Abstract
An epithelial-mesenchymal transition (EMT) characterizes the progression of many carcinomas and it is linked to the acquisition of an invasive phenotype. Given that the tumor microenvironment is an active participant in tumor progression, an important issue is whether a reactive stroma can modulate this process. Using a novel EMT model of colon carcinoma spheroids, we demonstrate that their transforming-growth factor-β1 (TGF-β)-induced EMT is accelerated dramatically by the presence of activated macrophages, and we identify tumor necrosis factor-α (TNF-α) as the critical factor produced by macrophages that accelerates the EMT. A synergy of TNF-α and TGF-β signaling promotes a rapid morphological conversion of the highly organized colonic epithelium to dispersed cells with a mesenchymal phenotype, and this process is dependent on enhanced p38 MAPK activity. Moreover, exposure to TNF-α stimulates a rapid burst of ERK activation that results in the autocrine production of this cytokine by the tumor cells themselves. These results establish a novel role for the stroma in influencing EMT in colon carcinoma, and they identify a selective advantage to the stromal presence of infiltrating leukocytes in regulating malignant tumor progression.
INTRODUCTION
Many epithelial tumors undergo an epithelial-mesenchymal transition (EMT) that facilitates their invasion. The EMT is also an essential component of embryonic development, tissue remodeling, and wound repair (reviewed in Arias, 2001; Thiery, 2002). During this transition, the epithelial phenotype, characterized by strong cell-cell junctions and polarity, is replaced by a mesenchymal phenotype, with reduced cell-cell interactions, a fibroblastic morphology and increased motility. Given the importance of the EMT in carcinoma progression, there is considerable interest in understanding the mechanisms that contribute to this complex process. Although the exact mechanisms that underlie the EMT have not been elucidated, TGF-β has been implicated as a key inducer of the process (Oft et al., 1998; Portella et al., 1998; Lehmann et al., 2000; Bhowmick et al., 2001a; Ellenrieder et al., 2001; Fujimoto et al., 2001). TGF-β stimulates proliferation of many cell types, particularly those of mesenchymal origin, and it is also a potent inhibitor of epithelial cell proliferation. Contrary to an early ascribed role as a tumor suppressor (Markowitz and Roberts, 1996), TGF-β has been found to be abundantly expressed in many epithelial tumors and it acts in both an autocrine manner on the tumor cells themselves and as a paracrine modulator of the stroma (reviewed in Gold, 1999; de Caestecker et al., 2000; Yue and Mulder, 2001). In colon carcinoma, TGF-β also acts differently depending on the differentiation stage of the tumor, in general by switching from an early inhibitor of proliferation to a stimulator of growth and invasion during tumor progression (Hsu et al., 1994).
TGF-β has been implicated as a major factor in the EMT, but this is likely to be a “multifactorial” process. Moreover, the stroma is an active participant in tumor progression, with complex interactions between tumor and stromal cells enhancing tumorigenesis by supporting cancer cell proliferation, survival, and migration (reviewed in Liotta and Kohn, 2001; Tuxhorn et al., 2001), prompting the question of whether signals from this source may modulate EMT sensitivity. One feature of the reactive stromal phenotype of many solid tumors is the influx of inflammatory cells, such as tumor infiltrating lymphocytes (TILs) and macrophages. Indeed, focal macrophage infiltration has been linked to increased angiogenesis in human breast and colorectal cancer (Leek et al., 1996; Etoh et al., 2000). Lymphocyte infiltration is more common in primary colon carcinomas than in metastases (Barth et al., 1996) and it was recently shown that overexpression of TGF-β itself can induce a local secretion of immunomodulating cytokines in a rat colon carcinoma model (Schiott et al., 2000), through increased leukocyte infiltration. Yet, the functional significance of cytokines produced in situ by stromal and tumor cells in human colon carcinoma is still unclear. Some functions are likely to inhibit tumor growth, such as the release of cytotoxic factors or the initiation of an immune response. However, the chemotactic recruitment of leukocytes to tumors suggests that these cells provide a selective advantage. Macrophages, in particular, have been shown to secrete growth factors that induce angiogenesis (Sunderkotter et al., 1994) but their effects on tumor cells are not well understood. We reasoned that cytokine release from these cells might serve to enhance the invasive step in colorectal carcinogenesis, through defined signaling pathways. Thus, this study was designed to determine whether stromally derived factors are capable of facilitating EMT and to understand the mechanisms involved.
Unfortunately, it is not possible to follow EMT in human tumors either temporally or spatially because of the great diversity of cellular organization displayed by neoplasms in vivo (Thiery, 2002). To address the role of stromal factors in EMT, therefore, we characterized a novel EMT model of colon carcinoma. Specifically, we report that LIM 1863 organoids undergo an EMT conversion from a well-differentiated spheroid structure to a migratory monolayer phenotype in response to TGF-β. Moreover, we found that a product of activated macrophages, which we identified as TNF-α, accelerates the TGF-β–mediated EMT dramatically. Furthermore, exposure of organoids to TNF-α results in the establishment of an autocrine loop of TNF-α that is dependent on ERK activation. Our findings reveal that TNF-α accelerates the EMT by a mechanism that involves p38 MAPK activation. Overall, the finding that stromally derived TNF-α can synergize with TGF-β to modulate a critical step in colon carcinogenesis has important implications for our understanding of how macrophages contribute to tumor development.
MATERIALS AND METHODS
Cell Culture
LIM 1863 cells (Whitehead et al., 1987; Bates et al., 1994) were routinely grown in RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 5% FCS. Clone A and HL-60 cell lines were cultured in RPMI 1640 plus 10% FCS.
Antibodies and Reagents
Recombinant human TNF-α and human TGF-β1 were purchased from R&D Systems (Minneapolis, MN), as were the neutralizing anti–hTNF-α mAb and isotype matched control IgG1. Polyclonal antibodies directed against p38 MAPK and E-cadherin were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The N-cadherin antibody was purchased from BD Transduction Laboratories (San Diego, CA). Anti-ERK, phospho-ERK, and phosphop38 MAPK antibodies were obtained from Cell Signaling Technology (Beverly, MA) and the antibody against tubulin from Sigma Co. (St. Louis, MO).
Coculture Assay
LIM 1863 organoids were seeded into the lower wells of Costar Transwell plates (Corning, NY) in culture medium, with or without TGF-β1 at a final concentration of 2 ng/ml. Suspensions of HL-60 cells, either untreated or after a 2-h pretreatment with 20 nM phorbol ester (PMA), were added to the upper chambers. Medium alone was added to control wells. After 24 h the upper chambers containing the HL-60 cells were removed, and organoids were photographed using phase contrast optics. For the antibody inhibition experiments, cocultures were established as described, with the addition of anti–hTNF-α antibody (1 μg/ml) or an isotype matched control (1 μg/ml) to duplicate wells at the time of seeding. After 24 h, the upper chambers were again removed and morphological changes assessed by photography.
Cytokine and Inhibitor Assays
LIM 1863 cells were seeded in 24-well plates with either TNF-α (10 ng/ml) or TGF-β1 (2 ng/ml), or a combination of both. Morphological changes in the organoid phenotype were assessed by light microscopy. For inhibition assays, cells were pretreated for 20 min with the MEK inhibitor PD98059 (20 μM) or 1 h with the p38 MAPK inhibitor SB203580 (40 μM) and then seeded in the presence of cytokines. Cells were also pretreated with the PI3K inhibitors Wortmannin (200 nm) or LY294002 (2.5 μM) for 2 h, before the addition of cytokines and subsequent culture in 24-well plates. All inhibitors were purchased from Calbiochem Corp. (San Diego, CA).
Migration Assay
Migration assays were performed by assessing the ability of cells to migrate toward NIH-3T3–conditioned medium using laminincoated 6.5-mm Costar Transwell chambers (8-μm pore size) after cytokine treatment. LIM 1863 organoids were resuspended in medium containing either no cytokine, TNF-α, TGF-β, or the combination of cytokines and added to each well. Conditioned NIH-3T3 medium was added to the bottom wells of the chambers, supplemented with the corresponding cytokine. After 3 d, cells were removed from the upper face of the filters using cotton swabs, and the cells that had migrated to the lower surface were fixed in methanol. Filters were mounted onto microscope slides using Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and invasion quantified by visual counting using fluorescence microscopy. The means of five individual fields selected at random were obtained for each well.
Immunoblotting
Cells were extracted in a Triton-X lysis buffer (1% Triton-X, 50 mM Tris, 150 mM NaCl) containing protease inhibitors (pepstatin, PMSF, aprotinin, leupeptin) for 1 h. Extracts were clarified by centrifugation. Nuclear isolation and extraction were carried out as described previously (Bates et al., 1994). Whole cell lysates and nuclear isolates were analyzed by SDS-PAGE, and proteins were transferred to nitrocellulose by electrophoresis. Residual protein sites were blocked in Tween/Tris-buffered saline (TBST) containing 5% skim milk. The filters were incubated with primary antibodies in TBST plus 2.5% skim milk at recommended concentrations for 1 h and developed using enhanced chemical luminescence (ECL).
PCR
RNA was prepared using the RNeasy Mini Kit (Qiagen, Valencia CA). For reverse-transcription PCR (RT-PCR), the OneStep RT-PCR Kit (Qiagen) was used. The primers used to detect TNF-α were as follows: TNF-α Forward: 5′ CGAGTGACAAGCCTGTAGCC 3′; and TNF-α Reverse: 5′ GTTGACCTTGGTCTGGTAGG 3′. The PCR cycle protocol consisted of 35 cycles of 94°C for 1 min, 62°C for 1 min, and 72°C for 1 min.
The principle of real time quantitative PCR (RQ-PCR) has been described by Heid et al. (1996). RNA was prepared and reverse transcribed after DNAse1 treatment. The primers and probes used for TNF-α were designed using Primer Express software version 1.0, based on mRNA sequences obtained from the NCBI database. All reactions were performed in an ABI Prism 7700 Sequence Detection System (Perkin Elmer-Cetus Applied Biosystems). Reactions were carried out in triplicate in a 50-μl reaction volume containing 25 μl of 2× TaqMan PCR Master Mix, a 50 nM concentration of each forward and reverse primer, a 100 nM concentration of dual-labeled probe, and 1 μg of total cDNA. Conditions for all PCR reactions were as follows: 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 95°C for 10 s and 60°C for 1 min. All reactions were repeated in at least two separate experiments to ensure reproducibility of results. Normalization to GAPDH (housekeeping gene) was performed for each sample. Ct values were exported into a Microsoft Excel worksheet for calculation of fold changes according to the delta delta CT method.
The primers and dual-labeled probe (TET/TAMRA) are as follows: TNF-α Forward: 5′ ATCTTCTCGAACCCCGAGTGA 3′; and TNF-α Reverse: 5′ GAGCTGCCCCTCAGCTTG 3′; TNF-α Probe: 5′/5TET/AGCCTGTAGCCCATGTTGTAGCAAACC/36-TAM/3′.
RESULTS
EMT of LIM 1863 Organoids
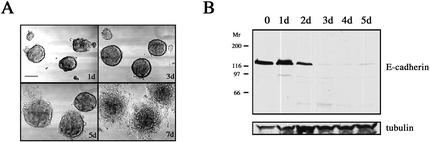
LIM 1863 cells are well-differentiated colon carcinoma cells that grow as structured spheroids, termed organoids, around a central lumen (Whitehead et al., 1987). Tight junctional complexes and epithelial polarity are hallmarks of this line that grows in suspension (Hayward and Whitehead, 1992; Bates et al., 1994). This remarkable degree of organization is not dependent on either exogenous basement membrane or culture within a three-dimensional matrix, unlike other spheroid tumor models (Weaver et al., 1997; Wang et al., 1998). The addition of TGF-β to LIM 1863 organoid cultures induced the phenotypical changes typical of EMT over a period of 5–7 d (Figure 1A). Although the organoids adhere to their substratum within 24 h of TGF-β treatment, it is not until 4–6 d later that cells “emerge” from the spheroid structures and migrate out as cellular sheets to form a monolayer (Figure 1A). Given that the LIM 1863 cell line switches from suspension culture to an adherent phenotype and must also overcome a highly organized threedimensional architecture, it is not surprising that these cells require a considerable time to undergo the transition.
Figure 1.
TGF-β induces EMT in LIM 1863 organoids. (A) TGF-β induces EMT. LIM 1863 organoids were seeded in the presence of TGF-β (2 ng/ml) and allowed to undergo transition in culture. Morphological changes were documented by light microscopy using phase contrast optics at 1, 3, 5, and 7 d after exposure to the cytokine. Bar, 150 μm. (B) E-cadherin loss characterizes EMT. Cell extracts were prepared over the time course shown after addition of TGF-β and immunoblotted with an E-cadherin–specific antibody. Relative molecular masses are shown to the left in kDa. Equal protein loading was confirmed by tubulin immunoblotting (bottom panel).
Loss of epithelial and gain of mesenchymal markers are widely used criteria to characterize bona fide EMT processes, though it has now emerged that changes in specific markers or gene expression may differ widely (Thiery and Chopin, 1999; Janda et al., 2002). However, one characteristic that is central to EMT, regardless of the cellular system, is loss of the adhesion molecule E-cadherin (Arias, 2001; Thiery, 2002). E-cadherin expression is necessary for the maintenance of the epithelial phenotype through the formation of adherens junctions and, importantly, there is a direct correlation between diminished E-cadherin expression and loss of the epithelial phenotype in vitro (Behrens et al., 1989). Further, E-cadherin is a target of the transcriptional repressor Snail, whose emerging role in EMT has identified it as a potential oncogenic regulator (Batlle et al., 2000; Cano et al., 2000). Our data showed that TGF-β treatment induced a loss of E-cadherin protein. Degradation products are discernable within 24 h, and complete downregulation is seen by 3 d (Figure 1B). In contrast, E-cadherin expression is not altered by culturing organoids in low-calcium medium (our unpublished results), a process that disrupts E-cadherin function and organoid structure although the cells remain in suspension (Bates et al., 1994). Taken together, TGF-β treatment of LIM1863 organoids results in a specific and rapid reduction in E-cadherin levels and concomittant induction of a mesenchymal phenotype.
Stromally Derived Factor(s) Augment TGF-β–directed EMT in Colon Carcinoma
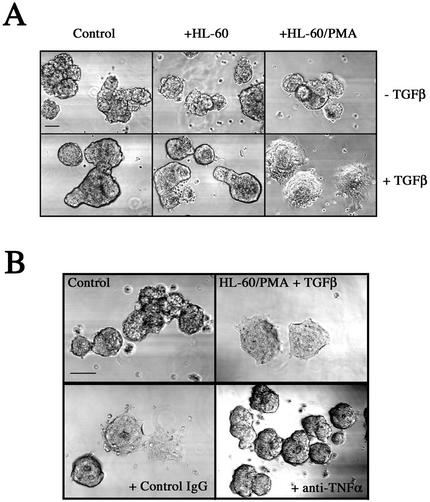
The long-term nature of EMT permitted the investigation of factors that may augment or promote the transition. Given that the tumor microenvironment is an active participant in tumor progression, an important issue is whether a reactive stroma can modulate this process. Therefore, we used the LIM 1863 model to examine the hypothesis that activated macrophages stimulate EMT. For this purpose, we cocultured organoids with HL-60 cells that were either unstimulated or activated by pretreatment with phorbol ester (PMA). Coculture with either resting or activated HL-60 cells in the absence of TGF-β had no effect on the organoid morphology of the LIM 1863 cells (Figure 2A, top panel). After addition of TGF-β, control organoids and those cultured with unactivated HL-60 cells became adherent after 24 h but with no spreading, with identical kinetics to that seen in Figure 1A. In stark contrast, TGF-β–treated organoids cultured in the presence of activated HL-60 cells underwent a rapid EMT, such that the cells were completely spread and flattened within 24 h (Figure 2A). These results infer that activated HL-60 cells secrete a soluble factor (or factors) that is responsible for cooperating with TGF-β to accelerate the EMT.
Figure 2.
Augmentation of LIM 1863 organoid EMT by a stromal factor. (A) Activated HL-60 cells secrete a factor that accelerates the EMT. LIM 1863 organoids were seeded in a coculture assay as described in MATERIALS AND METHODS. LIM 1863 cells were seeded in the absence (Control) or presence of HL-60 cells (+HL-60), or activated HL-60 cells that had been pretreated with PMA (+HL-60/PMA) as described in MATERIALS AND METHODS. Cells were cultured for 24 h in the absence or presence of TGF-β (top and bottom panels, respectively). Bar, 150 μm. (B) Anti–TNF-α antibody inhibits the stromally accelerated EMT. LIM 1863 cells were cultured in the presence of TGF-β for 24 h alone (Control), or in coculture with activated HL-60 cells (HL60/PMA + TGF-β). Either a neutralizing TNF-α antibody (1 μg/ml) or an isotype matched IgG control antibody was added to the cocultured cells as indicated. The degree of phenotypic transition was assessed by light microscopy. Bar, 150 μm.
TNF-α Is the Stromally Derived Factor that Synergizes with TGF-β
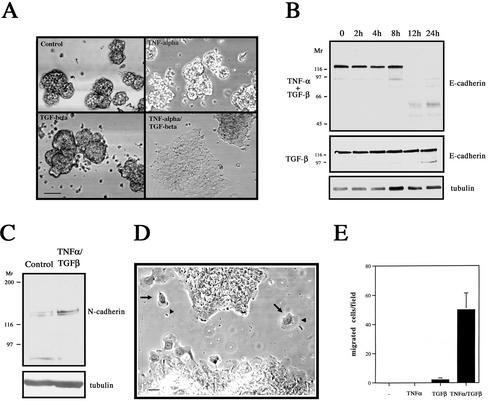
The activation of HL-60 cells and other macrophages induces the secretion of a wide variety of growth factors, such as interleukins and TNF-α. We focused on TNF-α because monocytes/macrophages are the largest source of TNF-α in the body (Papadakis and Targan, 2000) and PMA upregulates TNF-α in HL-60 cells (Lopez et al., 2000). Indeed, a TNF-α antibody inhibited the rapid EMT that is induced in the organoids by coculture with PMA-treated HL-60 cells and TGF-β (Figure 2B). This result strongly implicates TNF-α as the factor secreted by macrophages that is responsible for accelerating EMT. To substantiate this finding, we used recombinant human TNF-α. The addition of TNF-α to organoids in the absence of TGF-β had little or no effect on their morphology. However, the addition of both cytokines resulted in a rapid (24 h) and complete mesenchymal transition (Figure 3A). E-cadherin was again used as a marker of epithelial differentiation (Figure 3B). TGF-β treatment alone resulted in the appearance of degradation products at the 24-h time point, in agreement with the data presented in Figure 1B. Significantly, a time course analysis showed that E-cadherin expression was completely lost between 8 and 12 h in the presence of both cytokines, confirming that the addition of TNF-α rapidly accelerated the TGF-β–directed EMT response by these cells. Importantly, TNF-α treatment alone had no effect on E-cadherin protein levels (our unpublished results).
Figure 3.
TNF-α synergizes with TGF-β to promote EMT. (A) Recombinant human TNF-α recapitulates stromally derived TNF-α effect. LIM 1863 organoids were unstimulated (Control), treated with TNF-α (10 ng/ml), TGF-β (2 ng/ml) or the combination of cytokines for 24 h. The extent of morphological transformation was photographed under light microscopy. Bar, 150 μm. (B) Rapid and complete loss of E-cadherin during the TNF-α/TGF-β–induced EMT. Cell extracts were prepared over the time course shown after addition of TNF-α/TGF-β (top panel) or TGF-β alone (middle panel) and immunoblotted with an E-cadherin–specific antibody. Relative molecular masses are shown to the left in kDa. Equal protein loading was confirmed by tubulin immunoblotting (bottom panel). (C) Upregulation of the mesenchymal marker N-cadherin after EMT. Cell extracts from untreated organoids (Control) or cells treated with TNF-α/TGF-β for 24 h and immunoblotted with a N-cadherin–specific antibody. Relative molecular masses are shown to the left in kDa. Equal protein loading was confirmed by tubulin immunoblotting (bottom panel). (D) The EMT promotes a migratory phenotype in LIM 1863 cells. Photomicrograph of LIM 1863 cells after EMT induced by combination TNF-α/TGF-β treatment for 24 h. Individual migrating cells (arrows) exhibit broad lamellae (arrowheads). Bar, 10 μm. (E) The EMT induces chemotaxis. Chemotactic migration assay of LIM 1863 cells, treated with cytokines as indicated, for 3 d on laminin-coated Transwells toward conditioned NIH-3T3 medium. Data are expressed as the means and SDs of five individual fields randomly selected for each well.
Immunoblotting with the mesenchymal marker N-cadherin showed an upregulation of this protein after treatment with the two cytokines (Figure 3C), confirming this transition as a bona fide EMT process. In response to the EMT, individual LIM 1863 cells migrate out from the epithelial sheets, a behavior characteristic of a more invasive phenotype, and these motile cells exhibit broad lamellae (Figure 3D). To quantify the effects of TNF-α on migration, chemotaxis assays were performed (Figure 3E). As expected, control LIM 1863 organoids exhibited no migratory capacity because viable cells of this line only exist within the three-dimensional spheroid structure. Moreover, the addition of either TNF-α or TGF-β for 3 d did not induce significant migration (Figure 3E). The addition of both cytokines, however, triggered a robust increase in migration, substantiating the morphological observations shown in Figure 3D. Taken together, our data indicate that TNF-α cooperates with TGF-β to accelerate EMT and that this cooperation promotes a drastic and rapid disruption of organoid architecture. Further, the percentage of organoids undergoing EMT is 100%, because no cells remain in suspension and E-cadherin loss is complete.
TNF-α Induces Its Own Expression in an Autocrine Manner, Requiring ERK Activation
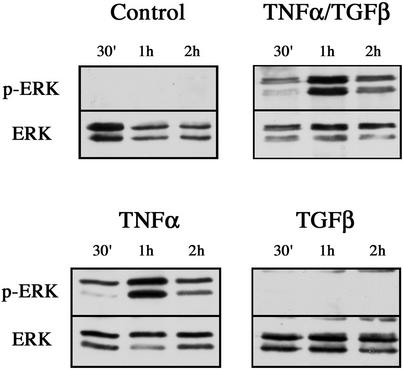
To elucidate the signaling pathways that are triggered by cytokine stimulation and that contribute to the EMT, we focused initially on the ERK pathway. This signaling cascade is known to be activated by TNF-α (Kyriakis and Avruch, 1996) and, importantly, has been implicated in directing epithelial cell plasticity and EMT induced by TGF-β (Ellenrieder et al., 2001; Zavadil et al., 2001). ERK 1/2 activation was assessed using a phospho-specific ERK antibody as shown in Figure 4. No activation of either ERK isoform was evident in control cells. In contrast, addition of both cytokines induced a robust activation of ERK 1/2. Phosphorylation of ERK 1 was evident within 30 min, and maximal activation of both isoforms was seen at 1 h. Moreover, TNF-α alone induced an ERK activation profile identical to that of both cytokines (Figure 4). Surprisingly, however, TGF-β failed to activate ERK over the 2-h time course. Thus it appears that TNF-α alone is responsible for a rapid activation of the ERK signaling pathway, raising the possibility that this activation is important for the accelerated EMT seen in the presence of this cytokine.
Figure 4.
ERK activation in response to cytokine stimulation. Cell extracts were prepared from untreated LIM 1863 organoids (Control), or cells treated with TNF-α, TGF-β, or the combination of cytokines, for the times indicated. ERK 1/2 activity was determined by immunoblotting with a phospho-specific ERK antibody (top panels). ERK protein expression was confirmed using an ERK antibody (bottom panels).
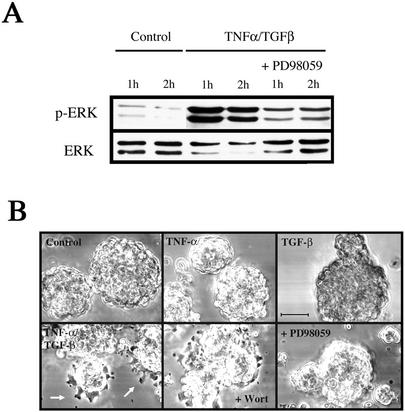
To investigate the functional consequences of ERK activation on the EMT, we used the MEK inhibitor PD98059. As shown in Figure 5A, this inhibitor prevented the burst of ERK activation that occurs after stimulation with TNF-α/TGF-β for 1 h. Subsequently, the effects of PD98059 on morphology were examined at 6 h after addition of cytokine, the time when the first discernible effects of the accelerated EMT can be observed (Figure 5B). At this time, organoids treated with both TNF-α and TGF-β were firmly attached to the substratum and individual cells could be seen emerging from the periphery of the organoids. However, treatment with PD98059 blocked the early transitional effects, completely preventing cellular adhesion and spreading. Over a longer time course (>24 h) the PD98059-treated cells underwent the EMT with kinetics similar to those treated with just TGF-β (our unpublished results). Given that TNF-α stimulation alone also induced the burst of ERK activity (Figure 4), but without spontaneous mesenchymal transition, the data indicate that ERK activation is necessary, but not sufficient, to promote the accelerated EMT process. In contrast, the EMT appears to be PI3K independent because neither wortmannin (Figure 5) nor LY294002 (our unpublished results) had any discernible effects on organoids treated with both cytokines.
Figure 5.
The ERK inhibitor PD98059 prevents the accelerated EMT induced by TNF-α. (A) PD98059 inhibits the 1-h peak of ERK activity induced by TNF-α treatment. Extracts were prepared from control LIM 1863 cells, or TNF-α/TGF-β–treated cells cultured in the absence or presence of the ERK inhibitor PD98059 for the times indicated. ERK 1/2 activation was determined using the phospho-specific ERK antibody (top panel). ERK protein expression was confirmed using an ERK antibody (bottom panel). (B) PD98059 prevents the accelerated EMT phenotype. Photomicrographs of LIM 1863 cells seeded in the cytokine assay for 6 h. Cells were untreated (Control) or stimulated with either TNF-α or TGF-β, or the combination of the two cytokines, as indicated. Individual cells can be seen emerging from the periphery of the organoids (arrows). Cells were pretreated with PD98059 for 20 min before stimulation with both cytokines and seeding in the assay (bottom left panel). The PI3K inhibitor wortmannin (Wort) was also used, after a 2-h pretreatment. Bar, 100 μm.
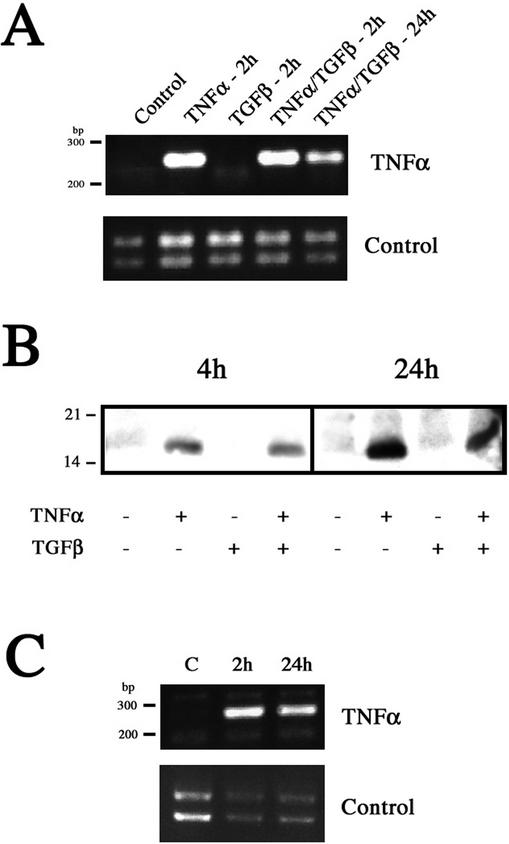
The ERK signaling cascade ultimately results in the activation of nuclear transcription factors, thereby altering gene expression. Although one possible explanation for the rapid EMT induction was that TNF-α stimulation, through ERK signaling, upregulates TGF-β expression, our results did not support this possibility (our unpublished results). Unexpectedly, we discovered that TNF-α induced its own expression, establishing an autocrine loop and the potential for constitutive TNF-α signaling (Figure 6). TNF-α expression was initially examined by RT-PCR (Figure 6A). Untreated LIM 1863 cells produced no TNF-α message. However, de novo synthesis of mRNA was observed 2 h after exposure to this cytokine, either alone or with TGF-β. TGF-β treatment alone had no effect. Significantly, these cells continued to produce TNF-α message at 24 h. To substantiate this finding, TNF-α protein levels were determined by immunoblotting, and these results corroborated the PCR data (Figure 6B). TNF-α protein was not detected in control LIM 1863 cells or in those cells treated only with TGF-β. However, exposure to TNF-α resulted in detectable levels of protein within 4 h. The possibility existed that this protein might be exogenous cytokine still bound to the cells. However, a comparative increase in TNF-α protein levels was seen at 24 h, confirming de novo protein synthesis. Importantly, we confirmed a functional role for autotropic TNF-α signaling by treating organoids with TNF-α for 24 h, thus establishing autocrine TNF-α production, and found that these cells also underwent an accelerated EMT in response to subsequent TGF-β stimulation (our unpublished results). This finding clearly demonstrates that autocrine TNF-α signaling is sufficient to promote a rapid EMT. Further, to ensure that autocrine production of TNF-α was not a cell-line–specific response, another colon carcinoma cell line (Clone A) was used (Figure 6C). These cells also induced new expression of mRNA for TNF-α after cytokine treatment, indicating that autocrine TNF-α production might be a more general response in colon cancer cells.
Figure 6.
TNF-α treatment induces its own expression in colon carcinoma cells. (A) RT-PCR for TNF-α was performed on RNA purified from untreated LIM 1863 cells (Control), cells treated with TNF-α or TGF-β for 2 h, and cells treated with both cytokines for 2 and 24 h, as described in MATERIALS AND METHODS. The predicted size of the PCR product is 254 base pairs. Control reactions were performed using integrin α6 primers (bottom panel). (B) TNF-α protein expression. Cell extracts were prepared from untreated, TNF-α–, TGF-β–, or TNF-α/TGF-β–treated LIM 1863 organoids harvested after 4 and 24 h. Extracts were analyzed by SDS-PAGE and immunoblotting with an anti–TNF-α antibody. Equal protein loading was confirmed by reprobing with tubulin (unpublished data). Relative molecular masses are shown to the left in kDa. (C) RT-PCR was performed as described above using RNA from Clone A colon carcinoma cells treated with TNF-α for 2 and 24 h. Control reactions were performed using integrin α6 primers (bottom panel).
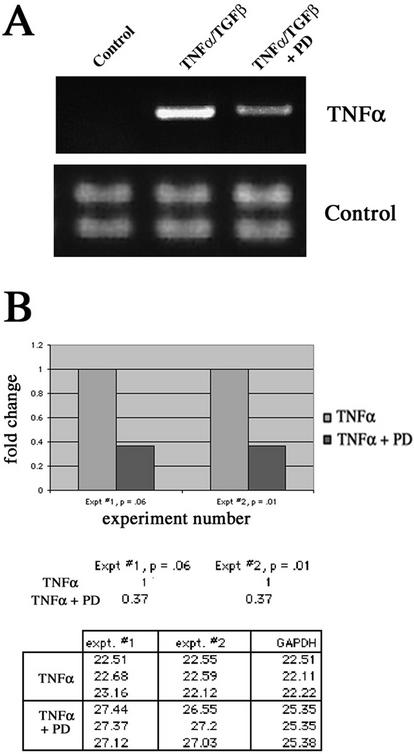
Given that TNF-α gene transcription can be regulated by ERK signaling (Zhu et al., 2000), we hypothesized that there may be a link between the peak of ERK activity seen after cytokine stimulation, and establishment of the autocrine loop. Indeed, RT-PCR indicated that treatment with the ERK inhibitor PD98059 substantially suppressed the de novo production of TNF-α message (Figure 7A). To quantify this result, we used real time quantitative PCR (Figure 7B). In two separate experiments, we observed a consistent 2.7-fold decrease in TNF-α message production in presence of the ERK inhibitor. Overall, our data reveal that TNF-α treatment of LIM 1863 cells results in autocrine TNF-α production and that this production is dependent, at least in part, on the activation of the ERK signaling cascade. Importantly, this finding does not preclude other roles for ERK activation in sensitizing the cells to the EMT-inducing effects of TGF-β.
Figure 7.
Inhibition of ERK activity reduces autocrine induction of TNF-α. (A) RT-PCR of untreated LIM 1863 cells (Control), or TNF-α/TGF-β–treated cells for 2 h in either the absence or presence (+PD) of the ERK inhibitor PD98059. Control reactions shown in bottom panel using integrin α6 primers. (B) Real time quantitative PCR (RQ-PCR) of cytokine-treated cells in the absence or presence of PD98059 for 2 h. Increases in fluorescence signal (ΔRn) from each PCR reaction were monitored in real time by the ABI Prism 7700 Sequence Detector. The fold change between treatments (a consistent 2.7-fold reduction in the presence of PD98059) in two separate experiments is represented graphically (top panel). Ct values, the PCR cycle at which a statistically significant difference in the ΔRn is first detected, are shown in the table. Ct values, when normalized to the internal reference gene (GAPDH), are inversely related to the magnitude of mRNA expression.
TNF-α Activates p38 MAPK Signaling to Promote EMT
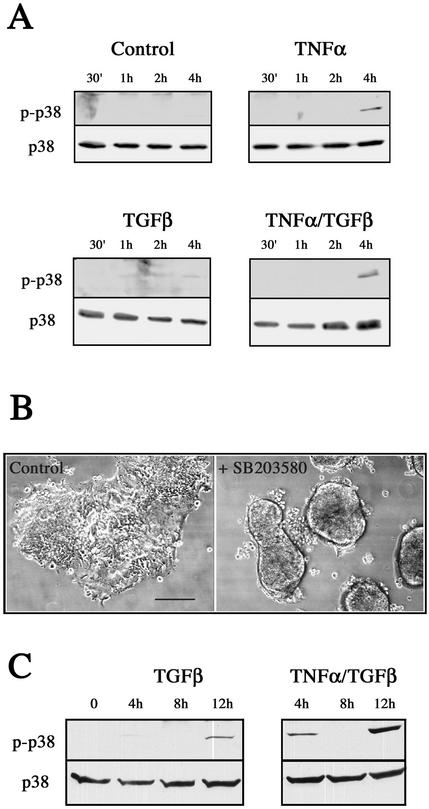
We sought to identify the signal pathways triggered by TNF-α in our model system that were required for this cytokine to facilitate the accelerated EMT. An apparent candidate was the p38 MAPK signaling pathway, which represents a convergence point for TNF-α and TGF-β signaling, because this pathway is responsive to both cytokines (Obata et al., 2000). Moreover, p38 activation has recently been described as necessary, but not sufficient, for EMT (Bhowmick et al., 2001b; Bakin et al., 2002; Yu et al., 2002). We therefore reasoned that elevated p38 MAPK activity, induced by TNF-α, might provide a mechanism by which the TGF-β/EMT effects were augmented. Immunoblotting with phospho-specific antibodies showed that p38 MAPK was indeed activated in response to TNF-α or TNF-α/TGF-β treatment (Figure 8A), and with distinct kinetics to that seen for ERK 1/2. In contrast to the ERK activation profile, p38 MAPK activity was not apparent until 4 h after TNF-α exposure. Further, TGF-β treatment alone had a negligible effect on p38 activity at this point. To characterize a functional role for this activation in the EMT, we used the specific p38 MAPK inhibitor SB203580 (Figure 8B). Treatment with SB203580 completely blocked the early (4–6 h) transitional effects by preventing organoid adhesion and spreading (our unpublished results), similar to that seen with the MEK inhibitor PD90859 (Figure 5B). EMT progression in inhibitor-treated cells was significantly retarded even after 24 h, as shown in Figure 8B. In addition, SB203580 also blocked the 24-h initial attachment of organoids stimulated with TGF-β alone (our unpublished results). Therefore, we investigated p38 MAPK activity over a longer time course and found that TGF-β alone activated p38 by 12 h (Figure 8C). Significantly, the addition of TNF-α led to a synergistic activation of p38, with a clear increase in activation seen at this time point in the presence of both cytokines. Taken together, our findings substantiate a role for p38 MAPK signaling in the EMT and demonstrate that elevated p38 activity in response to TNF-α stimulation accounts for the accelerated EMT process.
Figure 8.
p38 MAPK is activated in response to TNF-α and is required for the accelerated EMT. (A) p38 MAPK activation in response to cytokine stimulation. Cell extracts were prepared from untreated LIM 1863 organoids (Control) or cells treated with TNF-α, TGF-β, or the combination of cytokines, for the times indicated. p38 MAPK activity was determined by immunoblotting with a phospho-specific p38 MAPK antibody (top panels). p38 MAPK protein expression was confirmed using a p38 antibody (bottom panels). (B) SB203580 prevents the accelerated EMT phenotype. Photomicrographs of LIM 1863 cells seeded in the cytokine assay for 24 h. Control cells were treated with DMSO (left panel) or SB203580 (right panel) and stimulated with TNF-α/TGF-β. Bar, 150 μm. (C) TNF-α and TGF-β cause a synergistic activation of p38 MAPK. Cell extracts were prepared over the time course shown from cells treated with TGF-β alone or the combination of cytokines, as indicated. p38 MAPK activity was determined by immunoblotting as described above.
DISCUSSION
The multicellular nature of the EMT implies that its analysis in vitro requires cell systems such as spheroids that reiterate the epithelial phenotype. Indeed, in contrast to most traditional cell culture systems, spheroids provide a unique opportunity to recapitulate aspects of cell homeostasis and as such reflect in vivo tumor biology better (Bates et al., 2000). Much of our current understanding of the mechanisms and pathways that regulate EMT has arisen from the use of a handful of immortalized cell lines, such as EpH4/EpRas, NMuMG, MDCK, and MCF-10 (Reichmann et al., 1992; Oft et al., 1996; Lehmann et al., 2000; Bhowmick et al., 2001a; Schulze et al., 2001). Although these cell lines can manifest an epithelial phenotype and undergo EMT, they suffer from several limitations: genesis of an epithelial phenotype often requires culture in three-dimensional matrix, EMT usually takes several days to complete, and few common carcinoma cell types with a well-defined epithelial phenotype (such as colon cancer) can undergo EMT in vitro (Thiery, 2002). In this regard, our findings demonstrate clear advantages in the use of the LIM 1863 organoid cell line for studies on EMT, especially because the remarkable architecture of these highly differentiated colon carcinoma cells is intrinsic to the cells and does not require extraneous culture conditions. Moreover, as we have shown, these organoids are capable of undergoing an EMT that mimics the progression to invasive colon carcinoma.
The data we obtained using LIM 1863 spheroids reveal a novel role for stroma in stimulating EMT and in the genesis of invasive carcinoma. Specifically, we demonstrate that their TGF-β–induced EMT is accelerated dramatically by the presence of activated macrophages, and we identify TNF-α as the critical factor produced by macrophages that accelerates EMT. TNF-α promotes EMT by a mechanism that involves the establishment of autocrine TNF-α production by the tumor cells themselves, which is dependent on ERK stimulation, and activation of the p38 MAPK signaling pathway.
Our results implicate a critical role for TNF-α in stimulating the EMT, a function that contrasts with its more established role in inducing apoptosis. TNF-α, a proinflammatory cytokine, plays a predominant role in the immune system, where it regulates such cellular processes as cytokine induction, proliferation, differentiation, and apoptosis (Papadakis and Targan, 2000). Apoptosis in target cells is induced via the activation of death domain proteins, analogous to Fasmediated apoptotic signaling (Darnay and Aggarwal, 1999). In contrast to this role, however, several studies have suggested a possible function for TNF-α in tumor progression that may be explained by our data. It was recently demonstrated that TNF-α mRNA transcripts are more abundant in colorectal tumor cells than in their normal epithelial counterparts and that there is a positive correlation between expression level and Dukes' stages (Csiszar et al., 2001). A similar finding for TNF-α expression and tumor grade was previously reported for serous ovarian tumors (Naylor et al., 1993). Of particular note, Wu et al. (1993) showed that ascites ovarian cancer cells isolated directly from patients expressed endogenous TNF-α, a feature not shared by normal or malignant ovarian cells in culture, and further postulated that autocrine production of TNF-α by the ovarian cells in situ was the result of paracrine stimulation by infiltrating monocytes. Integrating these themes, our results advocate a model in which paracrine stimulation by stromal cell-derived cytokines induces autocrine TNF-α production within the tumor itself, thus promoting EMT sensitivity.
Although ERK signaling has been implicated in the EMT (Ellenrieder et al., 2001), our data link ERK signaling to TNF-α and the genesis of autocrine TNF-α production. We obtained unequivocal evidence that stimulating organoids with TNF-α results in de novo synthesis of TNF-α, demonstrated at both the transcriptional and protein levels (Figure 6). Mechanistically, we found that this autocrine TNF-α production was itself dependent on ERK activity. Exogenous TNF-α rapidly activated the ERK 1/2 pathway, with a peak of activity at 1 h, that could be suppressed by the MEK inhibitor PD98059. Treatment with the inhibitor resulted in an approximate threefold decrease in TNF-α mRNA synthesis, suggesting that transcription is dependent, at least in part, on this initial burst of ERK activity. In addition, the early morphological changes associated with an accelerated EMT were blocked by this inhibitor, with the organoids eventually undergoing an EMT with kinetics similar to TGF-β treatment alone. Taken together, the data strongly implicate ERK activity as being required for TNF-α induction, and promotion of the EMT phenotype. Importantly, this result does not preclude additional roles for ERK activation in promoting the EMT. Indeed, transcriptional profiling has identified ERK-dependent genetic programs as underlying the onset of TGF-β–mediated EMT (Zavadil et al., 2001). It is likely that we did not observe activation of the ERK pathway in response to TGF-β alone over the 2-h time course because of the long onset of EMT in those cells (5–7 d). Also, constitutive ERK cascade activation induces an invasive phenotype in MDCK cells (Montesano et al., 1999) and promotes cell motility through phosphorylation of myosin light chains (Klemke et al., 1997). Thus, TNF-α directed ERK activation may further “sensitize” LIM 1863 cells to the plasticity effects of TGF-β signals.
What is the mechanism by which TNF-α augmented the TGF-β–directed EMT in the colon cells? The signaling pathways that regulate EMT in response to TGF-β are still poorly defined but appear to be independent of Smad signaling. Evidence indicates that there is cooperativity between TGF-β and the Ras/Raf signaling pathways to induce EMT (Oft et al., 1996; Lehmann et al., 2000; Park et al., 2000; Fujimoto et al., 2001; Janda et al., 2002), and a role for increased Rho activity has been demonstrated (Zondag et al., 2000; Bhowmick et al., 2001a). Recently, it was shown that activation of the p38 MAPK pathway was required, although not sufficient, to induce the process (Bhowmick et al., 2001b; Bakin et al., 2002; Yu et al., 2002). Typically activated in response to environmental stresses, the p38 signaling cascade also plays important roles in differentiation, proliferation, and survival (Nebreda and Porras, 2000). Treatment of organoids with TNF-α alone activated this pathway but was not sufficient to induce an EMT. However, elevated p38 activity observed in the presence of both cytokines contributed to the rapid EMT, because this effect could be blocked by the inhibitor SB203580. Moreover, organoids undergoing EMT in response to TGF-β alone (over the longer time course) were also affected by the inhibitor. Taken together, these findings support a role for p38 MAPK activity as being required, but not sufficient, for EMT and that TNF-α stimulation of the pathway augments TGF-β/EMT effects. Moreover, our data are in agreement with those reported by Haas et al. (1999), whereby overexpression of a membrane-bound mutant form of TNF-α in HeLa cells resulted in continuous autotropic signaling with permanent activation of the p38 MAPK pathway. Although p38 MAPK activity has recently been shown to be required for EMT (Bakin et al., 2002; Yu et al., 2002), the downstream targets of p38 have not been determined to date. Interestingly, p38 can affect actin polymerization through regulation of hsp27 (Obata et al., 2000) and, given that the 4-h time point immediately precedes the initial attachment and spreading of organoids undergoing an accelerated EMT, this type of activity may be involved.
In summary, we have described a novel model for EMT of colon carcinoma and identified a new pathway that modulates this critical transition. Furthermore, our study reveals a potential for infiltrating leukocytes to regulate malignant tumor progression. One implication of our data is that the influx and activation of macrophages that secrete TNF-α might facilitate the progression of tumors that produce TGF-β. Similarly, establishment of autotropic TNF-α signaling in a developing tumor would be expected to sensitize those cells for subsequent exposure to TGF-β to promote an accelerated conversion. Moreover, understanding the mechanisms that regulate the EMT of carcinomas may offer new perspectives in designing therapies for metastatic disease. Stromal therapy is emerging as a viable approach to cancer intervention (Liotta and Kohn, 2001), and our findings may have important implications for understanding the biological activities of new stromally directed agents such as Pirfenidone, which has been shown to reduce the influx of activated macrophages and inflammatory cells and to downregulate the overexpression of TGF-β (Iyer et al., 1999).
Acknowledgments
We thank Mark Roberts for expert assistance with the RQ-PCR. This work was supported by National Institutes of Health Grant CA80789.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02-09-0583. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02-09-0583.
References
- Arias, A.M. (2001). Epithelial mesenchymal interactions in cancer and development. Cell 105, 425–431. [DOI] [PubMed] [Google Scholar]
- Bakin, A.V., Rinehart, C., Tomlinson, A.K., and Arteaga, C.L. (2002). p38 mitogen-activated kinase is required for TGF beta-mediated fibroblastic transdifferentiation, and cell migration. J. Cell Sci. 115, 3193–3206. [DOI] [PubMed] [Google Scholar]
- Barth, R.J., Jr., Camp, B.J., Martuscello, T.A., Dain, B.J., and Memoli, V.A. (1996). The cytokine microenvironment of human colon carcinoma. Lymphocyte expression of tumor necrosis factor-alpha and interleukin-4 predicts improved survival. Cancer 78, 1168–1178. [DOI] [PubMed] [Google Scholar]
- Bates, R.C., Buret, A., van Helden, D.F., Horton, M.A., and Burns, G.F. (1994). Apoptosis induced by inhibition of intercellular contact. J. Cell Biol. 125, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, R.C., Edwards, N.S., and Yates, J.D. (2000). Spheroids and cell survival. Crit. Rev. Oncol. Hematol. 36, 61–74. [DOI] [PubMed] [Google Scholar]
- Batlle, E., Sancho, E., Franci, C., Dominguez, D., Monfar, M., Baulida, J., and de Herreros, A.G. (2000). The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumor cells. Nat. Cell Biol. 2, 84–89. [DOI] [PubMed] [Google Scholar]
- Behrens, J., Mareel, M.M., Van Roy, F.M., and Birchmeier, W. (1989). Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J. Cell Biol. 108, 2435–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick, N.A., Ghiassi, M., Bakin, A., Aakre, M., Lundquist, C.A., Engel, M.E., Artega, C.L., and Moses, H.L. (2001a). Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a Rho-A-dependent mechanism. Mol. Biol. Cell 12, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick, N.A., Zent, R., Ghiassi, M., McDonnell, M., and Moses, H.L. (2001b). Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J. Biol. Chem. 276, 46707–46713. [DOI] [PubMed] [Google Scholar]
- Cano, A., Perez-Moreno, M.A., Rodrigo, I., Locascio, A., Blanco, M.J., del Barrio, M.G., Portillo, F., and Nieto, M.A. (2000). The transcription factor Snail controls epithelia-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83. [DOI] [PubMed] [Google Scholar]
- Csiszar, A., Szentes, T., Haraszti, B., Zou, W., Emilie, D., Petranyi, G., and Pocsik, E. (2001). Characterization of cytokine mRNA expression in tumor-infiltrating mononuclear cells and tumor cells freshly isolated from human colorectal carcinomas. Eur. Cytokine Network 12, 87–96. [PubMed] [Google Scholar]
- Darnay, B.G., and Aggarwal, B.B. (1999). Signal transduction by tumor necrosis factor and tumor necrosis factor related ligands and their receptors. Ann. Rheum. Dis. 58, I2–I13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Caestecker, M.P., Piek, E., and Roberts, A.B. (2000). Role of transforming growth factor-β signaling in cancer. J. Natl. Cancer Inst. 92, 1388–1402. [DOI] [PubMed] [Google Scholar]
- Ellenrieder, V., Hendler, S.F., Boeck, W., Seufferlein, T., Menke, A., Ruhland, C., Adler, G., and Gress, T.M. (2001). Transforming growth factor β1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular-signal regulated kinase 2 activation. Cancer Res. 61, 4222–4228. [PubMed] [Google Scholar]
- Etoh, T., Shibuta, K., Barnard, G.F., Kitano, S., and Mori, M. (2000). Angiogenin expression in human colorectal cancer: the role of focal macrophage infiltration. Clin. Cancer Res. 6, 3545–3551. [PubMed] [Google Scholar]
- Fujimoto, K., Sheng, H., Shao, J., and Beauchamp, R.D. (2001). Transforming growth factor-β1 promotes invasiveness after cellular transformation with activated ras in intestinal epithelial cells. Exp. Cell Res. 266, 239–249. [DOI] [PubMed] [Google Scholar]
- Gold, L.I. (1999). The role of transforming growth factor-beta (TGF-beta) in human cancer. Crit. Rev. Oncog. 10, 303–360. [PubMed] [Google Scholar]
- Haas, E., Grell, M., Wajant, H., and Scheurich, P. (1999). Continuous autotropic signaling by membrane-expressed tumor necrosis factor. J. Biol. Chem. 274, 18107–18112. [DOI] [PubMed] [Google Scholar]
- Hayward, I.P., and Whitehead, R.H. (1992). Patterns of growth and differentiation in the colon carcinoma cell line LIM 1863. Int. J. Cancer 51, 1–8. [DOI] [PubMed] [Google Scholar]
- Heid, C.A., Stevens, J., Livak, K.J., and Williams, P.M. (1996). Real time quantitative PCR. Genome Res. 6, 986–994. [DOI] [PubMed] [Google Scholar]
- Hsu, S., Huang, F., Hafez, M., Winawer, S., and Friedman, E. (1994). Colon carcinoma cells switch their response to transforming growth factor beta 1 with tumor progression. Cell Growth Differ. 5, 267–275. [PubMed] [Google Scholar]
- Iyer, S., Gurujeyalakshimi, G., and Giri, S.N. (1999). Effects of pirfenidone on transforming growth factor-β gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J. Pharmacol. Exp. Therapeut. 291, 367–373. [PubMed] [Google Scholar]
- Janda, E., Lehmann, K., Killisch, I., Jechlinger, M., Herzig, M., Downward, J., Beug, H., and Grunert, S. (2002). Ras, and TGFβ cooperatively regulate epithelial cell plasticity, and metastasis. dissection of ras signaling pathways. J. Cell Biol. 156, 299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke, R.L., Cai, S., Giannini, A.L., Gallagher, P.J., de Lanerolle, P., and Cheresh, D.A. (1997). Regulation of cell motility by mitogenactivated protein kinase. J. Cell Biol. 137, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis, J.M., and Avruch, J. (1996). Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays 18, 567–577. [DOI] [PubMed] [Google Scholar]
- Leek, R.D., Lewis, C.E., Whitehouse, R., Greenall, M., Clarke, J., and Harris, A.L. (1996). Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 56, 4625–4629. [PubMed] [Google Scholar]
- Lehmann, K., Janda, E., Pierreux, C.E., Rytomaa, M., Schulze, A., McMahon, M., Hill, C.S., Beug, H., and Downward, J. (2000). Raf induces TGFβ production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 14, 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta, L.A., and Kohn, E.C. (2001). The microenvironment of the tumor-host interface. Nature 411, 375–379. [DOI] [PubMed] [Google Scholar]
- Lopez, S., Peiretti, F., Bonardo, B., Juhan-Vague, I., and Nalbone, G. (2000). Tumor necrosis factor α upregulates in an autocrine manner the synthesis of plasminogen activator inhibitor Type-1 during induction of monocytic differentiation of human HL-60 leukemia cells. J. Biol. Chem. 275, 3081–3087. [DOI] [PubMed] [Google Scholar]
- Markowitz, S.D., and Roberts, A.B. (1996). Tumor suppressor activity of the TGF-beta pathway in human cancers. Cytokine Growth Factor Rev. 7, 93–102. [DOI] [PubMed] [Google Scholar]
- Montesano, R., Soriano, J.V., Hosseini, G., Pepper, M.S., and Schramek, H. (1999). Constitutively active mitogen-activated protein kinase kinase MEK1 disrupts morphogenesis and induces an invasive phenotype in Madin-Darby canine kidney epithelial cells. Cell Growth Differ. 10, 317–332. [PubMed] [Google Scholar]
- Naylor, M.S., Stamp, G.W., Foulkes, W.D., Eccles, D., and Balkwill, F.R. (1993). Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J. Clin. Invest. 91, 2194–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda, A.R., and Porras, A. (2000). p38 MAP kinases. beyond the stress response. Trends Biochem. Sci. 25, 257–260. [DOI] [PubMed] [Google Scholar]
- Obata, T., Brown, G.E., and Yaffe, M.B. (2000). MAP kinase pathways activated by stress: The p38 MAPK pathway. Crit. Care Med. 28, N67–77. [DOI] [PubMed] [Google Scholar]
- Oft, M., Peli, J., Rudaz, C., Schwarz, H., Beug, H., and Reichmann, E. (1996). TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 10, 2462–2477. [DOI] [PubMed] [Google Scholar]
- Oft, M., Heider, K.H., and Berg, H. (1998). TGFβ signaling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 8, 1243–1252. [DOI] [PubMed] [Google Scholar]
- Park, B.J., Park, J.I., Byun, D.S., Park, J.H., and Chi, S.G. (2000). Mitogenic conversion of transforming growth factor-beta1 effect by oncogenic Ha-Ras-induced activation of the mitogen-activated protein kinase signaling pathway in human prostate cancer. Cancer Res. 60, 3031–3038. [PubMed] [Google Scholar]
- Papadakis, K.A., and Targan, S.R. (2000). Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology 119, 1148–1157. [DOI] [PubMed] [Google Scholar]
- Portella, G., Cumming, S.A., Liddell, J., Cui, W., Ireland, H., Akhurst, R.H., and Balmain, A. (1998). Transforming growth factor beta is essential for spindle cell conversion of mouse skin carcinoma in vivo: implications for tumor invasion. Cell Growth Differ. 9, 393–404. [PubMed] [Google Scholar]
- Reichmann, E., Schwarz, H., Deiner, E.M., Leitner, I., Eilers, M., Busslinger, M., and Beug, H. (1992). Activation of an inducible c-fos ER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid conversion. Cell 71, 1103–1116. [DOI] [PubMed] [Google Scholar]
- Schiott, A., Johansson, A.C.M., Widegreen, B., Sjogren, H.O., and Lindvall, M. (2000). Effects of transforming growth factor β1 expression in a rat colon carcinoma: growth inhibition, leukocyte infiltration and production of interleukin-10 and tumor necrosis factor α. Cancer Immunol. Immunother. 48, 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze, A., Lehmann, K., Jeffries, H.B.J., McMahon, M., and Downward, J. (2001). Analysis of the transcriptional program induced by Raf in epithelial cells. Genes Dev. 15, 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkotter, C., Steinbrink, K., Goebeler, M., Bhardwaj, R., and Sorg, C. (1994). Macrophages and angiogenesis. J. Leukoc. Biol. 55, 410–422. [DOI] [PubMed] [Google Scholar]
- Thiery, J.P., and Chopin, D. (1999). Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 18, 31–42. [DOI] [PubMed] [Google Scholar]
- Thiery, J.P. (2002). Epithelial-mesenchymal transitions in tumor progression. Nature Rev. Cancer 2, 442–454. [DOI] [PubMed] [Google Scholar]
- Tuxhorn, J.A., Ayala, G.E., and Rowley, D.R. (2001). Reactive stroma in prostate cancer progression. J. Urol. 166, 2472–2483. [PubMed] [Google Scholar]
- Wang, F. Weaver, V.M., Petersen, O.W., Larabell, C.A., Dedhar, S., Briand, P., Lupu, R., and Bissell, M.J. (1998). reciprocal interactions between beta-1 integrin and epidermal growth factor receptor in three-dimensional basement membrane breats cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA 95, 14821–14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, V.M., Petersen, O.W., Wang, F., Larabell, C.A., Briand, P., Dansky, C., and Bissell, M.J. (1997). Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137, 231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead, R.H., Jones, J.K., Gabriel, A., and Lukies, R.E. (1987). A new colon carcinoma cell line (LIM 1863) that grows as organoids with spontaneous differentiation into crypt-like structures in vitro. Cancer Res. 47, 2683–2689. [PubMed] [Google Scholar]
- Wu, S., Boyer, C.M., Whitaker, R.S., Berchuck, A., Wiener, J.R., Weinberg, J.B., and Bast, R.C., Jr. (1993). Tumor necrosis factor alpha as an autocrine and paracrine growth factor for ovarian cancer: monokine induction of tumor cell proliferation and tumor necrosis factor alpha expression. Cancer Res. 53, 1939–1944. [PubMed] [Google Scholar]
- Yu, L., Hebert, M.C., and Zhang, Y.E. (2002). TGF-beta receptor activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 21, 3749–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, J., and Mulder, K.M. (2001). Transforming growth factor-β signal transduction in epithelial cells. Pharm. Therapeut. 91, 1–34. [DOI] [PubMed] [Google Scholar]
- Zavadil, J., Bitzer, M., Liang, D., Yang, Y.C., Massimi, A., Kneitz, S., Piek, E., and Bottinger, E.P. (2001). Genetic programs of epithelial cell plasticity directed by transforming growth factor-β. Proc. Natl. Acad. Sci. USA 98, 6686–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W., Downey, J.S., Gu, J., Di Padova, F., Gram, H., and Han, J. (2000). Regulation of TNF expression by multiple mitogen-activated protein kinase pathways. J. Immunol. 164, 6349–6358. [DOI] [PubMed] [Google Scholar]
- Zondag, G.C., Evers, E.E., ten Klooster, J.P., Janssen, L., van der Kammen, R.A., and Collard, J.G. (2000). Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity, and epithelial-mesenchymal transition. J. Cell Biol. 149, 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]