cause the proteinuria by immunological attack of LRP2. However, no significant correlation between proteinuria and the anti-LRP2 autoAbs was found in SLE, even though nephritis is one of the major manifestations of SLE. Therefore, the autoimmunity to LRP2 is probably not a critical factor for lupus nephritis, and other factors, such as anti-DNA autoAbs, would be more important. Further studies are needed to elucidate the different mechanisms of proteinuria between RA and SLE.
Conclusion
Our study indicated that LRP2 was a major autoantigen in systemic autoimmune diseases. The anti-LRP2 autoantibodies may play pathological roles by affecting functions of LRP2
Competing interests
None declared.
Abbreviations
autoAb = autoantibody; BP = base pairs; BSA = bovine serum albumin; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; LDLR = low-density-lipoprotein receptor; LRP(1, 2) = low-density-lipoprotein-receptor-related protein (1, 2); MBP = maltose-binding protein; OA = osteoarthritis; PBS = phosphate-buffered saline; RA = rheumatoid arthritis; RF = rheumatoid factor; rLRP2 = recombinant LRP2; SSc = systemic sclerosis; SLE = systemic lupus erythematosus; WBC = white blood cell.
Acknowledgments
Acknowledgements
This work was supported by in part by grants-in-aid from the Ministry of Health and Welfare and the Ministry of Education, Science, and Culture of Japan, and the Japan Rheumatism Foundation.
References
- Winfield JB, Mimura T. Pathogenetic significance of antilymphocyte autoantibodies in systemic lupus erythematosus. Clin Immunol Immunopathol. 1992;63:13–16. doi: 10.1016/0090-1229(92)90085-3. 1591875. [DOI] [PubMed] [Google Scholar]
- Sakane T, Steinberg AD, Reeves JP, Green I. Studies of immune functions of patients with systemic lupus erythematosus. T-cell subsets and antibodies to T-cell subsets. J Clin Invest. 1979;64:1260–1269. doi: 10.1172/JCI109581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C, Schlossman SF. Antilymphocyte antibodies and systemic lupus erythematosus. Arthritis Rheum. 1987;30:225–228. doi: 10.1002/art.1780300215. [DOI] [PubMed] [Google Scholar]
- Czyzyk J, Fernsten P, Shaw M, Winfield JB. Cell-type specificity of anti-CD45 autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 1996;39:592–599. doi: 10.1002/art.1780390408. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kurokawa M, Kobata T, Oki S, Azuma M, Tohma S, Inoue T, Yamamoto K, Nishioka K, Kato T. Autoantibodies to T cell costimulatory molecules in systemic autoimmune diseases. J Immunol. 1999;162:4328–4335. [PubMed] [Google Scholar]
- Yu X, Matsui T, Otsuka M, Sekine T, Yamamoto K, Nishioka K, Kato T. Anti-CD69 autoantibodies cross-react with low density lipoprotein receptor-related protein 2 in systemic autoimmune diseases. J Immunol. 2001;166:1360–1369. doi: 10.4049/jimmunol.166.2.1360. [DOI] [PubMed] [Google Scholar]
- Hussain MM. Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front Biosci. 2001;6:D417–D428. doi: 10.2741/hussain1. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol. 2001;280:F562–F573. doi: 10.1152/ajprenal.2001.280.4.F562. [DOI] [PubMed] [Google Scholar]
- Willnow TE, Hilpert J, Armstrong SA, Rohlmann A, Hammer RE, Burns DK, Herz J. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci USA. 1996;93:8460–8464. doi: 10.1073/pnas.93.16.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D, Farquhar MG. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci USA. 1982;79:5557–5581. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Chiovato L, Friedlander JA, Latrofa F, Pinchera A, McCluskey RT. Serum antibodies against megalin (GP330) in patients with autoimmune thyroiditis. J Clin Endocrinol Metab. 1999;84:2468–2474. doi: 10.1210/jcem.84.7.5837. [DOI] [PubMed] [Google Scholar]
- Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Criteria for diagnosis of Behcet's disease. International Study Group for Behçet's Disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, Howell D, Kaplan D, Koopman W, Longley S, Mankin H, Mcshane DJ, Medsger TJ, Meenan R, Mikkelsen W, Moskowitz R, Murphy W, Rothschild B, Segal M, Sokoloff L, Wolfe F. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- Orlando RA, Exner M, Czekay RP, Yamazaki H, Saito A, Ullrich R, Kerjaschki D, Farquhar MG. Identification of the second cluster of ligand-binding repeats in megalin as a site for receptor-ligand interactions. Proc Natl Acad Sci USA. 1997;94:2368–2373. doi: 10.1073/pnas.94.6.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalm G, Murray E, Crumley G, Harazim W, Lundgren S, Onyango I, Ek B, Larsson M, Juhlin C, Hellman P, Davis H, Akerstrom G, Rask L, Morse B. Cloning and sequencing of human gp330, a Ca(2+)-binding receptor with potential intracellular signaling properties. Eur J Biochem. 1996;239:132–137. doi: 10.1111/j.1432-1033.1996.0132u.x. [DOI] [PubMed] [Google Scholar]
- Tsuruha J, Masuko-Hongo K, Kato T, Sakata M, Nakamura H, Sekine T, Takigawa M, Nishioka K. Autoimmunity against YKL-39, a human cartilage-derived protein in patients with osteoarthritis. J Rheumatol. 2002;29:1459–1466. [PubMed] [Google Scholar]
- Tsuruha J, Masuko-Hongo K, Kato T, Sakata M, Nakamura H, Nishioka K. Implication of cartilage intermediate layer protein (CILP) in cartilage destruction in subsets of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001;44:838–845. doi: 10.1002/1529-0131(200104)44:4<838::AID-ANR140>3.3.CO;2-3. [DOI] [PubMed] [Google Scholar]


