Abstract
Epstein-Barr virus (EBV) infects human resting B cells and transforms them in vitro into continuously growing lymphoblastoid cell lines (LCLs). EBV nuclear antigen 2 (EBNA2) is one of the first viral proteins expressed after infection. It is able to transactivate viral as well as cellular target genes by interaction with cellular transcription factors. EBNA2 target genes can be studied easily by using an LCL (ER/EB2-5) in which wild-type EBNA2 is replaced by an estrogen-inducible EBNA2. Since the cell surface molecule CD83, a member of the immunoglobulin superfamily and a marker for mature dendritic cells, appeared on the surface of ER/EB2-5 cells within 3 h after the addition of estrogen, we analyzed the regulation of CD83 induction by EBV in more detail. Despite its rapid induction, CD83 turned out to be an indirect target gene of EBNA2. We could show that the viral latent membrane protein 1 (LMP1) is responsible for the induction of CD83 by using an LCL expressing a ligand- or antibody-inducible recombinant nerve growth factor receptor-LMP1 fusion protein. The inducibility of the CD83 promoter by LMP1 was mediated by the activation of NF-κB, as seen by use of luciferase reporter assays using the CD83 promoter and LMP1 mutants. Additionally, fusion constructs of the transmembrane domain of LMP1 and the intracellular signaling domain of CD40, TNF-R1, and TNF-R2 likewise transactivated the CD83 promoter via NF-κB. Our studies show that CD83 is also a target of the NF-κB signaling pathway in B cells.
Epstein-Barr virus (EBV) is a B-lymphotropic gammaherpesvirus that is associated with several human malignant diseases, including lymphoproliferations in immunocompromised individuals, Burkitt's lymphoma, nasopharyngeal carcinoma, and Hodgkin's disease. In vitro, EBV transforms resting human B lymphocytes into continuously proliferating lymphoblastoid cell lines (LCLs). In these cells, EBV expresses six nuclear proteins (EBNA1, -2, -3A, -3B, -3C, and -LP), three integral latent membrane proteins (LMP1, LMP2A, and LMP2B), two small nuclear RNAs (EBV-encoded RNAs EBER1 and EBER2), and BamHI-A rightward transcripts. EBNA1, -2, -3A, and -3C as well as LMP1 seem to be absolutely required for B-cell immortalization by EBV (for review, see references 6 and 30).
Together with EBNA-LP, EBNA2 is the first viral protein expressed in infected cells and is absolutely required for initiation and maintenance of immortalization (29). EBNA2 is a master regulator that transactivates viral (e.g., LMP1 and LMP2) and cellular (e.g., CD21, CD23, and c-myc) genes involved in B-cell activation and induction of proliferation (27, 29). Additional B-cell activation markers and adhesion molecules such as CD39, CD40, CD54, and CD58 are induced by EBNA2 target genes (6, 30). EBNA2 by itself does not bind to DNA directly. It activates its target genes by interacting with cellular proteins, such as RBP-Jκ, PU.1, and AUF1, directly or indirectly (13, 17, 21, 37, 54, 62).
To dissect the early steps in B-cell immortalization, we established an LCL in which the function of EBNA2 has been rendered inducible. EBNA2 was fused to the hormone binding domain of the estrogen receptor (ER), and the fusion gene was used to rescue immortalization by the transformation-incompetent P3HR1 virus, generating a conditional LCL (ER/EB2-5 [29]). In the presence of estrogen the ER-EBNA2 fusion protein is located in the nucleus and can transactivate its target genes, whereas in the absence of estrogen the ER-EBNA2 fusion protein is sequestered to the cytoplasm. ER/EB2-5 cells proliferate in the presence of estrogen, and their growth is arrested when estrogen is withdrawn.
LMP1 is a direct target gene of EBNA2. It is an integral membrane protein of 386 amino acids (aa) composed of a short intracellular N terminus, six hydrophobic transmembrane domains, and an intracellular C terminus including three functional domains, CTAR1, CTAR2, and CTAR3 (for C-terminal activator region). LMP1 is the only EBV protein able to transform rodent fibroblasts (4, 42, 58). It binds tumor necrosis factor receptor (TNFR)-associated factors (TRAF1, -2, -3, and -5) directly via the PxQxT site in CTAR1 (7, 9, 10, 43, 50). TNFR-associated death domain protein (TRADD) interacts with tyrosines 384 and 385 in the CTAR2 domain, thereby recruiting TRAF2 to the complex (25). In addition, TRAF6 is recruited to the complex by indirect interaction with CTAR1 and CTAR2 (52). By virtue of its transmembrane domain, LMP1 acts as a constitutively active receptor of the TNFR family (12, 16, 43). Members of the TNFR family play an important role in the functional regulation of lymphocytes, including their activation, differentiation, proliferation, and survival. LMP1 thus shares some functional homology with CD40 (16, 20, 33, 57).
LMP1 activates its target genes via different signaling pathways that include NF-κB, AP-1, p38-MAPK/ATF, and STAT (11, 15, 18, 32, 36). Both CTAR1 and CTAR2 contribute to NF-κB activation, although to a different extent (23, 41). Upon recruitment of TRAF2 and TRAF6 to LMP1, the inhibitor of κB kinase complex becomes activated and phosphorylates I-κBα at Ser-32 and Ser-36. I-κBα is ubiquitinated and targeted for proteasomal degradation (19, 22). NF-κB is additionally phosphorylated and translocated into the nucleus to activate its target genes (28).
CD83 is a cell surface glycoprotein with a molecular weight of 40 to 45 kDa that belongs to the immunoglobulin (Ig) superfamily. It consists of an N-terminal extracellular V-type Ig-like domain, a transmembrane domain, and a short 39-aa cytoplasmic tail (38, 61). CD83 has been described as a marker of mature dendritic cells (DCs), including Langerhans' cells and interdigitating reticulum cells in the T-cell zones of the lymph nodes, and of activated B and T lymphocytes (60). In immature monocyte-derived (Mo) DCs CD83 is upregulated after stimulation with lipopolysaccharide, tumor necrosis factor alpha (TNF-α), or CD40 ligand (2, 3, 8, 39). Furthermore, CD83 is expressed on Hodgkin cells (55). Granulocyte-macrophage colony-stimulating factor and TNF-α CD83 expression is induced on monocytes, granulocyte-precursor cells, and myelocytes by the addition of a high dose of interleukin-4 (44, 60). CD83 has also been reported to be expressed on polymorphonuclear neutrophils (24, 59).
Recently, a role for CD83 in CD4+-T-cell development was demonstrated by researchers studying CD83−/− mice (14). CD83 plays a regulatory role during T-cell differentiation and affects the decision that governs the development of singly positive (SP) CD4+ T cells in the thymus. Reduction of SP CD4+ T cells in CD83−/− mice in the thymus also resulted in a reduction of naïve CD4+ T cells in the periphery. CD83 was also shown to be expressed on thymus epithelial cells (TEC) (14).
By studying the induction of cellular genes upon addition of estrogen to estrogen-deprived ER/EB2-5 cells, we have observed that CD83 is induced by viral proteins. The rapid induction of CD83 within 2 hours after the addition of estrogen prompted us to investigate the mechanism of CD83 induction. By using a derivative ER/EB2-5 cell line expressing LMP1 constitutively and an LCL expressing a conditional LMP1 gene, we were able to demonstrate that CD83 is a target of LMP1. The analysis of LMP1 mutants and CD83 promoter deletion constructs revealed that NF-κB is the critical transcription factor responsible for LMP1-mediated upregulation of CD83 expression.
MATERIALS AND METHODS
Cell lines and culture conditions.
ER/EB2-5 cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (Dynamics, Heidelberg, Germany), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1 mM sodium pyruvate, 1% l-glutamine (all from Invitrogen/Life Technologies), and 2 μM β-estradiol (Sigma Aldrich). For depletion of estrogen, cells were washed three times in phosphate-buffered saline supplemented with 10% fetal calf serum and were resuspended in supplemented RPMI 1640 medium without β-estradiol. 293-T cells were cultured in Dulbecco's modified Eagle medium with the same supplements as those used for ER/EB2-5 cells.
The LCL B2264-19/3 was generated by infection of primary human B lymphocytes (isolated from cord blood) with recombinant EBV in which LMP1 had been replaced by a nerve growth factor receptor (NGF-R)-LMP1 fusion gene consisting of aa 1 to 279 of the human low-affinity NGF-R fused to aa 192 to 386 of LMP1. The cells were grown in supplemented RPMI 1640 medium on γ-irradiated WI38 human fibroblasts (obtained from American Type Culture Collection) as a feeder layer. For induction of the NGF-R-LMP1 fusion protein, B2264-19/3 cells were removed from the WI38 feeder layer for 1 week and then 5 × 105 cells/ml were treated with 0.5 μg of anti-NGF-R antibody (hybridoma supernatant no. HB8737; American Type Culture Collection) per ml. For cross-linking, 5 μg of goat anti-mouse secondary antibody (IgG plus IgM [heavy-plus-light chains]; Dianova) per ml was added 1 h later for the indicated times.
FACS analysis.
For fluorescence-activated cell sorter (FACS) analysis cells were washed once and incubated with the indicated primary antibodies or isotype controls (IgG1, IgG2a, and IgG2b). Antibodies for human CD10, CD16, CD21, CD23, CD38, CD39, CD40, CD54, CD58, and CD95 and IgM were purchased from Dianova; antibodies for human CD80, CD86, HLA-A, -B, -C, -DR, -DP, and -DQ were purchased from Pharmingen/Becton Dickinson and anti-CD83 (HB15A) antibody was purchased from Coulter Immunotech (Marseilles, France). Goat anti-mouse antibody was used as secondary antibody (Dianova). Dead cells were excluded by propidium iodide staining. Samples were analyzed with the FACS Calibur instrument (Becton Dickinson).
Northern blot analysis.
Total RNA was prepared using the Qiagen RNeasy Midi kit according to the manufacturer's instructions (Qiagen, Hilden, Germany). Ten micrograms of total RNA per lane was subjected to electrophoresis and afterwards blotted onto a nylon membrane (Hybond N+; Amersham Pharmacia) as described previously (49). Membranes were hybridized with a CD83 full-length cDNA probe (kindly provided by J. Hauber, Hamburg, Germany) or a PCR product of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (primers 5′-TCCACCACCCTGTTGCTGTA-3′ and 5′-ACCACAGTCCATGCCATCAC-3′) radiolabeled with [α32-P]dCTP according to the manufacturer's protocols (Rediprime II; Amersham Pharmacia).
Cell fractionation and Western blot analysis.
All subcellular fractionations were done as described previously (51). Briefly, 1 × 108 to 3 × 108 proliferating or estrogen-depleted ER/EB2-5 cells were resuspended in homogenization buffer (10 mM Tris-acetate [pH 7.0], 250 mM sucrose, 1 mM phenylmethylsulfonyl fluoride, 5 μg of pepstatin A per ml, 10 μg of leupeptin per ml), subjected to Dounce homogenization, and fractionated into plasma membrane (PM)-nucleus (2 min at 4,000 × g), endosomal-lysosomal (2 min at 100,000 × g), and microsomal (10 min at 400,000 × g) compartments by differential centrifugation. The supernatant after centrifugation at 400,000 × g was defined as cytosol.
For Western blot analysis, 100 μg of the protein extracts was electrophoretically separated on sodium dodecyl sulfate-10 and 15% polyacrylamide gels under reducing conditions and transferred onto a polyvinylidene difluoride membrane (Hybond P; Amersham Pharmacia). Membranes were blocked for 1 h in TBST buffer (10 mM Tris-HCl [pH 7.5], 20 mM NaCl, 1% Tween 20) containing 5% dry milk (Merck) and then incubated in TBST containing 3% dry milk overnight at 4°C with either anti-CD83, anti-CD86, anti-HLA-A, -B, or -C, or anti-LMP1 (Becton Dickinson) antibodies. After being washed, membranes were incubated with peroxidase-labeled goat anti-mouse secondary antibody (Dianova) in TBST containing 3% dry milk for 1 h at room temperature. Finally, blots were washed three times in TBST containing 3% dry milk. Proteins were visualized with the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Uppsala, Sweden).
Plasmid construction and mutagenesis.
The expression plasmids pSV-LMP1, pSV-LMP1 (PQT-AAA), pSV-LMP1 (PQT-AAA/Y384G), pSV-LMP1 Δ194-386, p35, pSV-LMP1:CD40, and pSV-LMP1:TNF-R2 have been described previously (16, 19, 31, 32, 53). The expression plasmid pSV-LMP1:HA/TNF-R1 was cloned by a PCR approach from a TNF-R1 cDNA into the pSV-LMP1 plasmid. The β-galactosidase reporter PGKβGal was kindly provided by S. Wagener. The plasmids pGL CD83 (−3037), pGL CD83 (−261), pGL CD83 (−123), and pGL CD83 (−123mut) have been described previously (5). pGL Basic was purchased from Promega.
Transient transfection and luciferase reporter assay.
293-T cells (4 × 105 per well) were seeded in a six-well plate and transfected the following day with 2 μg of DNA using Lipofectamine (Life Technologies/Invitrogen) according to the manufacturer's protocol. Vector DNA was added to adjust for equal amounts of transfected DNA. After 16 to 20 h, cells were harvested, lysed in extraction buffer (10% [wt/vol] glycerine, 1% [wt/vol] Triton X-100, 2 mM EDTA [pH 8.0], 25 mM Tris-HCl [pH 7.8], 2 mM dithiothreitol), and centrifuged, and the supernatant was collected. Ten microliters of the protein extract was measured in a Mikro Lumat LB96P (Berthold, Wildbach, Germany) by adding 50 μl of the luciferase assay buffer [20 mM Tricin, 107 mM 4(MgCO3) · Mg(OH)2 · 5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 33.3 mM dithiothreitol, 270 μM acetyl-coenzyme A (Roche), 530 μM ATP (Roche), and 470 μM d-Luciferin (Roche)]. For the β-galactosidase assay, 10 μl of protein extract was incubated for 20 min in 100 μl of reaction buffer A (100 mM Na2HPO4-NaH2PO4 [pH 8.0], 0.1 mM MgCl, 1% Galacton-Plus [Applied Biosystems]) and measured in a Mikro Lumat LB96P with the addition of 50 μl of reaction buffer B (0.2 M NaOH, 10% Emerald-Enhancer [Applied Biosystems]). The reporter activity was calculated relative to that of β-galactosidase expression. All data were obtained from at least three independent experiments, and each experiment was performed in duplicate. The relative promoter activities were depicted as means ± standard deviations (SD).
RESULTS
CD83 is strongly induced in ER/EB2-5 cells after addition of estrogen.
To study the regulation of cellular genes by EBNA2, we used the LCL ER/EB2-5 in which wild-type EBNA2 is replaced by estrogen-inducible EBNA2. ER/EB2-5 cells were deprived of estrogen for 4 days, and the expression of a panel of representative B-cell surface markers was determined in these cells as well as in cells that had been reinduced by the addition of estrogen (Fig. 1). Several genes, those for CD38, IgM (26), and HLA-A, -B, and -C, were found to be upregulated upon withdrawal of estrogen. Several surface markers known to be induced by EBV were poorly, if at all, downregulated, indicating that the half-lives of these proteins are probably too long to see pronounced effects in this experimental setting (CD10, CD16, CD21, CD23, CD39, CD40, CD54, CD58, CD80, and CD95). The pattern of expression was heterogeneous for CD86 upon withdrawal of estrogen. Only CD83 and HLA-DR, -DP, and -DQ were uniformly downregulated. We focused our attention on CD83, because CD83 had completely disappeared from the surfaces of estrogen-deprived ER/EB2-5 cells (Fig. 1) and was rapidly induced upon the addition of estrogen (see Fig. 3B). To determine whether CD83 was degraded or just removed from the cell surface, estrogen-deprived and -induced cells were fractionated and the fractions representing the various subcellular compartments were subjected to gel electrophoresis and Western blotting. As shown in Fig. 2, CD83 was present mainly in the endosomal-lysosomal fraction of estrogen-treated ER/EB2-5 cells and was almost undetectable in any of the fractions after estrogen deprivation. In contrast, the representation of CD86 in the various cellular compartments remained virtually unaltered despite the fact that CD86 was downregulated to a considerable extent when estrogen was withdrawn. Notably, CD83 visualized by Western blotting appeared as many distinct bands in the gel, presumably reflecting the large and variable degree of glycosylation. This indicated that the regulated expression of CD83 on the cell surface is not a consequence of cellular redistribution; rather, it represents rapid degradation and resynthesis of CD83 upon withdrawal and then addition of estrogen, respectively.
FIG. 1.
CD83 disappears from the surface of ER/EB2-5 cells upon estrogen deprivation. Expression of cell surface markers on ER/EB2-5 cells proliferating continuously in the presence of estrogen (shaded) or deprived of estrogen for 96 h (thick lines) as measured by flow cytometry. Isotype controls are presented as dotted lines.
FIG. 3.
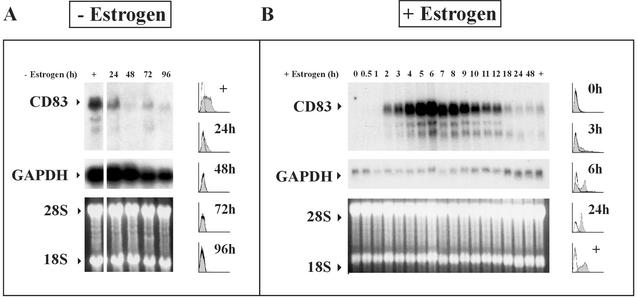
CD83 mRNA disappears rapidly after estrogen withdrawal (A) and accumulates upon addition of estrogen to hormone-deprived ER/EB2-5 cells (B). Proliferating ER/EB2-5 cells (+) were depleted of estrogen for 24 to 96 h (A) and then estrogen was added to hormone-deprived cells for 0 to 48 h as indicated (B). Cells were harvested at the given time points for FACS analysis and RNA preparation. Northern blots with 10 μg of RNA were hybridized with 32P-labeled CD83 (upper panel) and GAPDH (middle panel) probes. Equal loading of the gel is documented by ethidium bromide staining (lower panel) of the 18S and 28S ribosomal RNAs. To better visualize the decrease of CD83 RNA over time, the Northern blot shown in panel A was exposed about 10 times longer than the one in panel B. The shaded histograms represent FACS analysis for CD83 expression of cells after estrogen depletion (A) or estrogen addition (B).
FIG. 2.
CD83 is degraded and not internalized upon withdrawal of estrogen. A Western blot for detection of LAMP-1, HLA-A, -B, and -C, CD83, and CD86 in various subcellular compartments (PM-nucleus, endosomes-lysosomes, microsomes, and cytosol, as indicated at the top) of estrogen-treated ER/EB2-5 cells (+ estrogen) and estrogen-depleted cells (depleted for 4 days) (− estrogen) is shown. Estrogen-treated and estrogen-deprived cells were fractionated into different subcellular compartments as described in Materials and Methods, and the respective proteins were detected by Western blotting. LAMP-1 was included as a marker for the endosomal-lysosomal compartment.
To look at CD83 expression at the transcriptional level, Northern blots were performed with total RNA isolated from estrogen-deprived and estrogen-treated ER/EB2-5 cells at different times after withdrawal and addition of estrogen, respectively. As shown in Fig. 3A, CD83 RNA declined rapidly and continuously within 24 to 48 h after estrogen deprivation and reappeared as early as 2 h after the addition of estrogen (Fig. 3B). CD83 RNA expression strongly increased up to 6 h and then gradually declined until a low steady-state level was reached at about 18 h upon addition of estrogen. The overshooting expression of CD83 RNA to a very high level between 2 and 12 h after the addition of estrogen does not seem to be represented to the same extent at the level of the CD83 protein (Fig. 3B, histogram). In summary, CD83 is rapidly induced after induction of EBNA2 in ER/EB2-5 cells and completely degraded after repression of EBNA2 by estrogen withdrawal.
CD83 is a LMP1 target gene.
To analyze whether CD83 is directly or indirectly transactivated by EBNA2, estrogen-depleted ER/EB2-5 cells were treated with estrogen in the presence and absence of cycloheximide (CHX) and with CHX alone. RNA was isolated after 3, 6, and 12 h. Induction of CD83 was only marginally affected by treatment with CHX plus estrogen compared to with CHX alone (data not shown). This indicated that CD83 is not a direct EBNA2 target gene and raised the question of which of the EBNA2 target genes is responsible for induction of CD83. c-myc could be ruled out as a mediator of CD83 expression since CD83 is absent from A1 (data not shown) and P493-6 (45) cells. A1 and P493-6 are ER/EB2-5 derivative cell lines that express c-myc constitutively from the Ig κ regulatory elements or under the control of tetracycline and are able to proliferate in the absence of estrogen (46, 47).
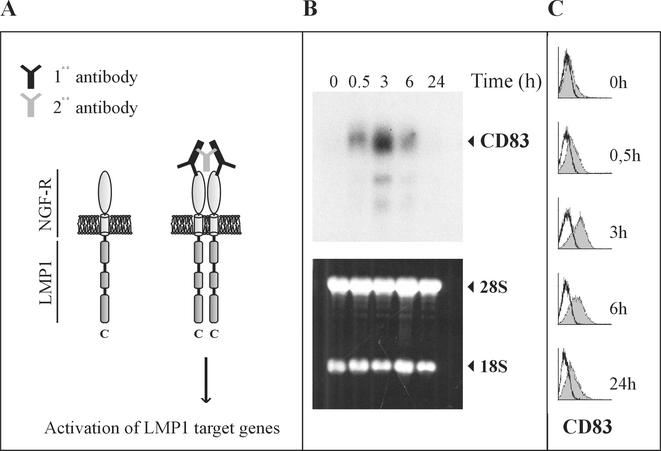
A second direct target gene of EBNA2 is viral LMP1. To demonstrate the effect of LMP1 on CD83 expression, we made use of a conditional LMP1 system developed by U. Dirmeier et al. (submitted). In this system primary B cells have been immortalized by recombinant B95-8 virus in which the viral LMP1 gene is replaced by an NGF receptor-LMP1 fusion gene consisting of the transmembrane and extracellular domains of the NGF receptor and the intracellular signaling portion of LMP1 (16). Signaling through LMP1 was rendered inducible by the ligand (NGF) or by the addition of a monoclonal antibody directed against the receptor (Fig. 4A) (16, 52). In this cell line CD83 expression is induced as early as 30 min after cross-linking. It reaches a maximum after 3 h and declines to low levels similarly to estrogen-deprived ER/EB2-5 cells that have been restimulated by the addition of estrogen (Fig. 4B and C). These data show that CD83 expression is activated by LMP1 signaling.
FIG. 4.
CD83 is induced by LMP1. (A) Experimental system. LMP1 was activated by antibody-mediated NGF-R cross-linking in a cell line immortalized by recombinant EBV and expressing a fusion protein consisting of the extracellular and transmembrane domains (light gray) of the NGF-R and the intracellular signaling domain of LMP1, including CTAR1, -2, and -3 (dark gray cylinder) at the C terminus. (B) RNA was prepared at different time points after receptor cross-linking and was analyzed for CD83 expression by Northern blot analysis (upper panel). The lower panel shows the ethidium bromide-stained gel with rRNAs indicated at the right to show equal loading. (C) At the same time points, CD83 cell surface expression was monitored by FACS analysis. The shaded histograms represent the measurements of CD83 protein on the cell surface after the addition of estrogen.
The CD83 promoter is induced by LMP1 through activation of NF-κB.
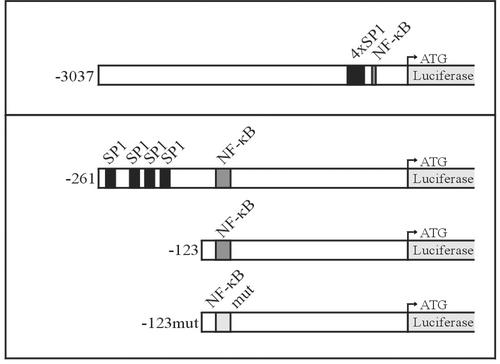
To study the mechanism of CD83 induction, we cloned the CD83 promoter in front of the luciferase reporter gene and studied its induction by LMP1 by transient transfection experiments in 293-T cells. Four SP1 binding sites and one NF-κB binding site are located in the TATA-less CD83 promoter within 260 bp upstream of the translation start site (5, 34, 40). Four different constructs were used (Fig. 5) as follows. The first represents the CD83 wild-type promoter and consists of 3,037 bp upstream of the translation start site (−3,037). The second consists of 261 bp upstream of the translation start site, including the four SP1 sites and the NF-κB binding sequence (−261). The third construct carries the NF-κB site without the four SP1 sites (−123). The last one has a mutated NF-κB site (−123mut).
FIG. 5.
CD83 promoter constructs. The genomic sequence of the human CD83 promoter was cloned into the vector pGL Basic containing the luciferase gene without promoter sequences. The pGL CD83 (−3,037) plasmid contains 3,037 bp upstream of the translation initiation site. The start codon of luciferase is indicated by a bent arrow. The CD83 promoter contains an NF-κB site (dark gray box) and four SP1 binding sequences (black boxes). pGL CD83 (−261) contains the CD83 promoter sequence up to position −261, including the four SP1 and NF-κB sites. pGL CD83 (−123) contains only the NF-κB site. In pGL CD83 (−123mut), the NF-κB site is mutated at position −115 to −106 (light gray box) so that NF-κB cannot bind (5). All CD83 promoter mutants were generated by PCR and verified by sequencing.
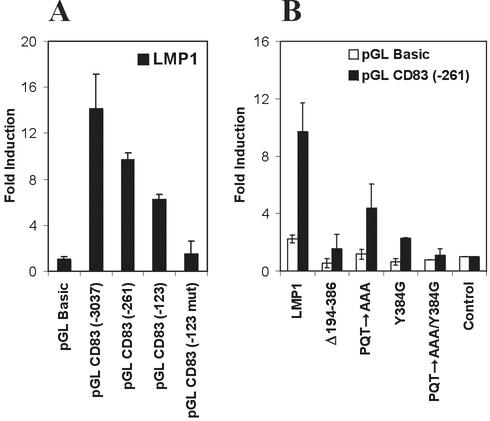
As shown in Fig. 6A, cotransfection of the CD83 promoter-luciferase reporter constructs with LMP1 resulted in a 14- and 10-fold induction of the 3,037- and 261-bp constructs. LMP1 itself had a twofold effect on the promoterless pGL luciferase vector containing a small unspecific induction luciferase by LMP1. Deletion of the SP1 sites had no effect on the long 3,037-bp construct (data not shown) and reduced transactivation by LMP1 in the 123-bp construct only slightly. Mutation of the NF-κB site abolished transactivation by LMP1 completely (Fig. 6A), suggesting that NF-κB is an important factor mediating the effect of LMP1 on the CD83 promoter. This was confirmed by an independent line of evidence. LMP1 mutants were cotransfected with the CD83 promoter-luciferase reporter constructs in which either the whole C terminus, the CTAR1 domain (PQT to AAA), the CTAR2 domain (Tyr384 to Gly384), or both CTAR domains were mutated. The data were virtually identical for the 261-bp (Fig. 6B) and 3,037-bp constructs (data not shown). Mutation of each CTAR domain individually decreased the level of transactivation of the CD83 promoter by LMP1 to a different extent (about fourfold for CTAR2 and about twofold for CTAR1), whereas mutation of both sites abolished transactivation by LMP1 completely, similar to the deletion of the complete C terminus. These data show that LMP1 induces CD83 expression through activation of NF-κB.
FIG. 6.
The CD83 promoter is activated by LMP1 expression plasmids in transient transfections. (A) Fifty nanograms of CD83 promoter-reporter constructs were transfected into 293-T cells together with 0.5 μg of LMP1 and 10 ng of β-galactosidase expression plasmids (black bars). (B) The LMP1 expression plasmid (pSV-LMP1) or LMP1 mutant expression plasmid pSV-LMP1 (Δ194-386), pSV-LMP1 (PQT→AAA), pSV-LMP1 (Y384G), or pSV-LMP1 (PQT→AAA/Y384G) (0.5 μg) and 10 ng of β-galactosidase reporter plasmid were cotransfected with 50 ng of the CD83 promoter-reporter construct (−261) or pGL Basic, as indicated by black and white bars, respectively. Transfections were harvested the following day, and the luciferase values were normalized, with β-galactosidase as a standard. The CD83 promoter activity is given as fold induction versus mock-transfected controls. Error bars show standard deviations for at least three independent experiments.
The CD83 promoter is also activated by chimeric LMP1-CD40, LMP1-TNFR1, and LMP1-TNFR2.
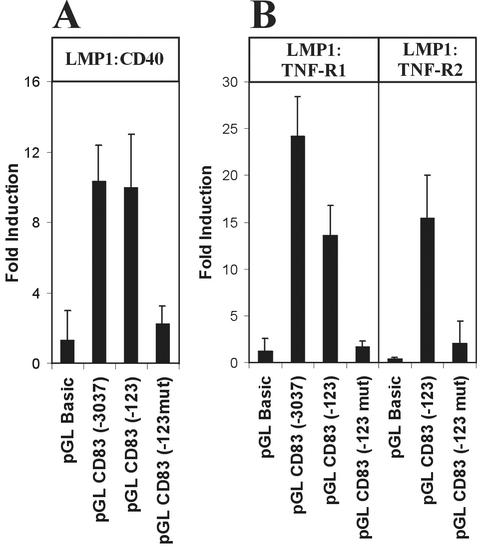
LMP1 acts like a constitutively active member of the TNFR family and shares with other members of the family a number of signaling molecules that are involved in signal transmission from the cell surface to the nucleus, including TRAF2, TRAF6, and TRADD. In vivo, these receptors have overlapping yet distinct functions in the regulation of the immune response and apoptosis. CD83 expression can be induced on dendritic cells by CD40 ligand (8) or by TNF-α (5, 39, 48). Therefore, we made constitutive signaling fusion proteins of the intracellular signaling domains of CD40, TNFR1, and TNFR2 with the transmembrane domain of LMP1 (16) to test whether these receptor domains could also contribute to CD83 promoter activation. All three fusion constructs (LMP1-CD40, LMP1-TNFR1, and LMP1-TNFR2) transactivated the CD83 promoter-luciferase reporter constructs to the same extent as LMP1 (Fig. 7), indicating that activation of NF-κB is the critical step for CD83 induction through different receptors.
FIG. 7.
The CD83 promoter is also activated by TNFR family members via NF-κB. CD83 promoter-reporter constructs (50 ng) as described for Fig. 5 were cotransfected into 293-T cells with 0.5 μg of LMP1-CD40 (A), LMP1-TNFR1, or LMP1-TNFR2 expression plasmids (B). In the case of LMP1-TNFR1, 1.5 μg of p35 plasmid was cotransfected to avoid apoptosis. The CD83 promoter activity is shown as fold induction compared to mock-transfected cells (black bars). Error bars indicate the standard deviations for three independent experiments.
DISCUSSION
To study the phenotypic changes induced by gene products of EBV in B cells, we have made use of a conditional EBV-immortalized B-cell line in which the function of the viral gene product EBNA2 is controllable by estrogen. As expected, many of the activation markers and adhesion and costimulatory molecules were upregulated by EBV. Only a few of the surface markers studied (i.e., CD38, IgM, and HLA-A, -B, and -C) were found to be regulated in an opposite fashion, i.e., they were downregulated by viral gene products and upregulated when estrogen was withdrawn. Among the surface markers studied, CD83 attracted our particular attention because it was the only surface molecule that totally disappeared from the cell surface after estrogen withdrawal and rapidly reappeared after the addition of estrogen. The rapid time course of disappearance and reappearance of CD83 on the cell surface prompted us to study the mechanism of regulation of CD83 expression by viral gene products in more detail. The rapid removal of CD83 from the cell surface is not due to redistribution of CD83 inside the cell. In fact, CD83 has a half-life that is significantly shorter than those of most other surface molecules and is rapidly degraded and resynthesized upon removal and addition of estrogen, respectively. This regulation at the protein level is also reflected at the RNA level. CD83 RNA declined within 24 h after removal of estrogen and was virtually gone after 48 h. CD83 RNA was detected as soon as 2 h after the addition of estrogen, peaked at 6 h, and leveled off to reach a constant level of expression after about 18 h.
The rapid induction of CD83 after the addition of estrogen raised the question of whether CD83 might be a direct target gene of EBNA2. This turned out not to be the case. The CD83 RNA level did not increase to a significantly higher level in the presence of estrogen plus CHX compared to the level with CHX alone (data not shown). Twofold evidence indicated that CD83 is a target gene of LMP1. Firstly, CD83 remained expressed upon withdrawal of estrogen in a cell line in which LMP1 was constitutively expressed (data not shown), and secondly, in a cell line with a conditional LMP1 gene CD83 was induced when the NGF-R-LMP1 fusion protein was cross-linked on the cell surface (Fig. 4).
To identify the mechanism by which CD83 is induced by LMP1, we performed CD83 promoter studies. A 3-kb fragment encompassing the CD83 promoter as well as 261- and 123-bp derivatives thereof conferred inducibility by LMP1. Four potential SP1 sites located at a distance between −180 and −261 had no effect on the inducibility of the promoter by LMP1, whereas deletion or mutation of the NF-κB site completely abolished inducibility by LMP1. This is in agreement with the observation that NF-κB regulates CD83 expression in activated T lymphocytes, monocytic U937 cells, and dendritic DC2.4 cells (5, 40). McKinsey et al. (40) identified two NF-κB binding sites in the CD83 promoter at positions −115 to −106 and −91 to −82 (relative to the translation initiation start), but the NF-κB site at position −115 to −106 seems to be sufficient for the LMP1-mediated induction of the CD83 gene (Fig. 6 and 7). Another approach verified that NF-κB is indeed the critical player for mediating the effect of LMP1 on the CD83 promoter. Mutation of the CTAR1 or CTAR2 domain of LMP1 decreased inducibility by LMP1 by a factor of 2 and 4, respectively, whereas a double mutation affecting CTAR1 as well as CTAR2 completely abolished inducibility by LMP1 in a fashion similar to the deletion of the complete C terminus of LMP1.
Since LMP1 is a member of the TNFR family and stimulation of other family members through CD40 ligand or TNF-α also contributes to CD83 induction on dendritic cells, we asked whether these receptors use the same route to induce CD83 expression. This is in fact the case. Chimeric receptors consisting of the transmembrane domain of LMP1 and the signaling domains of CD40, TNFR1, and TNFR2 also induced the CD83 promoter through NF-κB activation.
The fact that the CD83 promoter is induced by LMP1 through NF-κB may partly explain the kinetics of CD83 RNA induction by LMP1. Induction of CD83 RNA is strongly overshooting, peaks at a very high level 6 h after the addition of estrogen, and reaches a fairly low steady-state expression level in continuously proliferating EBV-immortalized cells. This pattern of expression may be explained by the fact that NF-κB, by inducing its own inhibitor, I-κB, induces a negative feedback loop that limits the expression of its target genes under steady-state conditions.
A novel mechanism of regulation of CD83 expression has been described for dendritic cells (35) that affects the efficiency of RNA transport from the nucleus to the cytoplasm. In our cell system there is no evidence that CD83 expression might also be regulated at this level. The stringent regulation of CD83 RNA in estrogen-deprived versus estrogen-supplemented ER/EB2-5 cells rules out the idea that this mechanism contributes significantly to regulation of CD83 expression by LMP1.
Many viruses, including herpesviruses (e.g., herpes simplex virus and cytomegalovirus), have evolved a multitude of mechanisms that subvert the immune system (for review, see references 1 and 56). EBV is peculiar in the sense that it induces a strong immune response against virus-infected B cells and that it enhances rather than impairs antigen presentation. Assuming a role for CD83 in antigen presentation and T-cell stimulation, we propose that induction of CD83 is part of this virus-induced immune enhancement program. There are two possibilities to explain why EBV induces such a strong immune response against virus-infected B cells. Firstly, a virus that is as strongly transforming as EBV might eradicate its host and eliminate its own niche for survival unless it elicits a strong immune response that counters the transforming ability of the virus in vivo. Alternatively, induction of a strong immune response against virus-infected cells may be a prerequisite for the establishment of latency in the B-cell system in vivo. A better understanding of the mechanisms by which virus latency is established in vivo might help to discriminate between these possibilities.
Acknowledgments
This work was supported by grants to W.H. and G.W.B. from Deutsche Forschungsgemeinschaft (SFB455) and Fonds der Chemischen Industrie.
We are grateful to Josef Mautner and Franz Kohlhuber for helpful discussions and reagents.
REFERENCES
- 1.Alcami, A., and U. H. Koszinoski. 2000. Viral mechanisms of immune evasion. Immunol. Today 21:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardeshna, K. M., A. R. Pizzey, S. Devereux, and A. Khwaja. 2000. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96:1039-1046. [PubMed] [Google Scholar]
- 3.Armitage, R. J., D. T. Ulrich, B. M. Macduff, J. Zappone, M. Z. Kubin, and W. C. Fanslow. 1996. Induction of membrane-associated and soluble CD83 from B, T, and dendritic cells, p. 593-595. In T. Kishimoto, H. Kikutani, A. E. G. K. von dem Borne, S. M. Goyert, D. Y. Mason, M. Miyasaka, L. Moretta, K. Okumura, S. Shaw, T. A. Springer, K. Sugamura, and H. Zola (ed.), Leukocyte typing VI. Garland, New York, N.Y.
- 4.Baichwal, V. R., and B. Sugden. 1988. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene 2:461-467. [PubMed] [Google Scholar]
- 5.Berchtold, S., P. Muhl-Zurbes, E. Maczek, A. Golka, G. Schuler, and A. Steinkasserer. 2002. Cloning and characterization of the promoter region of the human CD83 gene. Immunobiology 205:231-246. [DOI] [PubMed] [Google Scholar]
- 6.Bornkamm, G. W., and W. Hammerschmidt. 2001. Molecular virology of Epstein-Barr virus. Phil. Trans. R. Soc. Lond. B. Biol. Sci. 356:437-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodeur, S. R., G. Cheng, D. Baltimore, and D. A. Thorley-Lawson. 1997. Localization of the major NF-kappaB-activating site and the sole TRAF3 binding site of LMP-1 defines two distinct signaling motifs. J. Biol. Chem. 272:19777-19784. [DOI] [PubMed] [Google Scholar]
- 8.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, C. Van Kooten, I. Durand, and J. Banchereau. 1994. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180:1263-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devergne, O., E. D. Cahir McFarland, G. Mosialos, K. M. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-kappaB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devergne, O., E. Hatzivassiliou, K. M. Izumi, K. M. Kaye, M. F. Kleijnen, E. Kieff, and G. Mosialos. 1996. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-kappaB activation. Mol. Cell. Biol. 16:7098-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliopoulos, A. G., N. J. Gallagher, S. M. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 274:16085-16096. [DOI] [PubMed] [Google Scholar]
- 12.Floettmann, J. E., and M. Rowe. 1997. Epstein-Barr virus latent membrane protein-1 (LMP1) C-terminus activation region 2 (CTAR2) maps to the far C-terminus and requires oligomerisation for NF-kappaB activation. Oncogene 15:1851-1858. [DOI] [PubMed] [Google Scholar]
- 13.Fuentes-Panana, E. M., R. Peng, G. Brewer, J. Tan, and P. D. Ling. 2000. Regulation of the Epstein-Barr virus C promoter by AUF1 and the cyclic AMP/protein kinase A signaling pathway. J. Virol. 74:8166-8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto, Y., L. Tu, A. S. Miller, C. Bock, M. Fujimoto, C. Doyle, D. A. Steeber, and T. F. Tedder. 2002. CD83 expression influences CD4+ T cell development in the thymus. Cell 108:755-767. [DOI] [PubMed] [Google Scholar]
- 15.Gires, O., F. Kohlhuber, E. Kilger, M. Baumann, A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gires, O., U. Zimber-Strobl, R. Gonnella, M. Ueffing, G. Marschall, R. Zeidler, D. Pich, and W. Hammerschmidt. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 16:6131-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman, S. R., E. Johannsen, X. Tong, R. Yalamanchili, and E. Kieff. 1994. The Epstein-Barr virus nuclear antigen 2 transactivator is directed to response elements by the J kappa recombination signal binding protein. Proc. Natl. Acad. Sci. USA 91:7568-7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammarskjold, M. L., and M. C. Simurda. 1992. Epstein-Barr virus latent membrane protein transactivates the human immunodeficiency virus type 1 long terminal repeat through induction of NF-kappa B activity. J. Virol. 66:6496-6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haskill, S., A. A. Beg, S. M. Tompkins, J. S. Morris, A. D. Yurochko, A. Sampson-Johannes, K. Mondal, P. Ralph, and A. S. Baldwin, Jr. 1991. Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell 65:1281-1289. [DOI] [PubMed] [Google Scholar]
- 20.Hatzivassiliou, E., W. E. Miller, N. Raab-Traub, E. Kieff, and G. Mosialos. 1998. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-kappa B, and stress-activated protein kinase. J. Immunol. 160:1116-1121. [PubMed] [Google Scholar]
- 21.Henkel, T., P. D. Ling, S. D. Hayward, and M. G. Peterson. 1994. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science 265:92-95. [DOI] [PubMed] [Google Scholar]
- 22.Henkel, T., U. Zabel, K. van Zee, J. M. Muller, E. Fanning, and P. A. Baeuerle. 1992. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-kappa B subunit. Cell 68:1121-1133. [DOI] [PubMed] [Google Scholar]
- 23.Huen, D. S., S. A. Henderson, D. Croom-Carter, and M. Rowe. 1995. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene 10:549-560. [PubMed] [Google Scholar]
- 24.Iking-Konert, C., C. Cseko, C. Wagner, S. Stegmaier, K. Andrassy, and G. M. Hansch. 2001. Transdifferentiation of polymorphonuclear neutrophils: acquisition of CD83 and other functional characteristics of dendritic cells. J. Mol. Med. 79:464-474. [DOI] [PubMed] [Google Scholar]
- 25.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jochner, N., D. Eick, U. Zimber-Strobl, M. Pawlita, G. W. Bornkamm, and B. Kempkes. 1996. Epstein-Barr virus nuclear antigen 2 is a transcriptional suppressor of the immunoglobulin mu gene: implications for the expression of the translocated c-myc gene in Burkitt's lymphoma. EMBO J. 15:375-382. [PMC free article] [PubMed] [Google Scholar]
- 27.Kaiser, C., G. Laux, D. Eick, N. Jochner, G. W. Bornkamm, and B. Kempkes. 1999. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J. Virol. 73:4481-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karin, M., and A. Lin. 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 29.Kempkes, B., D. Spitkovsky, P. Jansen-Durr, J. W. Ellwart, E. Kremmer, H. J. Delecluse, C. Rottenberger, G. W. Bornkamm, and W. Hammerschmidt. 1995. B cell proliferation and induction of early G1-regulating proteins by Epstein-Barr virus mutants conditional for EBNA2. EMBO J. 14:88-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Williams & Wilkins, Philadelphia, Pa.
- 31.Kieser, A., C. Kaiser, and W. Hammerschmidt. 1999. LMP1 signal transduction differs substantially from TNF receptor 1 signaling in the molecular functions of TRADD and TRAF2. EMBO J. 18:2511-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kieser, A., E. Kilger, O. Gires, M. Ueffing, W. Kolch, and W. Hammerschmidt. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J. 16:6478-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilger, E., A. Kieser, M. Baumann, and W. Hammerschmidt. 1998. Epstein-Barr virus-mediated B cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 17:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozlow, E. J., G. L. Wilson, C. H. Fox, and J. H. Kehrl. 1993. Subtractive cDNA cloning of a novel member of the Ig gene superfamily expressed at high levels in activated B lymphocytes. Blood 81:454-461. [PubMed] [Google Scholar]
- 35.Kruse, M., O. Rosorius, F. Kratzer, D. Bevec, C. Kuhnt, A. Steinkasserer, G. Schuler, and J. Hauber. 2000. Inhibition of CD83 cell surface expression during dendritic cell maturation by interference with nuclear export of CD83 mRNA. J. Exp. Med. 191:1581-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laherty, C. D., H. M. Hu, A. W. Opipari, F. Wang, and V. M. Dixit. 1992. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J. Biol. Chem. 267:24157-24160. [PubMed] [Google Scholar]
- 37.Laux, G., B. Adam, L. J. Strobl, and F. Moreau-Gachelin. 1994. The Spi-1/PU.1 and Spi-B ets family transcription factors and the recombination signal binding protein RBP-J kappa interact with an Epstein-Barr virus nuclear antigen 2 responsive cis-element. EMBO J. 13:5624-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lechmann, M., E. Zinser, A. Golka, and A. Steinkasserer. 2002. Role of CD83 in the immunomodulation of dendritic cells. Int. Arch. Allergy Immunol. 129:113-118. [DOI] [PubMed] [Google Scholar]
- 39.Lyakh, L. A., G. K. Koski, W. Telford, R. E. Gress, P. A. Cohen, and N. R. Rice. 2000. Bacterial lipopolysaccharide, TNF-alpha, and calcium ionophore under serum-free conditions promote rapid dendritic cell-like differentiation in CD14+ monocytes through distinct pathways that activate NK-kappa B. J. Immunol. 165:3647-3655. [DOI] [PubMed] [Google Scholar]
- 40.McKinsey, T. A., Z. Chu, T. F. Tedder, and D. W. Ballard. 2000. Transcription factor NF-kappaB regulates inducible CD83 gene expression in activated T lymphocytes. Mol. Immunol. 37:783-788. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell, T., and B. Sugden. 1995. Stimulation of NF-kappa B-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J. Virol. 69:2968-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moorthy, R. K., and D. A. Thorley-Lawson. 1993. All three domains of the Epstein-Barr virus-encoded latent membrane protein LMP-1 are required for transformation of rat-1 fibroblasts. J. Virol. 67:1638-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mosialos, G., M. Birkenbach, R. Yalamanchili, T. VanArsdale, C. Ware, and E. Kieff. 1995. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 80:389-399. [DOI] [PubMed] [Google Scholar]
- 44.Oehler, L., O. Majdic, W. F. Pickl, J. Stockl, E. Riedl, J. Drach, K. Rappersberger, K. Geissler, and W. Knapp. 1998. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J. Exp. Med. 187:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pajic, A., M. S. Staege, D. Dudziak, M. Schuhmacher, D. Spitkovsky, G. Eissner, M. Brielmeier, A. Polack, and G. W. Bornkamm. 2001. Antagonistic effects of c-myc and Epstein-Barr virus latent genes on the phenotype of human B cells. Int. J. Cancer 93:810-816. [DOI] [PubMed] [Google Scholar]
- 46.Pajic, A., D. Spitkovsky, B. Christoph, B. Kempkes, M. Schuhmacher, M. S. Staege, M. Brielmeier, J. Ellwart, F. Kohlhuber, G. W. Bornkamm, A. Polack, and D. Eick. 2000. Cell cycle activation by c-myc in a Burkitt lymphoma model cell line. Int. J. Cancer 87:787-793. [DOI] [PubMed] [Google Scholar]
- 47.Polack, A., K. Hortnagel, A. Pajic, B. Christoph, B. Baier, M. Falk, J. Mautner, C. Geltinger, G. W. Bornkamm, and B. Kempkes. 1996. c-myc activation renders proliferation of Epstein-Barr virus (EBV)-transformed cells independent of EBV nuclear antigen 2 and latent membrane protein 1. Proc. Natl. Acad. Sci. USA 93:10411-10416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook, J., and D. W. Russel. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 50.Sandberg, M., W. Hammerschmidt, and B. Sugden. 1997. Characterization of LMP-1's association with TRAF1, TRAF2, and TRAF3. J. Virol. 71:4649-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeter, C. J., M. Braun, J. Englert, H. Beck, H. Schmidt, and H. Kalbacher. 1999. A rapid method to separate endosomes from lysosomal contents using differential centrifugation and hypotonic lysis of lysosomes. J. Immunol. Methods 227:161-168. [DOI] [PubMed] [Google Scholar]
- 52.Schultheiss, U., S. Puschner, E. Kremmer, T. W. Mak, H. Engelmann, W. Hammerschmidt, and A. Kieser. 2001. TRAF6 is a critical mediator of signal transduction by the viral oncogene latent membrane protein 1. EMBO J. 20:5678-5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seshagiri, S., and L. K. Miller. 1997. Baculovirus inhibitors of apoptosis (IAPs) block activation of Sf-caspase-1. Proc. Natl. Acad. Sci. USA 94:13606-13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sjoblom, A., A. Jansson, W. Yang, S. Lain, T. Nilsson, and L. Rymo. 1995. PU box-binding transcription factors and a POU domain protein cooperate in the Epstein-Barr virus (EBV) nuclear antigen 2-induced transactivation of the EBV latent membrane protein 1 promoter. J. Gen. Virol. 76:2679-2692. [DOI] [PubMed] [Google Scholar]
- 55.Sorg, U. R., T. M. Morse, W. N. Patton, B. D. Hock, H. B. Angus, B. A. Robinson, B. M. Colls, and D. N. Hart. 1997. Hodgkin's cells express CD83, a dendritic cell lineage associated antigen. Pathology 29:294-299. [DOI] [PubMed] [Google Scholar]
- 56.Tortorella, D., B. E. Gewurz, E. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 57.Uchida, J., T. Yasui, Y. Takaoka-Shichijo, M. Muraoka, W. Kulwichit, N. Raab-Traub, and H. Kikutani. 1999. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 286:300-303. [DOI] [PubMed] [Google Scholar]
- 58.Wang, D., D. Liebowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831-840. [DOI] [PubMed] [Google Scholar]
- 59.Yamashiro, S., J. M. Wang, D. Yang, W. H. Gong, H. Kamohara, and T. Yoshimura. 2000. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood 96:3958-3963. [PubMed] [Google Scholar]
- 60.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou, L. J., R. Schwarting, H. M. Smith, and T. F. Tedder. 1992. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J. Immunol. 149:735-742. [PubMed] [Google Scholar]
- 62.Zimber-Strobl, U., L. J. Strobl, C. Meitinger, R. Hinrichs, T. Sakai, T. Furukawa, T. Honjo, and G. W. Bornkamm. 1994. Epstein-Barr virus nuclear antigen 2 exerts its transactivating function through interaction with recombination signal binding protein RBP-J kappa, the homologue of Drosophila Suppressor of Hairless. EMBO J. 13:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]