Abstract
Nasopharyngeal carcinoma, Kaposi's sarcoma, and B-cell lymphomas are human malignancies associated with gammaherpesvirus infections. Members of this virus family are characterized by their ability to establish latent infections in lymphocytes. The latent viral genome expresses very few gene products. The infected cells are therefore poorly recognized by the host immune system, allowing the virus to persist for long periods of time. We sought to identify the cell-specific factors that allow these viruses to redirect their life cycle from productive replication to latency. We find that the cellular transcription factor NF-κB can regulate this process. Epithelial cells and fibroblasts support active (lytic) gammaherpesvirus replication and have low NF-κB activity. However, overexpression of NF-κB in these cells inhibits the replication of the gammaherpesvirus murine herpesvirus 68 (MHV68). In addition, overexpression of NF-κB inhibits the activation of lytic promoters from MHV68 and human gammaherpesviruses Kaposi's sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV). In lymphocytes latently infected with KSHV or EBV, the level of NF-κB activity is high, and treatment of these cells with an NF-κB inhibitor leads to lytic protein synthesis consistent with virus reactivation. These results suggest that high levels of NF-κB can inhibit gammaherpesvirus lytic replication and may therefore contribute to the establishment and maintenance of viral latency in lymphocytes. They also suggest that NF-κB may be a novel target for the disruption of virus latency and therefore the treatment of gammaherpesvirus-related malignancies.
Herpesviruses are characterized by their ability to support two distinct life cycles: latency and lytic (productive) replication. Latency is established after infection of a nonpermissive cell and is characterized by persistence of the viral genome in the cell nucleus and expression of a small number of viral genes. Lytic replication occurs after de novo infection of a permissive cell or as a result of reactivation of latent virus within a nonpermissive cell. In both of these cases, replication involves the sequential activation of immediate-early, early, and late viral genes. In the gammaherpesvirus subfamily, this cascade is initiated by immediate-early viral transactivators (7, 15, 30, 38, 39, 43, 44), including RTA. The ectopic expression of RTA from Kaposi's sarcoma-associated herpesvirus (KSHV), murine herpesvirus 68 (MHV68), or Epstein-Barr virus (EBV) in latently infected B lymphocytes is sufficient to reactivate the latent virus and initiate replication (24, 30, 38, 43). Dominant-negative mutants have also shown that RTA is required for the reactivation of latent KSHV and MHV68 and for de novo replication of MHV68 (23, 42). In addition to transactivating viral genes required for replication, the RTA proteins have been shown to autoactivate their own promoters (9, 12, 30, 42). EBV encodes an additional immediate-early transactivator, Zebra, which synergizes with its RTA homologue to activate viral lytic genes (8, 15, 20). Zebra also autoactivates its own promoter and the EBV RTA promoter (11, 36).
The expression of RTA and Zebra therefore represents a highly sensitive switch for the initiation of virus replication by gammaherpesviruses. In light of this, we reasoned that mechanisms exist to suppress this switch in nonpermissive cells where these viruses establish latency. Because latency is established specifically in lymphocytes, we investigated the possibility that suppression involved a lymphocyte-specific factor. The NF-κB family of transcription factors have very low activity in most cell types, due to their association with the I-κB family of inhibitors, which promote their export to the cytoplasm (2, 3, 18). Inducers of the NF-κB pathway initiate the degradation of I-κB, allowing the NF-κB proteins to accumulate in the nucleus, where they bind to DNA and activate target genes (10, 40). In lymphocytes, however, the I-κB proteins are unstable, and high levels of NF-κB are constitutively present in the nucleus (27). In lymphocytes latently infected with gammaherpesviruses, NF-κB activity is further elevated by the expression of latent viral gene products that activate the NF-κB signaling pathway (6, 14, 21). These elevated NF-κB levels promote survival and proliferation of the infected cell (5, 6, 17, 19). We find that NF-κB also exerts dramatic effects on gammaherpesvirus replication. In epithelial cells permissive for virus replication, the overexpression of NF-κB can inhibit lytic gene promoter activation, lytic protein synthesis, and replication. Conversely, in some lymphocyte cell lines latently infected with KSHV or EBV, the inhibition of NF-κB leads to lytic protein synthesis consistent with virus reactivation. These results identify NF-κB as an inhibitor of gammaherpesvirus replication and therefore a potential regulator of the balance between lytic replication and latency in vivo.
MATERIALS AND METHODS
Plasmid constructs.
Reporter pRpluc contains the 3-kb region upstream of the KSHV RTA coding sequence in pGL2-Basic (Promega, Madison, Wis.) (9). 57pLuc contains a 565-nucleotide fragment of the MHV68 open reading frame 57 (ORF57) promoter cloned into pGL2-Basic (gift of S. Speck) (22). pPAN-69Luc contains a 69-nucleotide fragment of the polyadenylated nuclear RNA (PAN) promoter of KSHV in pGL3-Basic (Promega) (37). BHLF1Luc contains a 781-bp fragment of the BHLF1 promoter of EBV (26). pNF-κBLuc is an NF-κB-responsive reporter (Stratagene, La Jolla, Calif.). All reporter constructs express Photinus pyralis luciferase. In addition, the pRL-SV40 vector constitutively expressing Renilla reniformis luciferase was used as an internal control. cDNAs encoding KSHV RTA, EBV RTA, EBV Zebra, full-length human p65, and p65 deletion mutants or genomic DNA encoding MHV68 RTA was expressed from the pFLAG-CMV2 vector (Kodak). I-κBS32/36 is a dominant inhibitor of NF-κB (4).
Transient transfections.
293T cells (105 per well) were seeded onto a 24-well dish 16 h before transfection. A total of 600 ng of DNA per well was transfected by the calcium phosphate method. Twenty-five nanograms of pRpluc was transfected with 25 ng of pFLAGRTA (KSHV RTA), and 10 ng of pPAN-69Luc was transfected with 1 ng of pFLAGRTA. Ten nanograms of 57pLuc was transfected with 2 ng of pFLAG-MRTA (MHV-68 RTA). Five nanograms of pFLAG/EBVRTA and 5 ng of pFLAG/EBVZebra were transfected with 10 ng of BHLF1Luc. Cell extracts were harvested 24 h posttransfection and assayed by using the dual-luciferase reporter assay system (Promega, Madison, Wis.).
Reactivation assay.
A total of 107 KS-1 cells (KSHV-positive, EBV-negative primary effusion B-cell lymphoma; gift from J. Said, University of California at Los Angeles) or P3J-HR-1 cells (EBV-positive, KSHV-negative Burkitt’s lymphoma cells) were incubated with 20 ng of tetradecanoyl phorbol acetate (TPA) per ml (KS-1, BCBL1) or 1.5 mM sodium butyrate (HR-1) for 48 h or were incubated with the indicated amounts of Bay11-7082 (Calbiochem, San Diego, Calif.) for 30 min and returned to fresh medium for 48 h.
Western blotting.
Forty-eight hours after treatment, whole-cell extracts from 105 KS-1 or HR-1 cells were analyzed by Western blotting with Kaposi's sarcoma patient serum and polyclonal rabbit serum recognizing KSHV RTA or nasopharyngeal carcinoma patient serum and a monoclonal antibody (MAb) specific for EA-D (Chemicon International, Temecula, Calif.). Blots were reprobed with an actin MAb (Sigma, St. Louis, Mo.) as a loading control. After MHV68 DNA transfection (see Fig. 3A, 4, and 6A), whole-cell extracts from 293T cells were probed with polyclonal anti-MHV68 serum. Membranes were reprobed with MAbs recognizing p65 (Santa Cruz Biotechnology, Santa Cruz, Calif.) and actin (Sigma).
FIG. 3.
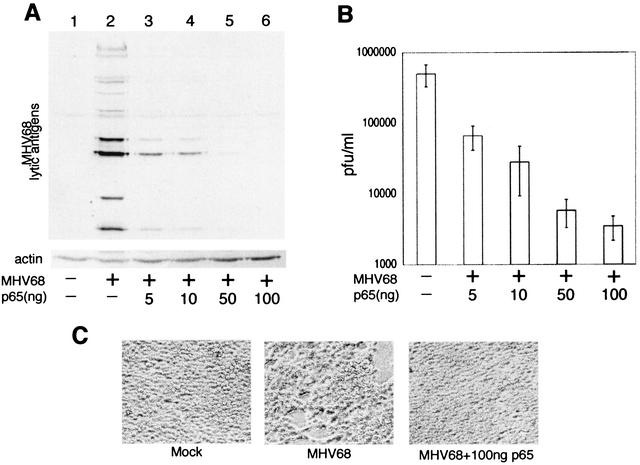
NF-κB(p65) inhibits replication of MHV68. (A) Western blot of 293T whole-cell extracts harvested 5 days posttransfection with purified MHV68 virion DNA and pCMVp65. Membranes were probed with polyclonal serum recognizing multiple MHV68 lytic antigens (upper panel) and actin MAb (lower panel). (B) MHV68 titer in supernatant from cells described for panel A. Viral titers from three separate transfection experiments are represented. (C) Phase-contrast images of cells described in panels A and B 5 days posttransfection.
FIG. 4.
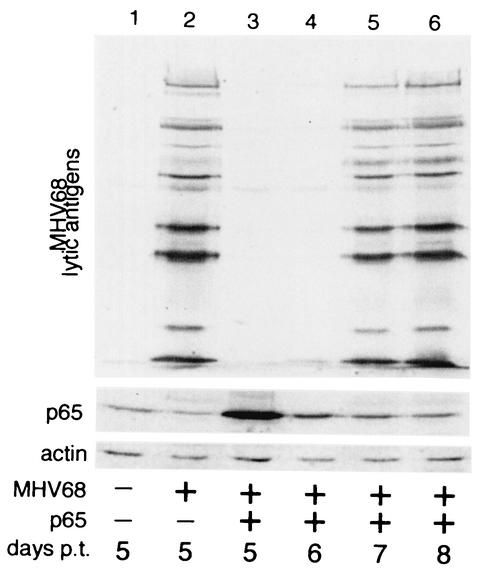
Inhibition of MHV68 replication by NF-κB(p65) is reversible. Western blot of 293T cells transfected with 1 μg of MHV68 virion DNA and 1 μg of pCMVp65 as in Fig. 3. Blots were probed with polyclonal serum against MHV68 lytic antigens (upper panel) and MAbs recognizing p65 (center panel) and actin (lower panel). p.t., posttransfection.
FIG. 6.
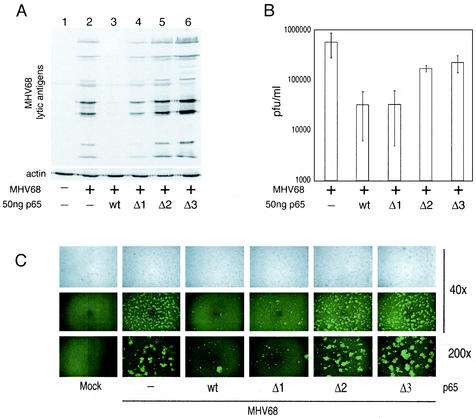
Both the activation domain and DNA binding domain of p65 are required for inhibition of MHV68 replication. (A) Western blot of 293T whole-cell extracts 5 days posttransfection with MHV68 virion DNA and pFLAGp65 constructs as described in the legend to Fig. 5. Blots were probed with polyclonal serum recognizing multiple MHV68 lytic antigens and a MAb recognizing actin. (B) MHV68 titers in supernatants from cells described for panel A. Average titers from three separate transfection experiments are represented. (C) Phase-contrast and fluorescence images of cells described for panel A, 2 days posttransfection with a recombinant MHV68 strain expressing GFP.
EMSA.
For the electrophoretic mobility shift assay (EMSA), 10 μg of nuclear extract, made from latently-infected lymphoma cell lines, was incubated with a consensus NF-κB oligonucleotide as previously described (19). To obtain supershifts, 200 ng of MAb recognizing p65 (Santa Cruz Biotechnology, Santa Cruz, Calif.) or FLAG (Sigma) was incubated with the cell extracts prior to addition of probe.
RESULTS
Gammaherpesvirus lytic promoter activation is inhibited by NF-κB(p65).
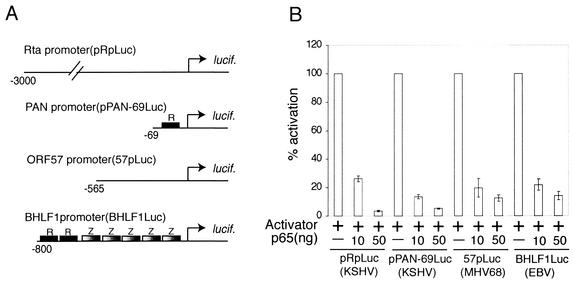
Herpesvirus replication involves the sequential activation of lytic genes, a cascade that is initiated by viral immediate-early transactivators. We therefore analyzed the effect of overexpression of NF-κB on the activity of lytic promoters selected from three gammaherpesviruses: KSHV, EBV, and MHV68 (Fig. 1A). pRpluc, pPAN-69Luc, and 57pLuc are all highly inducible by their respective RTA homologues (9, 22, 37). BHLF1Luc is activated synergistically by EBV RTA and Zebra (26). These reporters were cotransfected into 293T cells with their respective activators and increasing amounts of the p65 (transcriptionally active) subunit of NF-κB. The coexpression of p65 with the viral transactivators resulted in a dose-dependent inhibition of transcription activation on all four promoters (Fig. 1B). We found that p65 did not inhibit the activity of the constitutively active simian virus 40 reporter plasmid pRL-SV40, indicating that inhibition is not the result of cellular toxicity or global transcription repression.
FIG. 1.
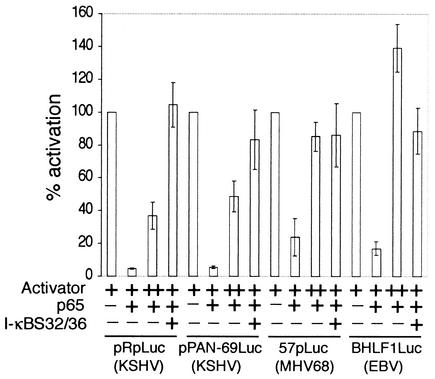
NF-κB(p65) inhibits activation of gammaherpesvirus lytic promoters. (A) Diagram of firefly luciferase (lucif.) reporter constructs used in transient-transfection assays. Constructs represent lytic genes from KSHV (pRpLuc and pPAN-69Luc), MHV68 (57pLuc), and EBV (BHLF1Luc). All reporters are highly inducible by viral immediate-early transactivators. Characterized binding sites for RTA (R) and Zebra (Z) are indicated. (B) 293T cells transiently transfected with the reporter constructs described in panel A. Cells were transfected with a fixed amount (+) of vectors expressing KSHV, MHV68, or EBV RTA/Zebra and increasing amounts of pFLAGp65. Luciferase assay data from three separate experiments are shown. Reporter activity is expressed as a percentage of activation, with activation by RTA or RTA and Zebra alone = 100%.
To further support the specificity of inhibition by p65, we determined whether inhibition could be reversed by either overexpressing the viral transactivators or coexpressing I-κBS32/36, a dominant inhibitor of NF-κB (4). We found that, in the presence of a fixed amount of p65, the 4- to 10-fold overexpression of RTA (pRpluc, pPAN-69Luc, and 57pLuc) or RTA with Zebra (BHLF1Luc) was sufficient to restore promoter activation (Fig. 2). In addition, the expression of I-κBS32/36 completely abolished the inhibitory activity of p65. The p50 subunit of NF-κB is a nuclear protein that can bind to NF-κB sites as a homodimer or as a heterodimer with p65. However, unlike p65, p50 lacks an activation domain and has no inherent activation capacity. We found that p50 did not inhibit activation of the KSHV pRpLuc promoter by RTA (data not shown). By Western blotting, we also confirmed that p65 did not inhibit the cytomegalovirus (CMV)-driven expression of RTA or Zebra (data not shown). These results suggest that NF-κB activity is a specific inhibitor of gammaherpesvirus lytic promoter activation.
FIG. 2.
Inhibition of transactivation by NF-κB(p65) is reversible. 293T cells were transfected with the reporter constructs shown in Fig. 1A and plasmids expressing transactivator(s) RTA or RTA and Zebra. Amounts of viral transactivators were increased 4-fold (pPAN-69Luc), 5-fold (BHLF1Luc), or 10-fold (pRpLuc and 57pLuc [++]) to restore activation in the presence of p65. I-κBS32/36 is a dominant inhibitor of NF-κB.
MHV68 lytic replication is inhibited by NF-κB(p65).
We then investigated whether the inhibition of lytic promoters by p65 correlated with an inhibition of virus replication. Of the three gammaherpesviruses represented in Fig. 1, only MHV68 replicates productively in cell culture. 293T (epithelial) cells are highly permissive for MHV68 replication following either infection or transfection of purified virion DNA (42). These cells also have low endogenous NF-κB activity. We therefore cotransfected 293T cells with MHV68 virion DNA and increasing amounts of the p65 expression vector and assayed the effect on virus replication by Western blotting (Fig. 3A) and by plaque assay (Fig. 3B). Five days after transfection with virion DNA alone, multiple lytic proteins were detected in whole-cell lysates, indicating productive MHV68 replication (Fig. 3A, lane 2). However, coexpression of p65 resulted in a dose-dependent inhibition of lytic protein synthesis (Fig. 3A, lanes 3 to 6). In addition, Western blotting showed that the levels of actin were higher in the cell extracts prepared from cells coexpressing p65 and virion DNA than in those from cells expressing virion DNA alone (Fig. 3A, compare lanes 2 and 6). This was a reflection of enhanced survival and proliferation of the cells overexpressing p65, consistent with their protection from the cytopathic effects of virus replication. Microscopic analysis of the transfected cells 5 days posttransfection also confirmed that the cells transfected with virion DNA alone showed visible cytopathic signs of virus replication, whereas the cells cotransfected with virion DNA and p65 did not (Fig. 3C). Plaque assays performed on the supernatants from these cells also demonstrated a dose-dependent decrease in virus production with increasing expression of p65 (Fig. 3B).
Inhibition of virus replication by NF-κB is reversible.
To confirm that the cells transfected with virion DNA and p65 were successfully transfected and were maintaining the viral DNA in the absence of significant replication, we also passaged a fraction of these cells (Fig. 4, lane 3) and monitored virus replication for several days. To minimize the transfer of infectious virus, the cell supernatant was removed before the cells were plated in fresh media. Cell extracts were then analyzed for virus replication 1, 2, and 3 days later (i.e., days 6, 7, and 8 posttransfection). Western blots showed that after the cells were passaged, the p65 levels gradually returned to their initial low level (Fig. 4, lanes 4 to 6). In addition, as the p65 levels declined, the expression of viral lytic proteins increased dramatically (Fig. 4, upper panel). There was therefore a strong inverse correlation between the levels of p65 in the cell and the extent of virus replication. In the presence of high levels of p65, the viral DNA was maintained in cells, but the level of virus replication was very low (Fig. 4, lane 3). As the cells proliferated in culture, expression of p65 from the plasmid vector declined, and this decrease in NF-κB activity correlated with viral reentry into the lytic cycle as the cells were restored to their permissive state (lane 6).
Both the DNA binding and activation domains of p65 are required for inhibition.
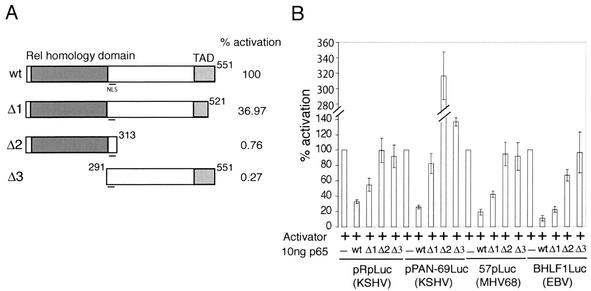
The p65 subunit of NF-κB is able to bind to consensus NF-κB binding sites and activate transcription (35). However, our data also suggests that it functions as a transcription inhibitor. To determine which domains of p65 are required for inhibition, we constructed p65 deletion mutants (Fig. 5A). The Δ1 mutant lacks 30 amino acids from the C terminus, a deletion that results in a 63% decrease in activation of an NF-κB-responsive reporter (pNF-κB-Luc). The Δ2 and Δ3 mutants lack the entire C-terminal activation domain or DNA binding/dimerization domain, respectively, and are essentially inactive for transactivation. However, both Δ2 and Δ3 mutants retain the nuclear localization signal, which is located between amino acids 291 and 313. We confirmed by Western blotting that these mutants were expressed efficiently in 293T cells (data not shown). We then tested their ability to inhibit activation of the lytic promoters described in the legend to Fig. 1A. On all reporters tested, the Δ1 p65 mutant retained some capacity to inhibit lytic promoter activity, although it was much less effective than the full-length protein (Fig. 5B). However, both the Δ2 and Δ3 mutants were unable to inhibit promoter activation. These results showed that both the DNA binding/dimerization domain and activation domain of p65 are required for its ability to inhibit lytic promoter activation.
FIG. 5.
Both the activation domain and DNA binding domain of p65 are required for inhibition of MHV68 lytic gene expression. (A) Diagram of pFLAGp65 deletion mutants used in panel B and Fig. 6. The N-terminal DNA binding/dimerization (Rel homology) domain and C-terminal activation domain (TAD) are indicated. Mutants were tested for transactivation by transient transfection into 293T cells with a reporter bearing five consensus NF-κB binding sites upstream of firefly luciferase (pNF-κBLuc). The percentage of activation represents the average results comparing 5, 10, and 50 ng of each pFLAGp65 construct in three separate experiments. (B) Transient transfection of 293T cells as described for Fig. 1B. Cells were transfected with RTA or RTA and Zebra (+) and 10 ng of wild type (wt), Δ1, or Δ2 pFLAGp65.
The p65 deletion mutants were then tested for their ability to inhibit virus replication after cotransfection into 293T cells with purified MHV68 DNA (as described in Fig. 3). The results were consistent with those from the transient transfection assay and demonstrated that the Δ1 mutant of p65 had a reduced capacity to inhibit lytic protein synthesis (Fig. 6A, compare lanes 3 and 4), whereas the Δ2 and Δ3 mutants had no inhibitory activity (lanes 5 and 6). The virion DNA used in the transient transfection assay was generated from a recombinant MHV68 strain expressing green fluorescent protein (GFP) from the CMV promoter in a nonessential region of the genome (42). This virus therefore allows the extent of virus replication to be visualized in the transfected cells (Fig. 6C). Two days posttransfection, the cells transfected with virion DNA alone showed high levels of GFP expression in the form of cell clusters, consistent with replication of the virus in transfected cells followed by release of new virions and infection of neighboring cells. However, in the cells transfected with virion DNA and either full-length or Δ1 p65, GFP expression was much lower and was in general confined to single cells, consistent with successful transfection of the cells but inefficient replication and therefore limited reinfection of neighboring cells. However, cells transfected with virion DNA and either the Δ2 or Δ3 p65 mutants expressed GFP in a pattern similar to virion DNA alone, with large clusters of fluorescent cells, in addition to single cells expressing GFP. The release of infectious MHV68 into the media of these transfected cells was also analyzed by plaque assay. Consistent with the previous assays, the cotransfection of either wild-type or Δ1 p65 with virion DNA resulted in a <10-fold decrease in the production of infectious virions (Fig. 6B). Unlike the transient transfection and Western blot assays, the Δ2 and Δ3 p65 mutants retained some inhibitory activity (inhibiting virus production by two- to threefold), although they were much less effective than the wild type or Δ1 mutant. The results of the transient transfection and Western blot assays were consistent with each other and demonstrated that both the N-terminal DNA binding/dimerization domain and C-terminal activation domain of p65 are required for its inhibitory activity. The extent of inhibition therefore closely mirrored the activation capacity of the p65 mutants on the NF-κB-dependent reporter (Fig. 5A).
Inhibition of NF-κB in lymphocytes leads to reactivation of latent virus.
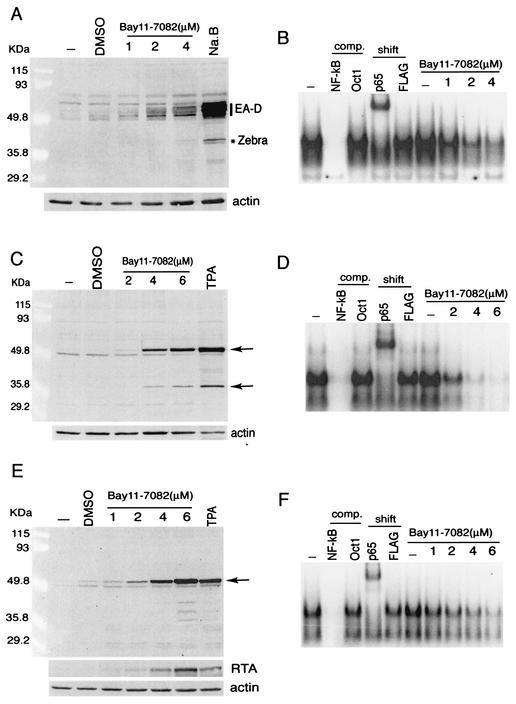
Our results support an inhibitory role for NF-κB in the initiation of gammaherpesvirus replication. This is consistent with the ability of this family of viruses to divert their transcription program from lytic replication to latency in cell types in which endogenous NF-κB activity is high. This model implies that high levels of NF-κB are required to maintain the latent infection. To test this, we treated B lymphocytes latently infected with EBV (HR-1) or KSHV (KS-1, BCBL1) with Bay11-7082, a specific inhibitor of NF-κB (29). Cells were incubated for 30 min in concentrations of Bay11-7082 previously reported to inhibit NF-κB in lymphocytes (19). After 30 min, cells were resuspended in fresh medium and incubated for a further 48 h. Cell extracts were assayed for lytic protein synthesis by Western blotting. NF-κB has been shown to be important for the survival of lymphocytes, with prolonged inhibition of NF-κB leading to apoptosis (5, 19, 28). However, after a brief incubation with the NF-κB inhibitor Bay11-7082 followed by 48 h of recovery, we observed an induction of EBV (Fig. 7A) and KSHV (Fig. 7C and E) lytic protein synthesis. After treatment with Bay11-7082, EBV-infected lymphocytes expressed early lytic protein EA-D and immediate-early lytic transactivator Zebra, similar to cells treated with sodium butyrate, a potent inducer of virus reactivation (34). Nuclear extracts analyzed by EMSA directly after the 30-min incubation period confirmed that the Bay11-7082 concentrations and conditions used were effectively inhibiting NF-κB in these cells (Fig. 7B). KSHV-infected cell lines were analyzed similarly and also showed induction of lytic antigens after treatment with Bay11-7082. In this case, the effect of Bay11-7082 was compared to treatment with TPA, a potent inducer of KSHV reactivation (32). Treatment of KS-1 cells resulted in expression of proteins identical to those induced by TPA (Fig. 7C). Immunofluorescence also confirmed the expression of the KSHV early lytic antigen coded for by ORF59 and the late antigen K8.1A (data not shown). Treatment of a second KSHV-infected cell line, BCBL1, with Bay11-7082 resulted in lytic protein synthesis to a higher level than that seen with TPA (Fig. 7E). In addition, inhibition of NF-κB in these cells resulted in the expression of the lytic transactivator RTA (Fig. 7E), which is known to be sufficient to initiate lytic replication in these cells. EMSA analysis of the KS-1 and BCBL1 nuclear extracts directly after treatment with Bay11-7082 confirmed that the concentrations and conditions were appropriate for inhibition of NF-κB (Fig. 7D and F). Other compounds known to inhibit NF-κB in lymphocytes such as anti-inflammatory prostaglandins and proteasome inhibitors were also found to be potent inducers of EBV and KSHV reactivation (H. Brown, unpublished observations). Our data therefore support the conclusion that high levels of NF-κB are required to maintain gammaherpesvirus latency. This is consistent with a report that signaling by EBV latent membrane protein 1 (LMP1) and cellular CD40, which activate the NF-κB pathway, inhibits reactivation of EBV from several latently infected cell lines (1).
FIG. 7.
NF-κB inhibitor Bay11-7082 induces lytic protein synthesis in latent KSHV- and EBV-infected cells. (A, C, and E) Western blots of HR-1 (A), KS-1 (B), and BCBL1 (C) whole-cell extracts. Cells were treated with sodium butyrate (A [Na.B]), TPA (C and E), or Bay11-7082. Blots were probed with nasopharyngeal carcinoma patient serum (A) or Kaposi's sarcoma patient serum recognizing several lytic antigens (C and E), polyclonal anti-RTA serum (E), and a MAb recognizing actin. DMSO, dimethyl sulfoxide. (B, D, and F) EMSA analysis of NF-κB activity in HR-1 (B), KS-1 (D), and BCBL1 (F) cells. Nuclear extracts were harvested directly after the 30-min treatment with Bay11-7082. Extracts were incubated with a consensus NF-κB binding site oligonucleotide. The specificity of the shifted complex was confirmed by using cold competitor oligonucleotides (comp.) and MAbs against p65 and FLAG (shift).
DISCUSSION
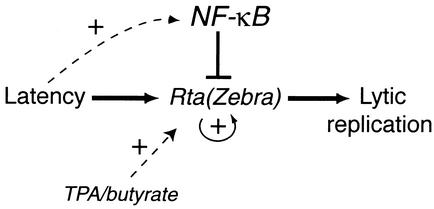
Immediate-early viral transactivators are known to play an important role in reactivating latent gammaherpesviruses (7, 15, 30, 38, 39, 43, 44). However, in specific cell types, gammaherpesviruses can maintain a latent infection for long periods of time. Therefore, cell-specific factors are also likely to be involved in regulating the balance between latency and lytic replication. We find that high levels of NF-κB inhibit gammaherpesvirus lytic promoter activation, lytic protein synthesis, and virus replication. In addition, the inhibition of NF-κB in latently infected cells leads to lytic protein synthesis. These data lead us to a model to describe the cell-specific regulation of gammaherpesvirus life cycles (Fig. 8). The induction of lytic replication is controlled by immediate-early viral transactivators (RTA of KSHV and MHV68 and RTA and Zebra of EBV). However, in lymphocytes, these transactivators are inhibited by high levels of NF-κB. This blocks both the expression of downstream lytic genes and the expression of RTA and Zebra themselves (via autoactivation). The inhibition of virus replication protects the cell from the cytopathic effects of viral protein synthesis and promotes the establishment of a latent infection. The expression of viral latent gene products then up-regulates NF-κB further (6, 14, 21), increasing the inhibition of lytic gene expression and stabilizing the latent infection. A decrease in NF-κB activity can release the inhibition on the lytic promoters, leading to reactivation. According to this model, transactivators are the initiators of reactivation and NF-κB regulates the levels of these proteins (i.e., the threshold) required to initiate this switch.
FIG. 8.
Model outlining a role for NF-κB in regulating gammaherpesvirus replication. (See text for details.)
Our data clearly demonstrate that different cell lines have different thresholds for reactivation via inhibition of NF-κB. This is consistent with well-established variations in the response of latently infected cell lines to chemical inducers and, in some cases, the necessity to combine chemical inducers in order to see robust reactivation. The difference in responsiveness between cell lines may be a reflection of variations in the NF-κB activity and/or RTA and Zebra regulation in different cells. Cell lines with high levels of NF-κB may be less sensitive to reactivation than those with less NF-κB activity or significant basal RTA or Zebra expression. Our data do not exclude reactivation by additional mechanisms. A signaling pathway leading directly to activation of the RTA or Zebra promoters may increase lytic gene expression sufficiently to overcome the inhibitory effect of NF-κB. This is exemplified by TPA and sodium butyrate, which activate the RTA and Zebra promoters but do not inhibit NF-κB (11) (H. Deng, unpublished observation). It is also consistent with our observation that increasing expression of RTA/Zebra is sufficient to restore activity to lytic promoters even in the presence of high levels of p65 (Fig. 2).
The mechanism by which NF-κB(p65) inhibits lytic promoters remains unclear. Our deletion analysis (Fig. 5) demonstrated that both the N-terminal DNA binding/dimerization domain and the C-terminal activation domain of p65 are required for inhibition. This may indicate that NF-κB is transactivating a cellular promoter and driving the expression of an inhibitor. Alternatively, as transcription factors interact with cofactors via their activation domains, p65 may be competing with RTA and Zebra for binding to common transcription targets (25, 31, 41). Although p65 has the capacity to bind directly to DNA via its N-terminal Rel homology domain, only two of the four promoters tested in the transient transfection assay (Fig. 1) contain consensus NF-κB binding sites (pPAN-69Luc and BHLF1Luc). This suggests that p65 does not require direct binding to the lytic promoters in order to inhibit promoter activation. This is supported by a report that p65 inhibits activation of the cellular interleukin-6 promoter by KSHV RTA and that this inhibition requires promoter sequences that do not include NF-κB binding sites (33). p65 may therefore be recruited to promoters indirectly via protein-protein interactions with RTA, Zebra, or other factors (13, 16). In this case, inhibition may result from stearic hindrance of the viral transactivators by p65 or competition for interactions with the basal transcription machinery. However, although p65 has been shown to interact directly with EBV Zebra (13), coimmunoprecipitation experiments did not detect any direct interaction between p65 and KSHV RTA (data not shown).
Current strategies to target latently infected tumor cells include reactivating latent virus in the presence of antiherpesvirus agents that block new virus production. Tumor cells would therefore succumb to the effects of viral protein synthesis without supporting the production of new virions. Our data suggest that NF-κB inhibitors may prove effective for the disruption of viral latency and therefore the destruction of latently infected tumor cells in vivo.
Acknowledgments
This work was supported by the National Institutes of Health (CA91791, DE14153), the Stop Cancer Foundation, the Concern Foundation, the UC Cancer Research Coordinating Committee, and the Jonsson Cancer Center Foundation (R.S.). H.J.B. was funded by a postdoctoral fellowship from the California Universitywide AIDS Research Program.
REFERENCES
- 1.Adler, B., E. Schaadt, B. Kempkes, U. Zimber-Strobl, B. Baier, and G. W. Bornkamm. 2002. Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1. Proc. Natl. Acad. Sci. USA 99:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arenzana-Seisdedos, F., P. Turpin, M. Rodriguez, D. Thomas, R. T. Hay, J. L. Virelizier, and C. Dargemont. 1997. Nuclear localization of IκBα promotes active transport of NF-κB from the nucleus to the cytoplasm. J. Cell Sci. 110:369-378. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle, P. A., and D. Baltimore. 1988. IκB: a specific inhibitor of the NF-κB transcription factor. Science 242:540-546. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 5.Cahir-McFarland, E. D., D. M. Davidson, S. L. Schauer, J. Duong, and E. Kieff. 2000. NF-κB inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 97:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhary, P. M., A. Jasmin, M. T. Eby, and L. Hood. 1999. Modulation of the NF-κB pathway by virally encoded death effector domains-containing proteins. Oncogene 18:5738-5746. [DOI] [PubMed] [Google Scholar]
- 7.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, M. A., J. Leahy, and J. M. Hardwick. 1990. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J. Virol. 64:313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng, H., A. Young, and R. Sun. 2000. Auto-activation of the rta gene of human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 81:3043-3048. [DOI] [PubMed] [Google Scholar]
- 10.Finco, T. S., and A. S. Baldwin. 1995. Mechanistic aspects of NF-κB regulation: the emerging role of phosphorylation and proteolysis. Immunity 3:263-272. [DOI] [PubMed] [Google Scholar]
- 11.Flemington, E., and S. H. Speck. 1990. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutsch, D. E., E. A. Holley-Guthrie, Q. Zhang, B. Stein, M. A. Blanar, A. S. Baldwin, and S. C. Kenney. 1994. The bZIP transactivator of Epstein-Barr virus, BZLF1, functionally and physically interacts with the p65 subunit of NF-κB. Mol. Cell. Biol. 14:1939-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammarskjold, M.-L., and M. C. Simurda. 1992. Epstein-Barr virus latent membrane protein transactivates the human immunodeficiency virus type 1 long terminal repeat through induction of NF-κB activity. J. Virol. 66:6496-6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong, Y., E. Holley-Guthrie, and S. Kenney. 1997. The bZip dimerization domain of the Epstein-Barr virus BZLF1 (Z) protein mediates lymphoid-specific negative regulation. Virology 229:36-48. [DOI] [PubMed] [Google Scholar]
- 17.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, C., D. Van Antwerp, and T. J. Hope. 1999. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IκBα. EMBO J. 18:6682-6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller, S. A., E. J. Schattner, and E. Cesarman. 2000. Inhibition of NF-κB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 96:2537-2542. [PubMed] [Google Scholar]
- 20.Kenney, S., E. Holley-Guthrie, E.-C. Mar, and M. Smith. 1989. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J. Virol. 63:3878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laherty, C. D., H. M. Hu, A. W. Opipari, F. Wang, and V. M. Dixit. 1992. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor κB. J. Biol. Chem. 267:24157-24160. [PubMed] [Google Scholar]
- 22.Liu, S., I. V. Pavlova, H. W. Virgin IV, and S. H. Speck. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J. Virol. 74:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 25.McKay, L. I., and J. A. Cidlowski. 1998. Cross-talk between nuclear factor-κB and the steroid hormone receptors: mechanisms of mutual antagonism. Mol. Endocrinol. 12:45-56. [DOI] [PubMed] [Google Scholar]
- 26.Mitsouras, K., B. Wong, C. Arayata, R. C. Johnson, and M. Carey. 2002. The DNA architectural protein HMGB1 displays two distinct modes of action that promote enhanceosome assembly. Mol. Cell. Biol. 22:4390-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyamoto, S., P. J. Chiao, and I. M. Verma. 1994. Enhanced IκBα degradation is responsible for constitutive NF-κB activity in mature murine B-cell lines. Mol. Cell. Biol. 14:3276-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori, N., Y. Yamada, S. Ikeda, Y. Yamasaki, K. Tsukasaki, Y. Tanaka, M. Tomonaga, N. Yamamoto, and M. Fujii. 2002. Bay 11-7082 inhibits transcription factor NF-κB and induces apoptosis of HTLV-I-infected T-cell lines and primary adult T-cell leukemia cells. Blood 100:1828-1834. [DOI] [PubMed] [Google Scholar]
- 29.Pierce, J. W., R. Schoenleber, G. Jesmok, J. Best, S. A. Moore, T. Collins, and M. E. Gerritsen. 1997. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272:21096-21103. [DOI] [PubMed] [Google Scholar]
- 30.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray, A., and K. E. Prefontaine. 1994. Physical association and functional antagonism between the p65 subunit of transcription factor NF-κB and the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 91:752-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 33.Roan, F., N. Inoue, and M. K. Offermann. 2002. Activation of cellular and heterologous promoters by the human herpesvirus 8 replication and transcription activator. Virology 301:293-304. [DOI] [PubMed] [Google Scholar]
- 34.Saemundsen, A. K., B. Kallin, and G. Klein. 1980. Effect of n-butyrate on cellular and viral DNA synthesis in cells latently infected with Epstein-Barr virus. Virology 107:557-561. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz, M. L., and P. A. Baeuerle. 1991. The p65 subunit is responsible for the strong transcription activating potential of NF-κB. EMBO J. 10:3805-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinclair, A. J., M. Brimmell, F. Shanahan, and P. J. Farrell. 1991. Pathways of activation of the Epstein-Barr virus productive cycle. J. Virol. 65:2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song, M. J., H. J. Brown, T.-T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by Rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma, I. M., J. K. Stevenson, E. M. Schwarz, D. Van Antwerp, and S. Miyamoto. 1995. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 41.Webster, G. A., and N. D. Perkins. 1999. Transcriptional cross talk between NF-κB and p53. Mol. Cell. Biol. 19:3485-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu, T.-T., L. Tong, T. Rickabaugh, S. Speck, and R. Sun. 2001. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J. Virol. 75:9262-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, T.-T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]