Abstract
The extreme thermophile Thermus thermophilus HB27 exhibits high frequencies of natural transformation. Although we recently reported identification of the first competence genes in Thermus, the molecular basis of DNA uptake is unknown. A pilus-like structure is assumed to be involved. Twelve genes encoding prepilin-like proteins were identified in three loci in the genome of T. thermophilus. Mutational analyses, described in this paper, revealed that one locus, which contains four genes that encode prepilin-like proteins (pilA1 to pilA4), is essential for natural transformation. Additionally, comZ, a new competence gene with no similarity to known genes, was identified. Analysis of the piliation phenotype revealed wild-type piliation of a pilA1-pilA3Δkat mutant and a comZ mutant, whereas a pilA4 mutant was found to be completely devoid of pilus structures. These findings, together with the significant similarity of PilA4 to prepilins, led to the conclusion that the T. thermophilus pilus structures are type IV pili. Furthermore, the loss of the transformation and piliation phenotype in the pilA4 mutant suggests that type IV pili are implicated in natural transformation of T. thermophilus HB27.
Analyses of complete genome sequences have suggested that large portions of bacterial genomes were acquired from archaea via lateral gene transfer; for example, it has been suggested that 16 and 24% of the genomes of the hyperthermophilic bacteria Aquifex aeolicus and Thermotoga maritima, respectively, are of archaeal origin (2, 6, 20, 21). Despite the substantial evidence that there has been massive DNA transfer between archaea and hyperthermophilic bacteria and the assumption that life originated in hot ecosystems, information on DNA transfer in hot environments and on the structure and function of transformation systems in extreme thermophiles is scarce.
Thermus thermophilus HB27 is an extremely thermophilic bacterium (23) which exhibits high frequencies of natural transformation (14, 17). The ability to take up free DNA has also been described for other representatives of the genus Thermus, such as T. thermophilus HB8, Thermus flavus AT62, Thermus caldophilus, and Thermus aquaticus YT1 (17). To gain insight into the mechanism of natural transformation in extremely thermophilic bacteria, we performed searches for homology in the genome sequence of T. thermophilus HB27 with sequences encoding known competence proteins of mesophilic model bacteria, such as Neisseria gonorrhoeae, Acinetobacter sp. strain BD413, Bacillus subtilis, and Streptococcus pneumoniae (4, 8, 18, 25, 26, 29), followed by gene disruption studies. These analyses led to identification of 11 competence genes in T. thermophilus HB27 (9, 10). Seven of these genes encode proteins that are similar to type IV pilus biogenesis proteins of Pseudomonas aeruginosa or N. gonorrhoeae, one of which exhibits significant similarity to prepilin peptidases (10). The latter finding suggests that prepilin-like components, which were not identified, are implicated in the transformation machinery of T. thermophilus HB27. However, the Thermus genome contains 12 distinct genes that encode prepilin-like proteins, distributed over three chromosomal loci. To analyze the implication of these genes in transformation, genes encoding prepilin-like proteins from each of the three chromosomal loci were subjected to mutational analyses.
Here we describe the results of these analyses, which revealed that genes that encode pilin-like competence proteins (PilA proteins) and a novel Thermus-specific competence gene are implicated in natural transformation of T. thermophilus. We provide evidence that one of the pilin-like competence proteins represents a major pilus subunit. Taken together, our results suggest that the type IV pili are implicated in transformation of T. thermophilus HB27.
MATERIALS AND METHODS
Strains, plasmids, DNA manipulation, and sequencing.
T. thermophilus HB27 wild-type and mutant strains were grown in a 1:1 mixture of Thermus medium and Luria-Bertani medium at 60 to 70°C (10). For transformation of T. thermophilus HB27 a modified protocol of Koyama et al. (17) was used. Escherichia coli strains were cultured at 37°C in Luria-Bertani medium. Antibiotics were added when appropriate (20 to 40 μg of kanamycin per ml, 100 μg of ampicillin per ml, and 100 to 500 μg of streptomycin per ml). The molecular and genetic procedures used were standard procedures. The complete genomic sequence of T. thermophilus HB27 was determined at the Göttingen Genomics Laboratory (G2L) as described recently (10). Generation of the Thermus gene bank by using the vector pTZ19r (MBI, Fermentas) has been described previously (9).
Generation of Thermus mutants.
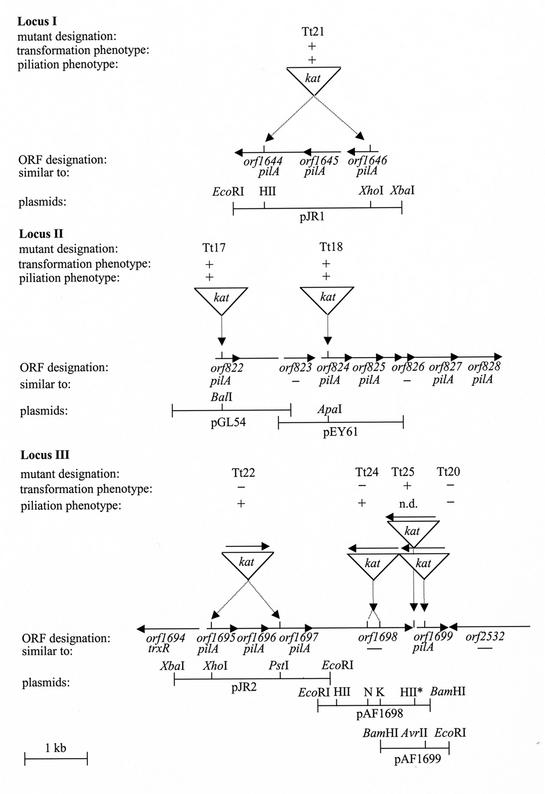
To analyze the role of the open reading frames (ORFs) encoding prepilin-like proteins in natural transformation, gene disruption or allelic replacement of selected DNA regions within distinct ORFs was performed by using a thermostable kanamycin resistance gene (kat), which was derived from the E. coli-Thermus shuttle vector pMK18 (5). For gene disruption either gene bank plasmids, such as pGL54 and pEY61, were used (Fig. 1) or the ORFs were amplified by PCR and subsequently inserted into pBluescript II KS or pGEM-7Zf(+). The latter approach resulted in recombinant plasmids pAF1699, pAF1698, pJR1, and pJR2 (Fig. 1). PCR primers used for amplification of these DNA fragments are listed in Table 1. To allow insertion of the kat gene into the intergenic region between orf1698 and orf1699, two adjacent HincII restriction sites upstream of orf1699 (Fig. 1) were used. To allow use of these HincII sites, an additional HincII site present in orf1698 had to be eliminated. Therefore, pAF1698 was digested with EcoRI and NcoI, treated with the Klenow enzyme, and religated. The kat gene was inserted into selected ORFs by using restriction sites within the ORFs in the recombinant plasmids, as indicated in Fig. 1. Mutant generation by allelic replacement of the wild-type loci by mutant loci was performed as described recently (10). Correct allelic replacement of chromosomal wild-type DNA by disrupted ORFs was verified by Southern hybridization and PCR. Plasmids that were generated for Thermus gene disruption studies were sequenced by using the standard primers or primers generated from the genomic sequence information (Table 1).
FIG. 1.
Organization of the ORFs detected in the Thermus genome sequence that encode 12 prepilin-like proteins and gene disruption of conserved ORFs within potential competence loci in the genome of T. thermophilus HB27. In the restriction maps of plasmids covering different conserved ORFs of the potential competence loci only selected restriction sites are shown. The triangle indicating the kat gene indicates the insertion site of the Kmr marker gene. The arrows indicate the directions of transcription. pilA, prepilin gene; trxR, putative thioredoxin reductase gene; HII, HincII; N, NcoI; K, KpnI; n.d., not determined. The asterisk indicates two closely associated HincII sites.
TABLE 1.
Primers designed for cloning of putative Thermus competence genes
| Plasmid generated | Primer designation | Primer sequence (5′-3′)a |
|---|---|---|
| pAF1699 | orf1699-BI | CCT CTT CGG ATC CGT CAT TAG |
| orf1699-EI | CGG CTG GAA TTC CCA CAA TGA G | |
| pAF1698 | orf1698-EI | GTT GCA GAA TTC CAA GGC TGC |
| orf1698-BI | GAT ACA TCC GGA TCC CGT TTG TC | |
| pJR1 | orf1644-EI | CGT GGC GGA ATT CGC GGA TGG |
| orf1646-XI | GAC GAG CTC TAG ACC CGC GTC | |
| pJR2 | orf1695-XI | CGG ATG CTC TAG ACC TCG GTG |
| orf1697-EI | GCA GCC TTG GAA TTC TGC AAC CC |
Modified base pairs are indicated in bold.
Electron microscopy.
Thermus wild-type and mutant strains grown overnight on freshly prepared Thermus medium plates were negatively stained with 4% (wt/vol) uranyl acetate or 3% (wt/vol) phosphotungstic acid. After the cells were dried on Formvar-coated copper grids, they were viewed with a Philips model EM301 transmission electron microscope at 80 kV.
Nucleotide sequence accession number.
The nucleotide sequence data have been deposited in the GenBank database under accession no. AY116643.
RESULTS AND DISCUSSION
Identification and analyses of genes that encode prepilin-like competence proteins.
Similarity searches with the genomic database of T. thermophilus HB27 led to detection of 12 conserved ORFs which encode prepilin-like proteins and are distributed over three distinct loci (Fig. 1). One of these loci (locus I) was found to be located on the Thermus megaplasmid pTT27, whereas locus II and locus III are located on the Thermus chromosome. To determine whether the ORFs that encode prepilin-like proteins are implicated in natural transformation, gene disruption and deletion studies were performed as indicated in Fig. 1.
Three of the resulting mutants, Tt17 (orf822::kat), Tt18 (orf824::kat), and Tt21 (orf1646-orf1644Δkat), exhibited wild-type transformation frequencies, which led to the conclusion that the ORFs are not implicated in natural transformation (Fig. 1 and Table 2). Simultaneous deletion of the 3′ end of orf1695, orf1696, and the 5′ end of orf1697 resulted in mutant Tt22 (orf1695-orf1697Δkat), which was found to be not competent (Fig. 1 and Table 2). From this finding we concluded that locus III is a competence-specific locus. For conclusions concerning functions of distinct ORFs that encode pilin-like proteins within this competence locus, the possibility of a potential polar effect of the kat marker had to be excluded. Northern studies have shown (33; J. Berenguer, personal communication) that analogous orientation of the kat marker gene and the gene disrupted by the kat marker prevents polar effects on the transcription of genes located downstream. Because the kat marker is in an orientation which does not result in any polar effects, we concluded that at least one of the ORFs examined (orf1695, orf1696, or orf1697) is essential for natural transformation. Disruption of orf1699, the last ORF that encodes a prepilin-like protein of locus III, resulted in the nontransformable mutant Tt20 (Fig. 1 and Table 2). Although the marker is inserted in the orientation opposite that of orf1699, which may cause polar effects, the possibility that there is a polar effect on genes located downstream can be excluded since orf2532, which is located immediately downstream of orf1699, is transcribed in the opposite direction. This clearly shows that orf1699 is essential for natural transformation. Based on the similarities of Orf1695, Orf1696, Orf1697, and Orf1699 to pilins (PilA of P. aeruginosa and PilE of N. gonorrhoeae) and their function in natural transformation, the ORFs encoding these proteins were designated pilA1, pilA2, pilA3, and pilA4.
TABLE 2.
ORFs analyzed in this studya
| ORF | Protein
|
Mutant | Mutant phenotype | Proposed function | ||
|---|---|---|---|---|---|---|
| No. of amino acids | Mol wt (103) | C-terminal Cys residues | ||||
| orf822 | 116 | 12 | C65-(27 aa)-C93b | Tt17 | Competent, piliated | Unknown |
| orf824 | 142 | 16 | C83-(17 aa)-C100 | Tt18 | Competent, piliated | Unknown |
| orf1644 | 305 | 33 | None | Tt21 | Competent, piliated | Unknown |
| orf1645 | 159 | 17 | C126-(8 aa)-C159 | Tt21 | Competent, piliated | Unknown |
| orf1646 | 145 | 16 | None | Tt21 | Competent, piliated | Unknown |
| orf1695 (pilA1) | 156 | 17 | None | Tt22 | Noncompetent, piliated | Prepilin-like protein of DNA transformation machinery |
| orf1696 (pilA2) | 193 | 21 | C170-(14 aa)-C185 | Tt22 | Noncompetent, piliated | Prepilin-like protein of DNA transformation machinery |
| orf1697 (pilA3) | 233 | 25 | C136-(22 aa)-C159, C170-(38 aa)-C209 | Tt22 | Noncompetent, piliated | Prepilin-like protein of DNA transformation machinery |
| orf1698 (comZ) | 554 | 59 | None | Tt24 | Noncompetent, piliated | Unknown function in DNA transformation machinery |
| orf1699 (pilA4) | 131 | 14 | C95-(34 aa)-C130 | Tt20 | Noncompetent, nonpiliated | Pilin-like subunit of pili and DNA uptake apparatus |
All deduced proteins except ComZ have the conserved cleavage motif for the prepilin endonuclease.
aa, amino acids.
The overall levels of amino acid identity of the deduced Thermus prepilin-like proteins to prepilins are rather low, ranging from 22 to 35%. There are significant similarities within the N-terminal domains. Particularly within the first 30 amino acids the levels of amino acid identity of Thermus prepilin-like proteins and prepilins are high, ranging from 44 to 73% with PilA of P. aeruginosa. All prepilins and prepilin-like proteins have a short leader peptide, a cleavage motif for the prepilin peptidase, a conserved glutamate residue at position 5 (E5) in the mature protein, and a characteristic size (145 to 160 amino acids) (15). For the E5 residue a critical role in polymerization of the pilin subunits has been demonstrated (24, 28). Additionally, most prepilins are characterized by a pair of cysteine residues near the C terminus, which form a disulfide loop (15). All these conserved features are also present in the pilA1 to pilA 4 gene products, suggesting that these pilin-like competence proteins are assembled in a pilus-like structure.
comZ, a novel competence gene.
The protein product of one ORF in the novel competence locus, orf1698, did not show any similarity to proteins in databases. Disruption of orf1698 resulted in the noncompetent mutant Tt24 (Fig. 1 and Table 2). Since the marker was found to be inserted into orf1698 in the opposite orientation, the possibility that the marker insertion had a polar effect on the downstream competence gene, pilA4, could not be excluded. To analyze the role of orf1698 in natural transformation, disruption of the intergenic space spanning 254 bp between orf1698 and pilA4 was performed by inserting the kat gene 171 bp upstream of the pilA4 start codon (Fig. 1). In contrast to marker insertion into orf1698, marker insertion into the intergenic region resulted in a wild-type transformation phenotype of mutant Tt25 (Fig. 1). This clearly shows that the noncompetent phenotype of orf1698 mutant Tt24 does not result from a polar effect and that orf1698 is essential for natural transformation. The novel competence gene, designated comZ, encodes a 59-kDa protein. The presence of one hydrophobic N-terminal domain suggests a membrane location for ComZ.
The detection of nonconserved genes in the Thermus transformation machinery, such as comZ in this study and pilW in previous studies (10), underlines the presence of distinct features in the transformation apparatus of Thermus, which might have evolved due to the extreme environment of Thermus and the specific outermost layer of Thermus cells, which consists of S-layer and lipid components (J. Berenguer, personal communication).
Piliation and transformation are linked in T. thermophilus HB27.
The significant similarities of the proteins encoded by 12 ORFs to prepilin-like proteins, together with the recent detection of pilus structures on the surface of Thermus cells (10), raised the question of whether the ORFs that encode prepilin-like proteins are implicated in pilus biogenesis. To answer this question, mutants having mutations in genes encoding prepilin-like proteins and a mutant having a mutation in comZ were analyzed to determine their piliation phenotypes. Tt17 (orf822::kat), Tt18 (orf824::kat), and Tt21 (orf1644-orf1646Δkat) were found to have a wild-type piliation phenotype (Fig. 1). These findings led to the conclusion that the ORFs are implicated in neither pilus biogenesis nor transformation. The transformation-defective mutants Tt22 (pilA1-3Δkat) and Tt24 (comZ::kat) were also found to have a wild-type piliation phenotype, suggesting that the pilin-like proteins encoded by the ORFs and ComZ play a role in natural transformation but are not essential for the pilus structures. Despite the wild-type piliation phenotype of these mutants, the data do not rule out the possibility that the orf822, orf824, orf1644 to orf1646, and pilA1 to pilA3 genes have a function in pilus biogenesis. In contrast, the transformation-defective mutant Tt20 having a mutation in pilA4 was found to be devoid of pilus structures (Fig. 1 and Table 2). From these findings we concluded that PilA4 has a dual function in transformation and pilus biogenesis. Since so far no other prepilin-like proteins have been found to be involved in pilus biogenesis in Thermus, we concluded that the prepilin-like competence protein PilA4 represents a pilus subunit and that the pilus structures are type IV pili. Although the finding that mutations of distinct genes in loci I and II that encode prepilin-like proteins (Fig. 1) had no effect on piliation suggests that these loci are not implicated in piliation, the possibility that other genes within these loci that encode prepilin-like proteins might be implicated in piliation cannot be completely excluded.
A connection between type IV pilus expression and competence for natural transformation has been reported for several competent bacteria, such as N. gonorrhoeae, Pseudomonas stutzeri, Dichelobacter nodosus, Legionella pneumophila, and Synechocystis sp. strain PCC6803 (11, 16, 27, 29, 32), but despite these findings it cannot be concluded in general that the expression of type IV pili is a prerequisite for natural transformation. Gram-positive competent bacteria, such as B. subtilis and S. pneumoniae, as well as the gram-negative organism Haemophilus influenzae, do not exhibit type IV pili, although their transformation apparatus consists of components that are similar to the pilus subunit (7, 8).
The central question that arises from the observation that a loss of pilus structures is accompanied by a loss of transformability is whether the pili themselves are involved in DNA uptake. The piliation and transformation defect of the Thermus pilA4 mutant, together with identification of other competence proteins with similarities to type IV pilus biogenesis proteins, suggests that transformation and the piliation apparatus are closely linked. However, this suggestion does not allow any conclusion concerning pilus-mediated DNA transfer. The possibility that the PilA4 protein plays a role in two distinct systems, the type IV pilus and the DNA uptake apparatus, cannot be excluded. The essential roles of several additional prepilin-like PilA proteins (PilA1 to PilA3) in transformation, together with the finding that these prepilin-like components are not essential for piliation, support the hypothesis that there is a distinct transformation apparatus consisting of several prepilin-like components plus the structural subunit of the pilus structures. It is tempting to speculate that such an apparatus resembles a rudimentary pilus structure. On the other hand, it is possible that a functional pilus is required for DNA binding followed by DNA transport via pilus retraction. The latter possibility is supported by the finding that pili retract in a PilT-dependent process (19) and the finding that the PilT protein, which is dispensable for pilus biogenesis, is essential for pilus-mediated twitching motility (pilus retraction) and natural transformation in N. gonorrhoeae, P. stutzeri, and Synechocystis sp. strain PCC6803 (13, 22, 30). The finding that a Thermus mutant defective in the pilF gene, which is similar to the gonococcal pilT gene, still has pilus structures but is defective in transformation (10) correlates with the described pilT mutant phenotypes. When these data are taken into account, it is tempting to speculate that retraction of Thermus pili might be important for DNA uptake in Thermus.
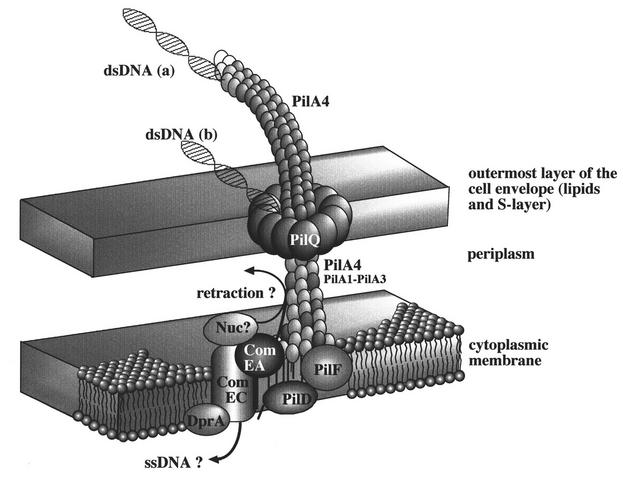
From the transformation-defective but pilus-positive phenotype of Thermus mutant Tt22 (pilA1-3Δkat), it is obvious that one of the proteins or even all of the proteins encoded by pilA1 to pilA3 are essential in natural transformation but are not essential in pilus biogenesis. From this finding we concluded that the pilA locus in Thermus consists of two classes of competence genes, one with a dual function in natural transformation and piliation (pilA4) and the other with a competence-specific function (pilA1 to pilA3 and comZ). A similar situation is encountered in Synechocystis sp. strain PCC6803; PilA1 is implicated in both pilus biogenesis and natural transformation, while PilA2 is a competence-specific protein (3, 32). In N. gonorrhoeae the structural subunit of the type IV pili (PilE) is required for transformation, a second pilin-like protein (ComP) is competence specific, and a third pilin-like protein (PilV) functions as an inhibitor of transformation as it acts as an antagonist of ComP (1, 31). An antagonistic effect was also reported for PilAII in P. stutzeri, which inhibits PilAI, the structural subunit (12). From these results it was concluded that pilin-like proteins have different functions, such as being major and minor structural components of the transformation and/or pilus apparatus and as regulatory components. From the results obtained in this study we concluded that PilA4 is the major subunit of the pilus and/or the transformation apparatus and that PilA1 to PilA3 are components of an associated structure rather than proteins that are involved in regulatory processes. A hypothetical model of a pilus-like DNA translocator taking into account all Thermus competence genes identified so far is presented in Fig. 2.
FIG. 2.
Hypothetical model of the transformation system in T. thermophilus HB27. Two possible DNA binding sites are indicated; they are at the tip of the pilus structures (a) and close to the potential ring-like structure consisting of secretin-like subunits in the outermost layer (b). dsDNA, double-stranded DNA; ssDNA, single-stranded DNA; Nuc, postulated but unidentified competence-specific endonuclease which cleaves double-stranded DNA upon transport through the inner membrane.
Acknowledgments
This work was supported by grants Av 9/4-5 and Av9/5-1 from the Deutsche Forschungsgemeinschaft. A. Friedrich was supported by the Stiftung Stipendien Fonds des Verbandes der Chemischen Industrie and the BMBF. The Göttingen Genomics Laboratory receives financial support from the Ministry of Science of the state of Lower Saxony.
We thank Caroline Wichmann and Michael Hoppert, Georg-August-Universität, Göttingen, Germany, for assistance with the electron microscopy studies.
REFERENCES
- 1.Aas, F. E., C. Lovold, and M. Koomey. 2002. An inhibitor of DNA binding and uptake events dictates the proficiency of genetic transformation in Neisseria gonorrhoeae: mechanism of action and links to type IV pilus expression. Mol. Microbiol. 46:1441-1450. [DOI] [PubMed] [Google Scholar]
- 2.Aravind, L., R. L. Tatusov, Y. I. Wolf, D. R. Walker, and E. V. Koonin. 1998. Evidence for massive gene exchange between archaeal and bacterial hyperthermophiles. Trends Genet. 14:442-444. [DOI] [PubMed] [Google Scholar]
- 3.Bhaya, D., N. R. Bianco, D. Bryant, and A. Grossman. 2000. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 37:941-9951. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, E. A., S. Y. Choi, and H. R. Masure. 1998. A competence regulon in Streptococcus pneumoniae revealed by genomic analysis. Mol. Microbiol. 27:929-939. [DOI] [PubMed] [Google Scholar]
- 5.de Grado, M., P. Castan, and J. Berenguer. 1999. A high-transformation-efficiency cloning vector for Thermus thermophilus. Plasmid 42:241-245. [DOI] [PubMed] [Google Scholar]
- 6.Doolittle, W. F. 1999. Lateral genomics. Trends Cell. Biol. 9:M5-M8. [PubMed] [Google Scholar]
- 7.Dougerthy, B. A., and H. O. Smith. 1999. Identification of Haemophilus infuenzae Rd transformation genes using cassette mutagenesis. Microbiology 145:401-409. [DOI] [PubMed] [Google Scholar]
- 8.Dubnau, D. 1999. DNA uptake in bacteria. Annu. Rev. Microbiol. 53:217-244. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich, A., T. Hartsch, and B. Averhoff. 2001. Natural transformation in mesophilic and thermophilic bacteria: identification and characterization of novel, closely related competence genes in Acinetobacter sp. strain BD413 and Thermus thermophilus HB27. Appl. Environ. Microbiol. 67:3140-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedrich, A., C. Prust, T. Hartsch, A. Henne, and B. Averhoff. 2002. Molecular analyses of the natural transformation machinery and identification of pilus structures in the extremely thermophilic bacterium Thermus thermophilus HB27. Appl. Environ. Microbiol. 68:745-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graupner, S., V. Frey, R. Hashemi, M. G. Lorenz, G. Brandes, and W. Wackernagel. 2000. Type IV pilus genes pilA and pilC of Pseudomonas stutzeri are required for natural genetic transformation, and pilA can be replaced by corresponding genes from nontransformable species. J. Bacteriol. 182:2184-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graupner, S., and W. Wackernagel. 2001. Pseudomonas stutzeri has two closely related pilA genes (type IV pilus structural protein) with opposite influences on natural genetic transformation. J. Bacteriol. 183:2359-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graupner, S., N. Weger, M. Sohni, and W. Wackernagel. 2001. Requirement of novel competence genes pilT and pilU of Pseudomonas stutzeri for natural transformation and suppression of pilT deficiency by a hexahistidine tag on the type IV pilus protein PilAI. J. Bacteriol. 183:4694-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidaka, Y., M. Hasegawa, T. Nakahara, and T. Hoshino. 1994. The entire population of Thermus thermophilus cells is always competent at any growth phase. Biosci. Biotechnol. Biochem. 58:1338-1339. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs, M., and J. S. Mattick. 1993. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol. 10:233-243. [DOI] [PubMed] [Google Scholar]
- 16.Kennan, R. M., O. P. Dhungyel, R. J. Whittington, J. R. Egerton, and J. I. Rood. 2001. The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion, and natural competence. J. Bacteriol. 183:4451-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama, Y., T. Hoshino, N. Tomizuka, and K. Furukawa. 1986. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J. Bacteriol. 166:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Link, C., S. Eickernjäger, D. Porstendörfer, and B. Averhoff. 1998. Identification and characterization of a novel competence gene, comC, required for DNA binding and uptake in Acinetobacter sp. strain BD413. J. Bacteriol. 180:1592-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merz, A. J., M. So, and M. P. Sheetz. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98-101. [DOI] [PubMed] [Google Scholar]
- 20.Nelson, K. E., R. A. Clayton, S. R. Gill, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, W. C. Nelson, K. A. Ketchum, L. McDonald, T. R. Utterback, J. A. Malek, K. D. Linher, M. M. Garrett, A. M. Stewart, M. D. Cotton, M. S. Pratt, C. A. Phillips, D. Richardson, J. Heidelberg, G. G. Sutton, R. D. Fleischmann, J. A. Eisen, C. M. Fraser, et al. 1999. Evidence for lateral gene transfer between archaea and bacteria from genome sequence of Thermotoga maritima. Nature 399:323-329. [DOI] [PubMed] [Google Scholar]
- 21.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto, S., and M. Ohmori. 2002. The cyanobacterial PilT protein responsible for cell motility and transformation hydrolyzes ATP. Plant Cell Physiol. 43:1127-1136. [DOI] [PubMed] [Google Scholar]
- 23.Oshima, T., and K. Imahori. 1974. Description of Thermus thermophilus comb. nov., a nonsporulating thermophilic bacterium (Yoshida and Oshima) from a Japanese thermal spa. Int. J. Syst. Bacteriol. 24:102-112. [Google Scholar]
- 24.Pasloske, B. L., D. G. Scraba, and W. Paranchych. 1989. Assembly of mutant pilins in Pseudomonas aeruginosa: formation of pili composed of heterologous subunits. J. Bacteriol. 171:2142-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pestova, E. V., and D. A. Morrison. 1998. Isolation and characterization of three Streptococcus pneumoniae transformation-specific loci by use of a lacZ reporter insertion vector. J. Bacteriol. 180:2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porstendörfer, D., O. Gohl, F. Mayer, and B. Averhoff. 2000. ComP, a pilin-like protein essential for natural competence in Acinetobacter sp. strain BD413: regulation, modification, and cellular localization. J. Bacteriol. 182:3673-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone, B. J., and Y. Abu Kwaik. 1999. Natural competence for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J. Bacteriol. 181:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strom, M. S., and S. Lory. 1991. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J. Biol. Chem. 266:1656-1664. [PubMed] [Google Scholar]
- 29.Tonjum, T., and M. Koomey. 1997. The pilus colonization factor of pathogenic neisserial species: organelle biogenesis and structure/function relationships. Gene 192:155-163. [DOI] [PubMed] [Google Scholar]
- 30.Wolfgang, M., P. Lauer, H. S. Park, L. Brossay, J. Herbert, and M. Koomey. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321-330. [DOI] [PubMed] [Google Scholar]
- 31.Wolfgang, M., J. P. Van Putten, S. F. Hayes, and M. Koomey. 1999. The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol. Microbiol. 31:1345-1357. [DOI] [PubMed] [Google Scholar]
- 32.Yoshihara S., X. Geng, S. Okamoto, K. Yura, T. Murata, M. Go, M. Ohmori, and M. Ikeuchi. 2001. Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 42:63-73. [DOI] [PubMed] [Google Scholar]
- 33.Zafra, O., S. Ramirez, P. Castan, R. Moreno, F. Cava, C. Valles, E. Caro, and J. Berenguer. 2002. A cytochrome c encoded by the nar operon is required for the synthesis of active respiratory nitrate reductase in Thermus thermophilus. FEBS Lett. 523:99-102. [DOI] [PubMed] [Google Scholar]