Abstract
The functional disturbance of self-renewing and multipotent hematopoietic stem cells (HSCs) in viral diseases is poorly understood. In this report, we have assessed the susceptibility of mouse HSCs to strain i of the autonomous parvovirus minute virus of mice (MVMi) in vitro and during persistent infection of an immunodeficient host. Purified 5FUr Lin− Sca-1+ primitive hematopoietic precursors were permissive for MVMi genome replication and the expression of viral gene products. The lymphoid and myeloid repopulating capacity of bone marrow (BM) cells was significantly impaired after in vitro infection, although the degree of functional effect proportionally decreased with the posttransplantation time. This indicated that MVMi targets the heterogeneous compartment of repopulating cells with differential affinity and suggests that the virus may persist in some primitive HSCs in the quiescent stage, killing those eventually recruited for proliferative activity. Immunodeficient SCID mice oronasally infected with MVMi were cured of the characteristic virus-induced lethal leukopenia by transplantation of immunocompetent BM grafts. However, two double-stranded viral DNA species, probably uncommon replicative intermediates, remained in the marrow of every transplanted mouse months after infectious virus clearance. Genetic analysis of the rescued mice showed that the infection ensured a stable engraftment of donor hematopoiesis by markedly depleting the pool of endogenous HSCs. The MVMi-induced suppression of HSC functions illustrates the accessibility of this compartment to infection during a natural viral hematological disease. These results may provide clues to understanding delayed hematopoietic syndromes associated with persistent viral infections and to prospective gene delivery to HSCs in vivo.
The hematopoietic stem cells (HSC) are multipotent, self-renewing, and long-term repopulating cells mainly located in the bone marrow (BM) and representing 0.05 to 0.1% of the hematopoietic BM cell population (reviewed in references 43 and 69). HSCs can develop the whole repertoire of proliferating cells committed to several differentiation lineages characterizing the hematopoietic system (39). The HSCs have also demonstrated their ability to generate a variety of tissue cell types in mice and humans (27, 28, 30, 40, 47). The important properties of these rare cells in the regulation of hematopoietic homeostasis and in the regeneration and maintenance of nonhematopoietic tissues make the susceptibility of HSCs to viral infections a matter of substantial interest. Moreover, the transduction of HSCs with viral vectors carrying exogenous genes is the basis of protocols aimed at permanent gene therapy for the hematopoietic system (46, 50, 58, 74).
In nature, many viral infections cause hematopoietic diseases by direct action of virus-encoded effectors on hematopoietic cells or indirect perturbation of the hematopoietic regulatory network (71). The viral etiology of the hematological diseases is commonly investigated in primary hematopoietic cultures in vitro, and in some cases the capacity of diverse viruses to directly infect and damage hematopoietic committed progenitors has been experimentally validated (see references 57, 68, and 73 as examples). Other viruses with immunosuppressive capacity can disrupt hematopoiesis in culture, reducing the expression of supportive cytokines by the BM stroma or targeting primitive precursors such as human CD34+ cells (1, 37, 45). However, the inherent difficulty of determining the biological properties of the genuine HSCs outside the mouse model, namely, their long-term repopulating capacity, has drastically limited the comprehensive investigation of virus-HSC interactions.
This technically complex quest has become experimentally attainable in mice with the assessment of HSC functionality by reconstitution assays (24), which have led to the enrichment and isolation of these cells by different techniques based on the expression of specific cell surface markers and resistance to cytotoxic drugs (33, 59, 61). But in addition to the viral tropism, the accessibility of the HSCs to infections in vivo may be restrained by their normal state in the G0 phase of the cell cycle under steady-state conditions (7) and their low proportion in anatomically restricted niches within the hematopoietic organs. Indeed, a disorder of HSC biological functions during systemic viral infection of a natural host has not been reported up to now.
We have studied the targeting of the self-renewing mouse HSCs having short and long-term repopulating capacity by the immunosuppressive strain of the parvovirus minute virus of mice (MVMi), both in culture and in the natural oronasal infection of an immunodeficient host. MVM is a molecular model of the Parvoviridae, a family of nonenveloped single-stranded DNA viruses requiring for their replication factors expressed during the S phase of the cell cycle (16, 70) and commonly associated with important hematopoietic disorders in humans and animals (71). Notably, a member of this family, the parvovirus B19, is an important human pathogen (reviewed in references 25 and 64) causing a variety of hematopoietic diseases such as the childhood rash called “erythema infectiosum” (2), erythroblastopenic crisis in patients with hemolytic disorders (49), chronic anemia in immunocompromised patients (29), and fetal anemia with hydrops fetalis in pregnant women (9). B19 preferentially infects hematopoietic cells of the erythroid lineage at the BFU-E and CFU-E stages (44, 63), via the glycolipid globoside of the blood group P antigens, which acts as a receptor of the virus (8), although the capacity of B19 to interact with more primitive human hematopoietic precursors is controversial (60). Animal parvoviruses, on the other hand, such as the feline panleukopenia virus, may target both myeloid and erythroid committed progenitors (71), and persistent immune dysfunctions are common among the rodent parvovirus group (26). MVMi can cytotoxically infect in vitro CFU-GM, BFU-E, CFU-MK, and primitive CFU-S clonogenic mouse hematopoietic precursors (31, 57). In newborn mice, the MVMi infection mediates a mild reduction of hematopoietic committed precursors (55) and an involution of hepatic erythropoietic foci (10). In adult immunodeficient SCID mice lacking T and B lymphocytes due to a deficiency in the rearrangement of the immunoglobulin and T-cell receptor genes (6), this virus causes a persistent BM infection leading to a lethal leukopenia with dysregulated erythropoiesis and megakaryopoiesis (31, 56). The hematopoietic disorders caused by MVMi in mice may provide insights into the B19-associated diseases in humans under different immunocompetence and developmental conditions.
Herein, the capacity of the parvovirus MVMi to target and damage mouse HSCs was studied (i) to determine virus replication and gene expression in highly purified hematopoietic precursors expressing a primitive surface phenotype (5FUr Lin− Sca-1+), (ii) to evaluate the short- and long-term repopulating capacity of BM cells upon MVMi infection, and (iii) to study the functionality of the HSC compartment during natural oronasal infection of the SCID mouse host. We demonstrate that MVMi infects in vitro and suppresses short- and long-term lymphohematopoietic repopulating cells of persistently infected mice.
MATERIALS AND METHODS
Virus and mice.
Purified clonal stocks of MVMi were prepared in the EL-4 lymphoma cell line (5) as recently described (35) and sterile filtered. Virus titers were determined by a PFU assay on the NB324K indicator cell line (57) cultured in Dulbecco's modified Eagle's medium (GIBCO Laboratories) supplemented with 5% inactivated fetal bovine serum (FBS; Life Technologies).
Eight- to twelve-week-old C57BL/6J mice (Ly-5.2 phenotype) and B6.SJL-PtprcaPep3b/BoyJ (Ly5a) mice (Ly-5.1 phenotype) were used to harvest BM cells. For the in vivo infections, 8- to 10-week-old immunodeficient CB-17 SCID mice (6) routinely handled under sterile conditions and maintained in microisolators were used. Breeding pairs, originally obtained from the Jackson Laboratory (Bar Harbor, Maine), were bred at the CIEMAT animal facility (registration number 28079-21A), allowed food and water ad libitum, and routinely screened for pathogens in accordance with Federation of European Laboratory Animal Science Associations procedures. For the enrichment in primitive hematopoietic precursors, Ly-5.1 mice were intravenously injected with 150 mg of 5-fluorouracil (5FU; Roche, Basel, Switzerland)/kg of body weight 5 days prior to the BM harvest. Recipients of BM transplants were irradiated for a myeloablative regimen with a Philips MG324 X-ray instrument (Philips, Hamburg, Germany) set at 300 kV and 10 mA and delivering a dose rate of 1.03 Gy/min.
BM sampling and HSC purification.
BM cells were obtained from the femora and tibiae of at least three mice and dispersed in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Paisley, Scotland). For cell sorting, cells were resuspended in Hanks' balanced salt solution (Life Technologies) supplemented with 10 mM HEPES buffer (pH 7.2; HH), dispersed by repeated flushing through a 25-gauge needle, and washed once in either medium supplemented with 10% FBS. For stem cell purification, BM cells from 5FU-treated mice were enriched for low-density cells by equilibrium centrifugation (400 × g for 15 min at 4°C) on a discontinuous Nycodenze gradient (1,090, 1,080, and 1,050 g/ml; Nycomed Pharma AS, Oslo, Norway). The upper layer together with the cells on the upper interface was collected, washed twice on HSA (HH supplemented with 5% FBS and 0.02% sodium azide), and resuspended at 2 × 107 cells/ml. Cells were incubated for 30 min at 4°C with saturating concentrations of monoclonal antibodies (MAbs) specific for antigens expressed in B cells (anti-B220; DNAX, Palo Alto, Calif.), T cells (anti-CD4 and anti-CD8, both from the European Collection of Cell Cultures), myeloid cells (anti-Mac-1, from the European Collection of Cell Cultures, and anti-Gr-1, from PharMingen, San Diego, Calif.), and erythroid cells (TER-119, kindly provided by Tatsuo Kina, Kyoto University, Kyoto, Japan). After washing, cells were incubated for 30 min at 4°C with an anti-rat immunoglobulin G-phycoerythrin (PE) antibody (Southern Biotechnology Associates, Inc., Birmingham, Ala.), spun down and resuspended in HSA supplemented with 20% rat serum, incubated for 10 min at room temperature to block the secondary antibody, and then centrifuged over a serum cushion and washed twice. Finally, to stain dead cells, a rat anti-Sca-1-fluorescein isothiocyanate (FITC)-conjugated MAb (PharMingen) was added to the cells for 30 min at 4°C, washed, and resuspended in HSA plus 2 μg of propidium iodide (PI)/ml. Cell sorting was done on an EPICS Elite ESP instrument (Coulter, Hialeah, Fla.), and cells positive for FITC signal and negative for PE and PI signals were sorted out. A sample of the sorted cells was removed for evaluation of sorting purity.
Virus infections and replication analyses.
Total BM or sorted Lin− Sca-1+ cells, at a concentration of 1 × 106 to 2 × 106 cells/ml in IMDM plus 2% FBS, were inoculated at the indicated multiplicity of infection (MOI) with purified MVMi for 1.5 h at 37°C and constant shaking. Samples were washed with IMDM plus 10% FBS to remove unadsorbed virus and resuspended in IMDM. The Lin− Sca-1+ cells were then incubated at 37°C in IMDM supplemented with 20% FBS and 100 ng of stem cell factor (SCF) (murine recombinant SCF; Genetics Institute, Cambridge, Mass.)/ml, 100 ng of interleukin-6 (IL-6; nonglycosylated purified human recombinant IL-6; specific activity, 2 × 107 U/ml; Pharmacia, Barcelona, Spain)/ml, and 100 ng of IL-3 (murine recombinant IL-3; Immunex, Seattle, Wash.)/ml. SCID animals were infected by intranasal inoculation of 106 PFU/mouse in 10 μl of phosphate-buffered saline with Ca2+ and Mg2+ (PBS+) or mock infected with the same volume of PBS+ as previously described (56). Mice were monitored for pathological signs (ruffled fur and hunched posture) and euthanized as moribund according to European Union guidelines (86/609/CEE).
At the indicated postinfection times, cells or BM samples were collected to monitor virus multiplication. MVMi genomic and intermediate replicative forms were obtained by a modified Hirt procedure (38) with carrier tRNA to ensure quantitative yields and analyzed by Southern blotting with an MVM-specific probe as previously described (57). For NS-1 protein expression, cells were washed with PAB (1× PBS− plus 0.1% Na3N plus 0.5% bovine serum albumin) and fixed in 1% paraformaldehyde at 4°C for 30 min. After being washed with PAB, cells were permeabilized in PABT (PAB plus 0.1% Triton X-100) for 3 min at 4°C, and then a mouse anti-NS-1 MAb (56) was added and incubated for 1 h at 4°C. Cells were stained with an anti-mouse-biotin antibody and streptavidin-Tricolor (Caltag, South San Francisco, Calif.) and analyzed for immunofluorescence in a flow cytometer (EPICS Elite ESP).
Competitive repopulation assay.
The repopulating ability of BM cells was determined according to a previously described methodology (24, 61, 62). In brief, mock-infected as well as MVMi-infected BM samples from Ly-5.1 mice (test BM samples) were mixed together with an equal number of fresh BM cells harvested from Ly-5.2 mice (reference BM samples). Aliquots of the chimeric BM consisting of 4 × 106 total cells were transplanted into four Ly-5.2 recipients per group, previously irradiated with an X-ray myeloablative regimen of two doses of 5 Gy spaced 4 h apart (66). At various times after transplantation, 50 to 200 μl of peripheral blood (PB) was sampled from the tail vein and mixed with 0.5 M EDTA (10%, vol/vol). The competitive repopulating abilities (CRA) of test samples were determined by fluorescence-activated cell sorting analysis of Ly-5.1-positive PB cells with the anti-Ly-5.1 MAb clone A20 (59). For multilineage reconstitution analysis, dual-color immunofluorescence analyses were made with a PE-conjugated antibody for Ly-5.1 antigen and FITC-conjugated antibodies specific for T-cell (Thy-1.2), B-cell (B220), and myeloid (Gr-1) lineages (PharMingen). For each analysis, 50 μl of 5,000 to 10,000 viable PB cells was incubated with the corresponding MAbs for 30 min at 4°C in the dark; erythrocytes were lysed with 2.5 ml of lysing solution (0.155 mM NH4Cl plus 0.01 mM KHCO3 plus 10−4 mM EDTA) for 10 min at room temperature; and cells were washed with PBA (1× PBS− plus 0.1% [wt/vol] bovine serum albumin plus 0.02% [wt/vol] Na3N), then resuspended in PBA plus 2 μg of PI stain/ml to exclude dead cells, and analyzed in an EPICS Elite ESP flow cytometer. Off-line analysis was done with the WinMDI free software package (J. Trotter, The Scripps Research Institute, La Jolla, Calif.).
Measurement of exogenous engraftment.
Mock-infected, MVMi-infected, or X-irradiated SCID females were transplanted with 5 × 106 BM cells from male BALB/c mice. Mobilized PB cells with increased representation of neutrophils, or BM, were used as a source of DNA for testing the presence of male sequences hybridizing with a Y-chromosome probe (32). The mobilization procedure (42) was performed by subcutaneously injecting recombinant human granulocyte colony-stimulating factor (Amgen, Barcelona, Spain) at 12-h intervals to yield a treatment dose of 250 μg/kg/day for 4 consecutive days. Three hours after the last injection, PB was collected from the tail vein. Prior to DNA extraction, myeloid cells from mobilized PB or BM were labeled with Gr-1-FITC and Mac-1-FITC antibodies, red cells were lysed, and Gr-1+/Mac-1+ and Gr-1−/Mac-1− cells were sorted out (EPICS Elite). Reanalysis of the sorted fractions yielded purities above 90%. Extraction of the DNA from cell samples and dot blot analyses to evaluate the exogenous reconstitution in the SCID recipients were performed as previously described (67).
RESULTS AND DISCUSSION
Mouse HSCs are permissive for parvovirus MVMi replication and gene expression.
The multipotent hematopoietic long-term repopulating cells of the mouse are resistant to the cytotoxic effects of 5FU, negative for the expression of differentiation markers, and positive for the Sca-1 antigen (48, 59, 61). To test whether MVMi was able to multiply in the primitive compartment of hematopoietic long-term repopulating cells, a BM population from 5FU-treated mice defined by the surface phenotype Lin− Sca-1+ was isolated by a sorter-based procedure with MAbs (see Materials and Methods). Figure 1A shows the phenotype of the obtained midsize and low-complexity 98% pure population (5FUr/Lin− Sca-1+), with respect to normal BM and to light-density cells from 5FU-treated mice. The purified population represented 0.36% of the normal mouse BM cells, consistent with earlier studies (61, 69).
FIG. 1.
Primitive HSCs support parvovirus MVMi multiplication. (A) Purification of Lin− Sca-1+ hematopoietic precursors by sorting BM cells from 5FU-treated mice. The panel shows dual staining for lineage markers (anti-CD4, anti-CD8, anti-B220, anti-Mac-1, anti-Gr-1, and anti-TER-119) and Sca-1 marker at different steps of the purification protocol. The square gate was used in sorting Lin− Sca-1+ cells. 5FUr LD cells, 5FU-resistant low-density cells. (B) Replication of viral DNA. Sorted cells were infected with MVMi (5 PFU/cell) and cultured in the presence of IL-3, IL-6, and SCF. Cell samples (2 × 104 cells) were collected at 0 and 17 hpi and analyzed for the presence of viral genomic and replicative forms by Southern blotting with an MVM 32P-DNA probe. The filter was exposed for 48 h with an intensifying screen. V, single-stranded DNA genomes extracted from purified MVMi preparations (equivalent to 0.02 and 0.2 ng of viral DNA); mRF and dRF, monomeric and dimeric viral replicative intermediates. Lin− Sca+, 5FUr Lin− Sca-1+ sorted cells; 0 h, 0 hpi; 17 h, 17 hpi; BMi, low-molecular-weight DNA obtained at 17 hpi from nonadherent myeloid cells of long-term bone marrow cultures (57) infected with MVMi at 5 PFU/cell. (C) Expression of the nonstructural NS-1 protein. 5FUr Lin− Sca-1+-infected cells (5 × 104) were labeled with an anti-NS-1 MAb and analyzed by flow cytometry at 0 hpi (open histogram, 4.1%) and 17 hpi (filled histogram, 20.4%). M1, region established as 1% of the secondary antibody nonspecific staining.
The permissiveness of the purified hematopoietic precursors for the MVMi life cycle was analyzed with purified MVMi inoculated at a multiplicity of 5 PFU/cell and incubated with IL-3, IL-6, and SCF in order to stimulate the proliferation of the primitive HSCs. Since restrictions of MVM infections may operate at postentry stages of the virus cycle, either before transcription (3, 23) or as a block on DNA replication uncoupled from gene expression (22, 53), we quantitatively determined both viral DNA replication and protein expression during a single round of infection. Viral DNA replicative intermediates (mainly monomeric replicative forms [mRF]) of the correct size accumulated in the infected cells by 17 h postinfection (hpi) (Fig. 1B), and single-stranded genomes resulting from the encapsidation of replicative forms into maturing virions (17) were synthesized to high levels in these cells as well. The expression of the nonstructural protein NS-1, a cytotoxic polypeptide (11, 41) essential for replication and packaging of parvovirus DNA (11, 14, 16) and for transactivation of the viral promoters (18, 19, 51), was monitored by a fluorescence-activated cell sorting-based procedure with an NS-1-specific MAb. In the example shown in Fig. 1C, close to 4% of the cells showed low detectable levels of NS-1 at 0 hpi, a signal likely contributed by the binding to these cells of a relatively higher number of viral particles carrying a copy of NS-1 linked to the 5′ end of the genome (17). However, by 17 hpi, the level of expression and the proportion of cells showing specific NS-1 staining significantly increased to 20%, as expected for a highly susceptible asynchronous cell population. The viral replication and the synthesis of the NS-1 polypeptide in a high proportion of 5Fur/Lin− Sca-1+ cells demonstrated that, upon in vitro proliferative stimuli, purified mouse HSCs are permissive for important processes of the MVMi life cycle.
The parvovirus MVMi differentially inhibits HSCs showing short- and long-term repopulating capacities.
Hematopoietic precursors purified on the basis of phenotypic markers are functionally heterogeneous (33). Moreover, the in vitro stimulation of purified 5FUr/Lin− Sca-1+ cells, although performed with a high concentration of early-acting cytokines to preserve their self-renewal (20), may lead to their differentiation to precursors with less totipotency and engraftment potential. These possibilities prompted us to analyze the potential inhibitory effects of MVMi infection on HSCs from freshly harvested and nonstimulated BM. To this aim, the functionality of mouse primitive hematopoietic precursors was assessed by means of a competitive repopulating assay (see Materials and Methods), measuring the short- and long-term repopulating abilities of BM cells infected at an MOI of 1 or 20 PFU/cell (test population) against those of nonmanipulated and genetically identifiable BM cells (reference population). As shown in Table 1, in the short-term analysis (15 days posttransplantation [dpt]) of PB samples, the CRA value of BM samples infected with 1 PFU/cell was reduced to 31.4% of the corresponding values observed in mock-infected BM and to 16.6% when the infection was conducted at 20 PFU of MVMi/cell, fairly corresponding to the expected percentages of surviving cells at these multiplicities. These data indicated a high susceptibility of the short-term repopulating cells to MVMi infection, consistent with evidence showing that short-term repopulation is mostly accomplished by rapidly proliferating cells (12), which would be highly sensitive to MVMi replication and to the expression of cytotoxic viral gene products.
TABLE 1.
CRA of mouse BM infected with the parvovirus MVMia
| Time of analysis (dpt) | % Ly-5.1+ cells in blood
|
CRA survivalb (%)
|
|||
|---|---|---|---|---|---|
| Mock | MOI 1 | MOI 20 | MOI 1 | MOI 20 | |
| 15 | 58.26 ± 3.09 | 30.86 ± 0.89c | 19.13 ± 0.30c | 31.45 ± 3.94 | 16.66 ± 2.00 |
| 30 | 56.21 ± 1.95 | 40.60 ± 0.98c | 33.33 ± 1.38c | 52.93 ± 4.77 | 38.77 ± 4.73 |
| 72 | 52.49 ± 2.16 | 46.96 ± 2.41 | 31.58 ± 1.07c | 80.16 ± 10.42 | 41.50 ± 4.17 |
| 100 | 54.57 ± 0.52 | 52.35 ± 4.42 | 34.47 ± 0.86c | 94.26 ± 15.26 | 43.84 ± 4.61 |
| 300 | 38.39 ± 4.32 | 42.20 ± 7.83 | 18.95 ± 2.97c | 130.7 ± 52.28 | 36.52 ± 3.85 |
Data are given as the means ± the standard errors of the means from two independent experiments.
CRA survival is defined by S = (CRAMVM/CRAmock) × 100, where CRA = % Ly-5.1/(100 − % Ly-5.1).
Significantly different from mock-infected BM at P < 0.01.
Notably, the CRA gradually increased in the samples infected at an MOI of 1 over longer periods of analysis, reaching the value of uninfected controls by 100 to 300 dpt (Table 1). However, in the BM infected at a high MOI (20 PFU/cell), a consistent reduction in the CRA to close to 40% of the control was observed in the first month, and this value remained essentially unchanged throughout the entire posttransplantation period. To evaluate whether the repopulation defect induced by MVMi infection involves the myeloid and lymphoid lineages, the ability of Ly-5.1 BM infected at 20 PFU/cell to develop myeloid (Gr-1+), T-cell (Thy-1.2+), and B-cell (B220+) populations in the recipient mice was quantitatively determined at several posttransplantation points. A representative example of the results obtained at 300 dpt is shown in Fig. 2. In all cases, a significant reduction of severalfold in the Ly-5.1 long-term repopulating capacity in respect to the uninfected BM values was observed in three lymphohematopoietic differentiation lineages. Taken together, these data indicated that the in vitro infection of BM with MVMi inhibited the functionality of the short- and long-term multipotent repopulating precursors. Interestingly, the extent of suppression was lower for the more primitive HSCs as the decrease in the long-term CRA was manifested only at a high MOI. These results suggest that most MVMi interactions with long-term repopulating HSCs do not lead to a productive infection under their normal quiescent stage, but at a high viral input some of the initially internalized particles may remain infectious for months and unleash their cytotoxic capacity whenever these cells are recruited into proliferative activity.
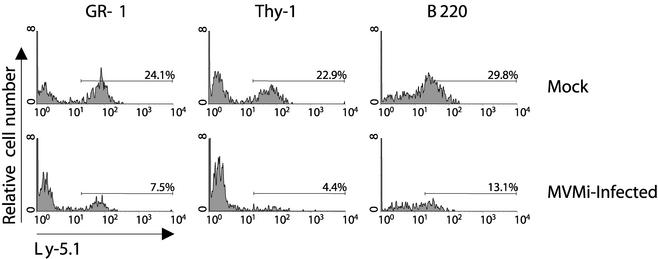
FIG. 2.
The lymphomyeloid repopulating ability of mouse HSCs is impaired by MVMi infection. Mock-infected and MVMi-infected (20 PFU/cell) BM cells (Ly-5.1 phenotype) were assayed for their CRAs against a reference nontreated BM (Ly-5.2 phenotype). At 300 dpt, PB samples from recipients were analyzed by double labeling to evaluate the contribution of the Ly-5.1 cells in the repopulation of T (Thy-1.2+), B (B220+), and myeloid (Gr-1+) cells. Numbers represent the proportions of Ly-5.1+ cells within each hematopoietic lineage.
BM transplantation (BMT) can rescue SCID mice from MVMi-induced lethal leukopenia.
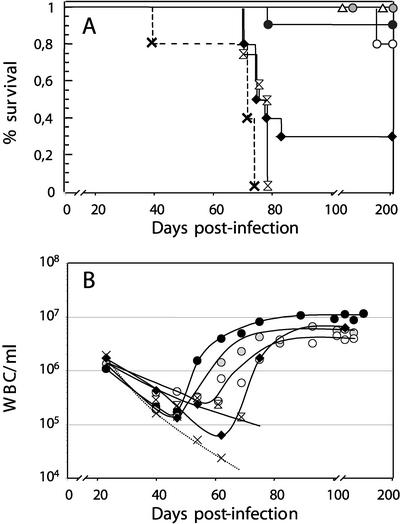
To investigate whether the capacity of MVMi to multiply and suppress HSC functions in vitro has any biological significance in the natural infection of the mouse host, the extent of damage to the HSC compartment was analyzed in mice with a severe combined immunodeficiency due to the absence of functional B and T lymphocytes (SCID mice) that were oronasally inoculated with MVMi. As an initial experiment the therapeutic capacity of exogenous BM transplants in lethally inoculated mice was quantitatively determined. Female MVMi-infected SCID mice were transplanted with different numbers of BM cells from syngeneic immunocompetent BALB/c male donors, and their survival and circulating white blood cells (WBC) were monitored during the posttransplantation period. As shown in Fig. 3A, consistent with our earlier studies (56), the nontransplanted MVMi-infected SCID mice developed evident pathological signs and an acute leukopenia by 40 days postinfection (dpi) with 100% incidence leading to death by 60 to 80 dpi, as did infected SCID mice receiving a large graft from immunodeficient donor mice. In contrast, most mice transplanted at 40 dpi with three doses of immunocompetent BM cells (ranging from 5 × 104 to 5 × 106) were rescued from the viral infection, changing the outcome of the disease from 100% lethality to up to 80 to 100% survival. Even at the height of the acute leukopenia (60 dpi), transplantation of 5 × 106 immunocompetent cells rescued about 20% of the moribund mice, which then survived for months afterwards.
FIG. 3.
Rescue of MVMi-induced fatal leukopenia by BMT. SCID mice intranasally inoculated with 106 PFU of MVMi/mouse were not transplanted (×, dotted line) or were transplanted at 40 dpi with either 5 × 104 (open circles), 5 × 105 (shaded circles), and 5 × 106 (solid circles) BALB/c or 5 × 106 SCID (double arrowheads) male BM cells or transplanted at 60 dpi with 5 × 106 (solid diamonds) male BALB/c BM cells. Control noninfected mice irradiated (2.5 Gy) and transplanted with 5 × 106 male BALB/c BM cells were also included (open triangles). (A) Percentage of survival of the mice analyzed at 200 dpi. Six to eight animals per group were used. (B) Concentration of WBC in PB. Each data point is the average value from at least five mice monitored weekly.
The survival of the mice was related to the number of circulating WBC during the BMT treatments. Thereby, mice rescued from the lethal infection by BMT showed a progressive recovery in their circulating WBC numbers (Fig. 3B), which in 40-dpi transplanted mice increased with kinetics correlating with the size of the exogenous immunocompetent graft. Furthermore, the number of WBC was stabilized at characteristic levels corresponding to the proportion of lymphoid precursors in the grafts, ranging from twice the average number found in SCID mice (in animals transplanted with 5 × 104 cells) to five times higher (animals transplanted with 5 × 106 cells), reaching the normal average value found in BALB/c mice. Thus, the damaged BM of SCID mice resulting from MVMi infection could be efficiently engrafted by immunocompetent lymphohematopoietic precursors, and the ensuing immune response controlled the lethal hematological disease and restored blood cell indices that remained stable for months. These results are in contrast to the inability of a repeated passive MAb therapy to control MVMi pathogenic capacity in the same virus-host system (35), suggesting the importance of a normal lymphohematopoietic cellular repertoire in controlling parvovirus evolutionary potential.
Persistence of two viral DNA species in the BM of transplanted mice.
We next investigated the fate of the virus in the BM of infected SCID recipients showing stabilized WBC levels. Infectious particles could not be detected in blood or BM homogenates at 100 dpi by plaque assay (data not shown), indicating that the virus present in hematopoietic organs of leukopenic mice (56) was cleared by the immune system following BMT. The prototype DNA replicative intermediates (mRF and dimeric replicative forms [dRF]) and single-stranded genomic forms accumulated to high levels in the BM of nontransplanted mice and in recipient mice transplanted with BM from SCID mice (Fig. 4). In agreement with the lack of infectious particles, these DNA forms were not demonstrable in mice cured by immunocompetent BMT. Instead, a remarkable uncommon pattern of low-molecular-weight viral DNA was consistently found in the BM of all the cured animals. As shown in Fig. 4, two low-abundance distinct DNA species were resolved in agarose gels after a long exposure of the blots: one migrated between the mRF and dRF with a size close to 8 kbp, and the other migrated as a molecule much larger than 20 kbp moving in the upper poorly resolved region of the gels. Both DNA species behaved as identical double-stranded DNAs (dsDNAs) in all the examined transplanted animals as judged by their cleavage with restriction endonucleases. The restriction analysis of the samples allowed the resolution of the EcoRI 2.3-kbp internal fragment of the MVM genome at submolar amounts (Fig. 4, “R” lanes) but of neither of the two predicted fragments generated by the single cutter enzyme XhoI (Fig. 4, bottom), which could be demonstrated only in the digested BM samples obtained from nontransplanted mice and mice transplanted with SCID marrow.
FIG. 4.
Persistence of parvovirus dsDNA species in reconstituted SCID mouse marrow. The figure shows a Southern analysis of low-molecular-weight DNA obtained from 105 BM cells of mice transplanted at 40 dpi with different amounts of immunocompetent cells (BALB/c-BM) or with SCID cells (SCID-BM). Analyses were performed at 100 and 60 dpi, respectively. Filters were exposed to autoradiography for 10 days (BALB/c donors), or 40 h (SCID donor) with an intensifying screen at −70°C. N.T., control BM sample from a nontransplanted MVMi-infected SCID mouse. Or, origin; N, undigested; R, EcoRI digested; X, XhoI digested; V, genomic forms isolated from purified MVMi virions. Open arrows, species of viral DNA present in the BALB/c reconstituted BM; open arrowheads, XhoI restriction fragments; solid arrowheads, EcoRI restriction fragments (the smallest 1.1-kbp fragment run out of the gel). At bottom is a map of the XhoI and EcoRI restriction sites in the mRF viral replicative form.
As for some helper-dependent members of the Parvovirinae that can integrate their genomes into a unique locus on human chromosome 19q13-qter (34, 72), MVM integration was demonstrated to occur at a similar frequency in an episomal model system (15), though never during productive or persistent infections of cell lines (52). The fact that the two MVM dsDNA molecules present in the rescued mice were recovered in the low-molecular-weight DNA fraction and their consistent size in agarose gels are incompatible with these species being viral DNA integrated in the host genome. Rather they may correspond to uncommon DNA replicative intermediates with heterogeneous ends. Indeed, the 8-kbp species may be related to a previously described partially replicated MVM dimer (21), and the large species may be related to a concatemer resolved in two-dimensional agarose gels (65). Further research is required to determine the exact nature of these dsDNA molecules and the BM cell types in which they persist in healthy mice long after infectious virus clearance. It would be highly interesting to study whether this DNA pattern represents a common latent genomic form for parvoviruses that, like the human B19 parvovirus, may persist in the BM (13), from which these viruses could reenter a productive cycle whenever the immune surveillance of the host was compromised.
MVMi suppresses long-term repopulating HSCs in naturally infected SCID mice.
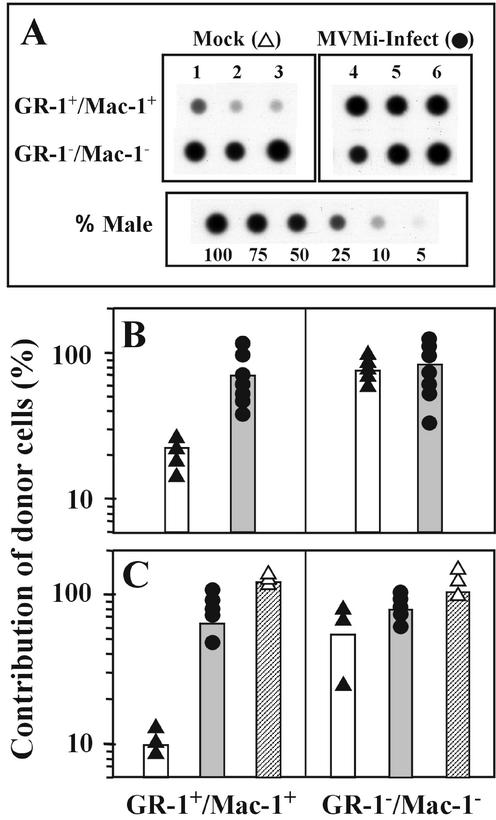
To investigate the degree of damage to the HSCs during the period of MVMi-induced leukopenia, healthy donor BM was transplanted into infected and noninfected SCID mice to evaluate the CRA of the stem cell compartment. For this purpose, the origin (exogenous or endogenous) of the BM cells of oronasally inoculated female SCID mice cured at 40 dpi by a BMT from a male immunocompetent graft was evaluated by measuring the level of engraftment of the donor cells at different posttransplantation times. Upon virus control by the immunocompetent cell boost, the hematopoiesis of these animals results from the functional competition between the endogenous primitive precursors that survived the infection and the transplanted repopulating cells. For a given number of transplanted cells, the rate of exogenous engraftment would therefore depend on the survival of endogenous HSCs. The recognized competitive repopulating advantage of donor BALB/c lymphoid cells over the host SCID cells (4) set the myeloid population as the only reliable indicator of donor stem cell engraftment, while the lymphoid cells were used as a general control of exogenous engraftment in the recipient mice. Thereby, hybridization analyses with a male-specific probe for the Y chromosome on purified myeloid (Gr-1+/Mac-1+) and nonmyeloid (Gr-1− Mac-1−) populations at 4 months posttransplantation indicated a very low engraftment of the exogenous BM in mobilized myeloid PB cells from mock-infected SCID mice, in contrast to the high myeloid reconstitution with donor repopulating cells in MVMi-infected mice (Fig. 5A). A densitometric measurement of the dot blots (Fig. 5B) showed a significant increase in the short-term engraftment of exogenous myeloid cells from an average close to 20% to as high as 70% by the MVMi infection of the SCID recipients.
FIG. 5.
Efficient long-term hematopoietic engraftment in MVMi-infected SCID mice. (A) Representative DNA dot blot analyses, with a Y-chromosome probe, of myeloid (Gr-1+/Mac-1+) and nonmyeloid (Gr-1−/Mac-1−) mobilized and sorted PB cells. Samples were obtained 4 months posttransplantation from mock- and MVMi-infected SCID mice transplanted at 40 dpi with donor male BM. Control samples hybridized in parallel harboring the indicated ratio of male cells are shown. (B and C) Quantitative densitometric analysis of dot blots hybridized with the Y-chromosome probe from mobilized PB cells sampled at 4 months posttransplantation (B) and from BM samples harvested at 7 months posttransplantation (C). All recipients were transplanted with the same graft of BM cells, including the positive-control group irradiated with a dose of 2.5 Gy. Each dot represents the analysis of a single mouse, and the bars indicate the mean values of the groups. Mouse groups were mock-infected recipients (solid triangles, open bars), MVMi-infected recipients (solid circles, shaded bars), and irradiated recipients (open triangles, crosshatched bars).
In the long-term repopulation studies, the extent of donor engraftment was genetically analyzed in a similar manner with BM samples harvested at 7 months posttransplantation. Interestingly, at this time of analysis the effect exerted on the exogenous engraftment by MVMi infection was even more pronounced. As illustrated in Fig. 5C, while most Gr-1−/Mac-1− cells were of donor origin, the extent of donor cells in the Gr-1+/Mac-1+ BM population increased from an average of close to 10% in the mock-infected mice to 60% in the MVMi-infected mice. Thus, the long-term myeloid reconstitution of MVMi-infected, but not that of mock-infected, SCID recipients is predominantly accomplished by exogenous repopulating cells. Taking into account that the 5 × 106 cells transplanted per mouse represent 3.5% of the BM content of the animal (54) and assuming a 10% seeding efficiency of the exogenous repopulating cells (36, 57), the ratio of the exogenous to the endogenous repopulating cells would be about 1:280. These data indicated that the intranasal inoculation of MVMi mediates a marked depletion of hematopoietic long-term repopulating cells (<0.3% survival), allowing the stable engraftment of an exogenous BM.
Concluding remarks.
We have demonstrated the susceptibility of the self-renewing HSCs to direct interaction with the cytotoxic parvovirus MVMi by testing in vitro their main biological characteristic not measurable in culture, namely, the multipotent repopulating capacity of the hematopoietic system. This remarkable tropism of an autonomous parvovirus may allow the development of lasting hematopoietic gene therapies based on transduction of HSCs in vitro as a strategy for transient expression in vivo long-term posttransplantation.
The much higher proportion of HSCs suppressed by MVMi in the BM of infected mice (Fig. 5) than in the direct in vitro interaction (Table 1) strongly suggests that the persistent severe leukopenia in the SCID mice triggers proliferative activity of an increasing number of cells from the pool of primitive quiescent HSCs, which become consequently susceptible to the viral cytotoxic infection. These hematological syndromes, though developed under a severe genetic immunodeficiency, may help to uncover delayed induced hematopoietic diseases in other viral systems with HSC targeting capacity.
A remarkable capacity of MVMi to persist in the BM is illustrated by two phenomena: (i) persistence of cytotoxic MVMi viral components for months in HSCs infected in vitro and transplanted into immunocompetent mice and (ii) prevalence of two uncommon viral dsDNA species for months in mice cured by BMT of a lethal MVMi infection. An understanding of the relevance of these patterns of persistence for the biology of parvovirus-host interactions deserves further study, but it is likely that the HSCs themselves may function as a reservoir of viruses in the immunocompetent hosts.
Finally, the sustained viral suppression of HSCs in vivo allowed mean long-term engraftments as high as those obtained in the group of 2.5-Gy X-irradiated control mice (Fig. 5C). Therefore, the intranasal inoculation of MVMi can mimic the effects produced by total body radiation conditioning in BMT. These results suggest that cytotoxic viruses with natural or engineered tropism to HSCs could potentially be implemented as biological agents for radiomimetic conditioning of recipients of BM transplants.
Acknowledgments
We thank G. Spangrude for providing us with anti-Ly-5.1 MAb (clone A20) and Juan C. Ramírez for critical reading of the manuscript. The technical assistance of I. Ormán, S. García, E. López, and J. Palacín is also gratefully acknowledged.
This work was supported by research grants from the Comisión Interministerial de Ciencia y Tecnología (CICYT) to J.A.B. and J.C.S. and SAF2001-1325 to J.M.A. and from the Dirección General de Investigación de la Comunidad de Madrid to G.G. and by an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular Severo Ochoa. J.M.G. was a fellow of the Gobierno Vasco.
REFERENCES
- 1.Almeida, G. D., C. D. Porada, S. St. Jeor, and J. L. Ascensao. 1994. Human cytomegalovirus alters interleukin-6 production by endothelial cells. Blood 83:370-376. [PubMed] [Google Scholar]
- 2.Anderson, M. J., E. Lewis, I. M. Kidd, S. M. Hall, and B. J. Cohen. 1984. An outbreak of erythema infectiosum associated with human parvovirus infection. J. Hyg. (London) 93:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonietti, J. P., R. Sahli, P. Beard, and B. Hirt. 1988. Characterization of the cell type-specific determinant in the genome of minute virus of mice. J. Virol. 62:552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blazar, B. R., P. A. Taylor, R. McElmurry, L. Tian, A. Panoskaltsis-Mortari, S. Lam, C. Lees, T. Waldschmidt, and D. A. Vallera. 1998. Engraftment of severe combined immune deficient mice receiving allogeneic bone marrow via in utero or postnatal transfer. Blood 92:3949-3959. [PubMed] [Google Scholar]
- 5.Bonnard, G. D., E. K. Manders, D. A. Campbell, Jr., R. B. Herberman, and M. J. Collins, Jr. 1976. Immunosuppressive activity of a subline of the mouse EL-4 lymphoma. Evidence for minute virus of mice causing the inhibition. J. Exp. Med. 143:187-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosma, G. C., R. P. Custer, and M. J. Bosma. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527-530. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, G. B., B. Williams, R. Rossi, and I. Bertoncello. 1997. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp. Hematol. 25:445-453. [PubMed] [Google Scholar]
- 8.Brown, K. E., S. M. Anderson, and N. S. Young. 1993. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114-117. [DOI] [PubMed] [Google Scholar]
- 9.Brown, T., A. Anand, L. D. Ritchie, J. P. Clewley, and T. M. Reid. 1984. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet ii:1033-1034. [DOI] [PubMed]
- 10.Brownstein, D. G., A. L. Smith, R. O. Jacoby, E. A. Johnson, G. Hansen, and P. Tattersall. 1991. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab. Investig. 65:357-364. [PubMed] [Google Scholar]
- 11.Caillet-Fauquet, P., M. Perros, A. Brandenburger, P. Spegelaere, and J. Rommelaere. 1990. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 9:2989-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cashman, J. D., T. Lapidot, J. C. Wang, M. Doedens, L. D. Shultz, P. Lansdorp, J. E. Dick, and C. J. Eaves. 1997. Kinetic evidence of the regeneration of multilineage hematopoiesis from primitive cells in normal human bone marrow transplanted into immunodeficient mice. Blood 89:4307-4316. [PubMed] [Google Scholar]
- 13.Cassinotti, P., G. Burtonboy, M. Fopp, and G. Siegl. 1997. Evidence for persistence of human parvovirus B19 DNA in bone marrow. J. Med. Virol. 53:229-232. [PubMed] [Google Scholar]
- 14.Cornelis, J. J., P. Becquart, N. Duponchel, N. Salome, B. L. Avalosse, M. Namba, and J. Rommelaere. 1988. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J. Virol. 62:1679-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corsini, J., J. Tal, and E. Winocour. 1997. Directed integration of minute virus of mice DNA into episomes. J. Virol. 71:9008-9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotmore, S. F., and P. Tattersall. 1987. The autonomously replicating parvoviruses of vertebrates. Adv. Virus Res. 33:91-174. [DOI] [PubMed] [Google Scholar]
- 17.Cotmore, S. F., and P. Tattersall. 1988. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5′ termini of duplex replicative-form DNA and progeny single strands. J. Virol. 62:851-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doerig, C., B. Hirt, J. P. Antonietti, and P. Beard. 1990. Nonstructural protein of parvoviruses B19 and minute virus of mice controls transcription. J. Virol. 64:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doerig, C., B. Hirt, P. Beard, and J. P. Antonietti. 1988. Minute virus of mice non-structural protein NS-1 is necessary and sufficient for trans-activation of the viral P39 promoter. J. Gen. Virol. 69:2563-2573. [DOI] [PubMed] [Google Scholar]
- 20.Ema, H., H. Takano, K. Sudo, and H. Nakauchi. 2000. In vitro self-renewal division of hematopoietic stem cells. J. Exp. Med. 192:1281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faust, E. A., and G. Gloor. 1984. Characterization of a metastable, partially replicated dimeric intermediate of minute virus of mice. J. Virol. 49:621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox, E., P. T. Moen, Jr., and J. W. Bodnar. 1990. Replication of minute virus of mice DNA in adenovirus-infected or adenovirus-transformed cells. Virology 176:403-412. [DOI] [PubMed] [Google Scholar]
- 23.Gardiner, E. M., and P. Tattersall. 1988. Evidence that developmentally regulated control of gene expression by a parvoviral allotropic determinant is particle mediated. J. Virol. 62:1713-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison, D. E., C. T. Jordan, R. K. Zhong, and C. M. Astle. 1993. Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple binomial, correlation and covariance calculations. Exp. Hematol. 21:206-219. [PubMed] [Google Scholar]
- 25.Heegaard, E. D., and K. E. Brown. 2002. Human parvovirus B19. Clin. Microbiol. Rev. 15:485-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacoby, R. O., L. Ball-Goodrich, D. G. Besselsen, M. D. McKisic, L. K. Riley, and A. L. Smith. 1996. Rodent parvovirus infections. Lab. Anim. Sci. 46:370-380. [PubMed] [Google Scholar]
- 27.Korbling, M., R. L. Katz, A. Khanna, A. C. Ruifrok, G. Rondon, M. Albitar, R. E. Champlin, and Z. Estrov. 2002. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N. Engl. J. Med. 346:738-746. [DOI] [PubMed] [Google Scholar]
- 28.Krause, D. S., N. D. Theise, M. I. Collector, O. Henegariu, S. Hwang, R. Gardner, S. Neutzel, and S. J. Sharkis. 2001. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 105:369-377. [DOI] [PubMed] [Google Scholar]
- 29.Kurtzman, G. J., K. Ozawa, B. Cohen, G. Hanson, R. Oseas, and N. S. Young. 1987. Chronic bone marrow failure due to persistent B19 parvovirus infection. N. Engl. J. Med. 317:287-294. [DOI] [PubMed] [Google Scholar]
- 30.LaBarge, M. A., and H. M. Blau. 2002. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111:589-601. [DOI] [PubMed] [Google Scholar]
- 31.Lamana, M. L., B. Albella, J. A. Bueren, and J. C. Segovia. 2001. In vitro and in vivo susceptibility of mouse megakaryocytic progenitors to strain i of parvovirus minute virus of mice. Exp. Hematol. 29:1303-1309. [DOI] [PubMed] [Google Scholar]
- 32.Lamar, E. E., and E. Palmer. 1984. Y-encoded, species-specific DNA in mice: evidence that the Y chromosome exists in two polymorphic forms in inbred strains. Cell 37:171-177. [DOI] [PubMed] [Google Scholar]
- 33.Li, C. L., and G. R. Johnson. 1992. Rhodamine123 reveals heterogeneity within murine Lin−, Sca-1+ hemopoietic stem cells. J. Exp. Med. 175:1443-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linden, R. M., P. Ward, C. Giraud, E. Winocour, and K. I. Berns. 1996. Site-specific integration by adeno-associated virus. Proc. Natl. Acad. Sci. USA 93:11288-11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López-Bueno, A., M. G. Mateu, and J. M. Almendral. 2003. High mutant frequency in populations of a DNA virus allows evasion from antibody therapy in an immunodeficient host. J. Virol. 77:2701-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lord, B. I., J. H. Hendry, J. P. Keene, B. W. Hodgson, C. X. Xu, M. Rezvani, and T. J. Jordan. 1984. A comparison of low and high dose-rate radiation for recipient mice in spleen-colony studies. Cell Tissue Kinet. 17:323-334. [DOI] [PubMed] [Google Scholar]
- 37.Manchester, M., K. A. Smith, D. S. Eto, H. B. Perkin, and B. E. Torbett. 2002. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J. Virol. 76:6636-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMaster, G. K., P. Beard, H. D. Engers, and B. Hirt. 1981. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J. Virol. 38:317-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metcalf, D. 1989. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature 339:27-30. [DOI] [PubMed] [Google Scholar]
- 40.Mezey, E., K. J. Chandross, G. Harta, R. A. Maki, and S. R. McKercher. 2000. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science 290:1779-1782. [DOI] [PubMed] [Google Scholar]
- 41.Moffatt, S., N. Yaegashi, K. Tada, N. Tanaka, and K. Sugamura. 1998. Human parvovirus B19 nonstructural (NS1) protein induces apoptosis in erythroid lineage cells. J. Virol. 72:3018-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molineux, G., Z. Pojda, I. N. Hampson, B. I. Lord, and T. M. Dexter. 1990. Transplantation potential of peripheral blood stem cells induced by granulocyte colony-stimulating factor. Blood 76:2153-2158. [PubMed] [Google Scholar]
- 43.Morrison, S. J., N. Uchida, and I. L. Weissman. 1995. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11:35-71. [DOI] [PubMed] [Google Scholar]
- 44.Mortimer, P. P., R. K. Humphries, J. G. Moore, R. H. Purcell, and N. S. Young. 1983. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. Nature 302:426-429. [DOI] [PubMed] [Google Scholar]
- 45.Moses, A. V., S. Williams, M. L. Heneveld, J. Strussenberg, M. Rarick, M. Loveless, G. Bagby, and J. A. Nelson. 1996. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood 87:919-925. [PubMed] [Google Scholar]
- 46.Orlic, D., L. J. Girard, C. T. Jordan, S. M. Anderson, A. P. Cline, and D. M. Bodine. 1996. The level of mRNA encoding the amphotropic retrovirus receptor in mouse and human hematopoietic stem cells is low and correlates with the efficiency of retrovirus transduction. Proc. Natl. Acad. Sci. USA 93:11097-11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orlic, D., J. Kajstura, S. Chimenti, I. Jakoniuk, S. M. Anderson, B. Li, J. Pickel, R. McKay, B. Nadal-Ginard, D. M. Bodine, A. Leri, and P. Anversa. 2001. Bone marrow cells regenerate infarcted myocardium. Nature 410:701-705. [DOI] [PubMed] [Google Scholar]
- 48.Osawa, M., K. Hanada, H. Hamada, and H. Nakauchi. 1996. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273:242-245. [DOI] [PubMed] [Google Scholar]
- 49.Pattison, J. R., S. E. Jones, J. Hodgson, L. R. Davis, J. M. White, C. E. Stroud, and L. Murtaza. 1981. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet i:664-665. [DOI] [PubMed]
- 50.Ponnazhagan, S., P. Mukherjee, X. S. Wang, K. Qing, D. M. Kube, C. Mah, C. Kurpad, M. C. Yoder, E. F. Srour, and A. Srivastava. 1997. Adeno-associated virus type 2-mediated transduction in primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. J. Virol. 71:8262-8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhode, S. L., III. 1985. trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J. Virol. 55:886-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ron, D., and J. Tal. 1985. Coevolution of cells and virus as a mechanism for the persistence of lymphotropic minute virus of mice in L cells. J. Virol. 55:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubio, M. P., S. Guerra, and J. M. Almendral. 2001. Genome replication and postencapsidation functions mapping to the nonstructural gene restrict the host range of a murine parvovirus in human cells. J. Virol. 75:11573-11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schofield, R., and L. J. Cole. 1968. An erythrocyte defect in splenectomized X-irradiated mice restored with spleen colony cells. Br. J. Haematol. 14:131-140. [DOI] [PubMed] [Google Scholar]
- 55.Segovia, J. C., J. A. Bueren, and J. M. Almendral. 1995. Myeloid depression follows infection of susceptible newborn mice with the parvovirus minute virus of mice (strain i). J. Virol. 69:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segovia, J. C., J. M. Gallego, J. A. Bueren, and J. M. Almendral. 1999. Severe leukopenia and dysregulated erythropoiesis in SCID mice persistently infected with the parvovirus minute virus of mice. J. Virol. 73:1774-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Segovia, J. C., A. Real, J. A. Bueren, and J. M. Almendral. 1991. In vitro myelosuppressive effects of the parvovirus minute virus of mice (MVMi) on hematopoietic stem and committed progenitor cells. Blood 77:980-988. [PubMed] [Google Scholar]
- 58.Sirven, A., F. Pflumio, V. Zennou, M. Titeux, W. Vainchenker, L. Coulombel, A. Dubart-Kupperschmitt, and P. Charneau. 2000. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood 96:4103-4110. [PubMed] [Google Scholar]
- 59.Spangrude, G. J., S. Heimfeld, and I. L. Weissman. 1988. Purification and characterization of mouse hematopoietic stem cells. Science 241:58-62. [DOI] [PubMed] [Google Scholar]
- 60.Srivastava, A., and L. Lu. 1988. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. J. Virol. 62:3059-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szilvassy, S. J., and S. Cory. 1993. Phenotypic and functional characterization of competitive long-term repopulating hematopoietic stem cells enriched from 5-fluorouracil-treated murine marrow. Blood 81:2310-2320. [PubMed] [Google Scholar]
- 62.Szilvassy, S. J., R. K. Humphries, P. M. Lansdorp, A. C. Eaves, and C. J. Eaves. 1990. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc. Natl. Acad. Sci. USA 87:8736-8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi, T., K. Ozawa, K. Takahashi, S. Asano, and F. Takaku. 1990. Susceptibility of human erythropoietic cells to B19 parvovirus in vitro increases with differentiation. Blood 75:603-610. [PubMed] [Google Scholar]
- 64.Torok, T. J. 1992. Parvovirus B19 and human disease. Adv. Intern. Med. 37:431-455. [PubMed] [Google Scholar]
- 65.Tullis, G., R. V. Schoborg, and D. J. Pintel. 1994. Characterization of the temporal accumulation of minute virus of mice replicative intermediates. J. Gen. Virol. 75:1633-1646. [DOI] [PubMed] [Google Scholar]
- 66.Varas, F., A. Bernad, J. M. Almendral, and J. A. Bueren. 1996. Relevance of myeloablative conditioning in the engraftment of limiting numbers of normal and genetically marked lympho-hematopoietic stem cells. Bone Marrow Transplant. 18:981-989. [PubMed] [Google Scholar]
- 67.Varas, F., A. Bernad, and J. A. Bueren. 1996. Granulocyte colony-stimulating factor mobilizes into peripheral blood the complete clonal repertoire of hematopoietic precursors residing in the bone marrow of mice. Blood 88:2495-2501. [PubMed] [Google Scholar]
- 68.Vuorinen, T., R. Vainionpaa, R. Vanharanta, and T. Hyypia. 1996. Susceptibility of human bone marrow cells and hematopoietic cell lines to coxsackievirus B3 infection. J. Virol. 70:9018-9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weissman, I. L. 2000. Stem cells: units of development, units of regeneration, and units in evolution. Cell 100:157-168. [DOI] [PubMed] [Google Scholar]
- 70.Wolter, S., R. Richards, and R. W. Armentrout. 1980. Cell cycle-dependent replication of the DNA of minute virus of mice, a parvovirus. Biochim. Biophys. Acta 607:420-431. [DOI] [PubMed] [Google Scholar]
- 71.Young, N. S. 1993. Viruses and bone marrow. Marcel Dekker, Inc., New York, N.Y.
- 72.Young, S. M., Jr., D. M. McCarty, N. Degtyareva, and R. J. Samulski. 2000. Roles of adeno-associated virus Rep protein and human chromosome 19 in site-specific recombination. J. Virol. 74:3953-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeldis, J. B., H. Mugishima, H. N. Steinberg, E. Nir, and R. P. Gale. 1986. In vitro hepatitis B virus infection of human bone marrow cells. J. Clin. Investig. 78:411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhong, Q., P. Oliver, W. Huang, D. Good, V. La Russa, Z. Zhang, J. R. Cork, R. W. Veith, C. Theodossiou, J. K. Kolls, and P. Schwarzenberger. 2001. Efficient c-kit receptor-targeted gene transfer to primary human CD34-selected hematopoietic stem cells. J. Virol. 75:10393-10400. [DOI] [PMC free article] [PubMed] [Google Scholar]